Short-Term l-arginine Treatment Mitigates Early Damage of Dermal Collagen Induced by Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatment Protocol
2.2. Skin Sample Preparation, pSHG Imaging, and β-Coefficient Calculation
2.3. Raman Instrumentation and Analysis
2.4. AFM Instrumentation and Analysis
2.5. Picrosirius Red Staining and Image Acquisition
3. Results and Discussion
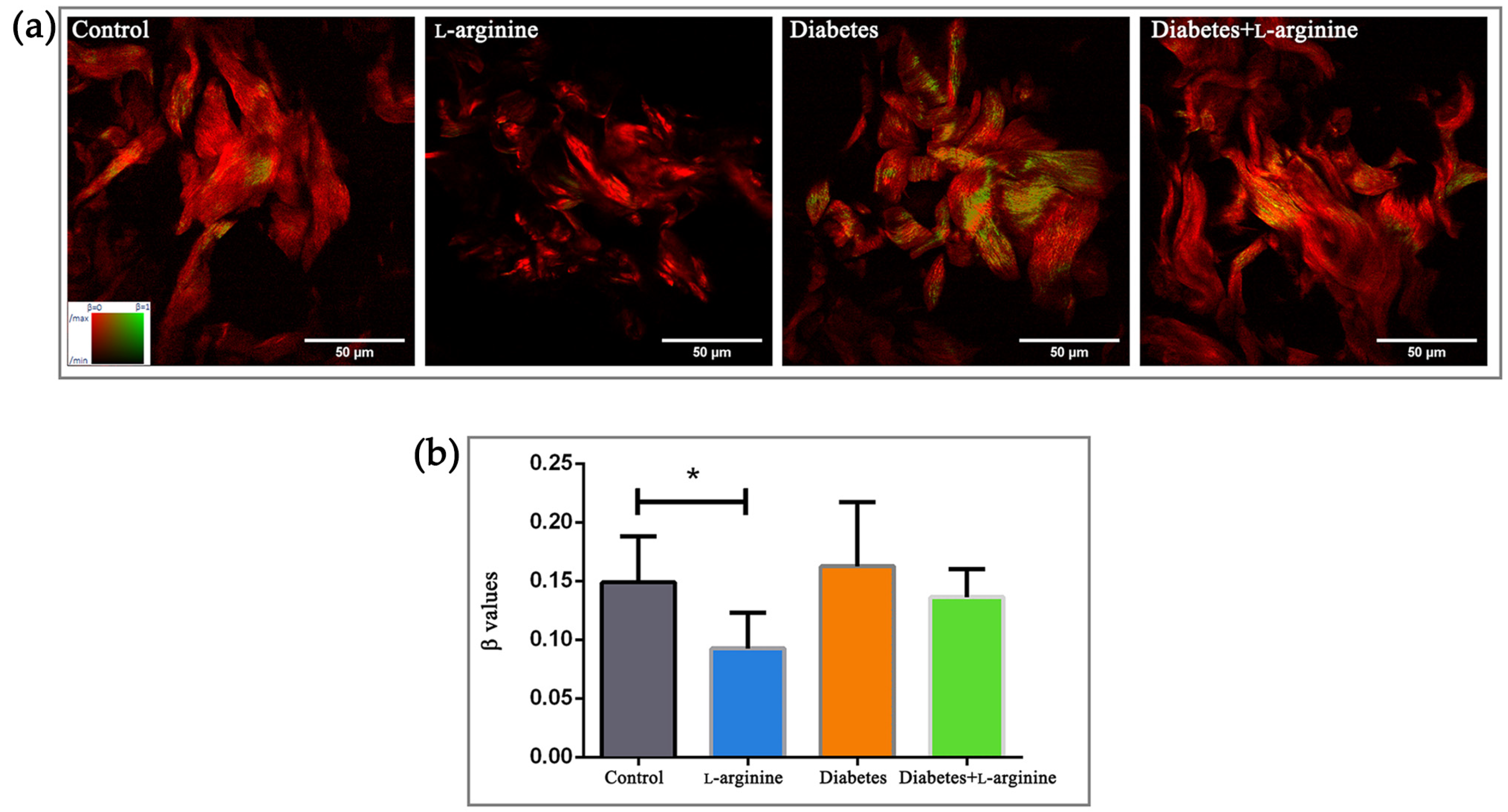
3.1. β-Coefficient Calculation from pSHG Images
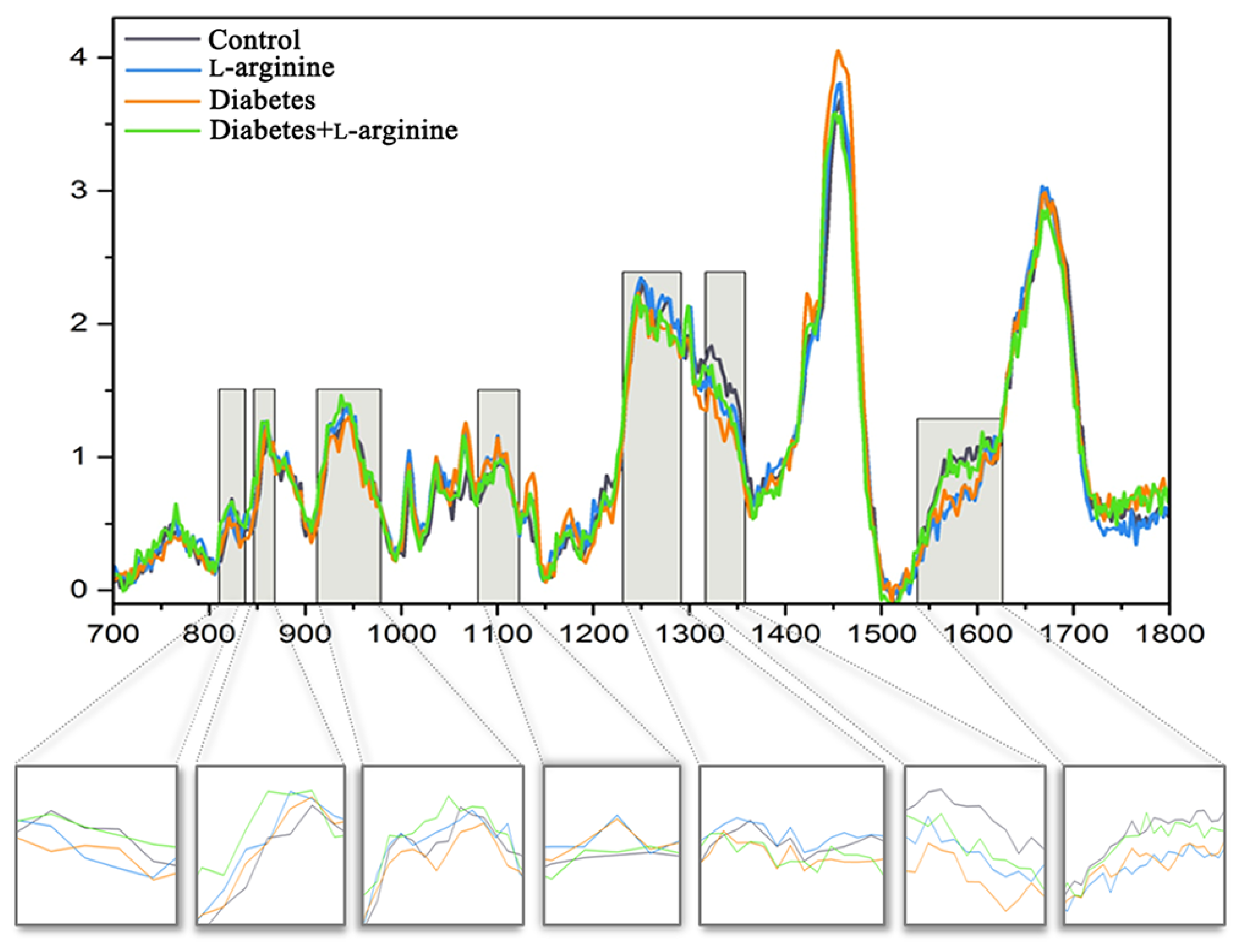
3.2. Raman Spectroscopy Analysis
3.3. AFM Analysis
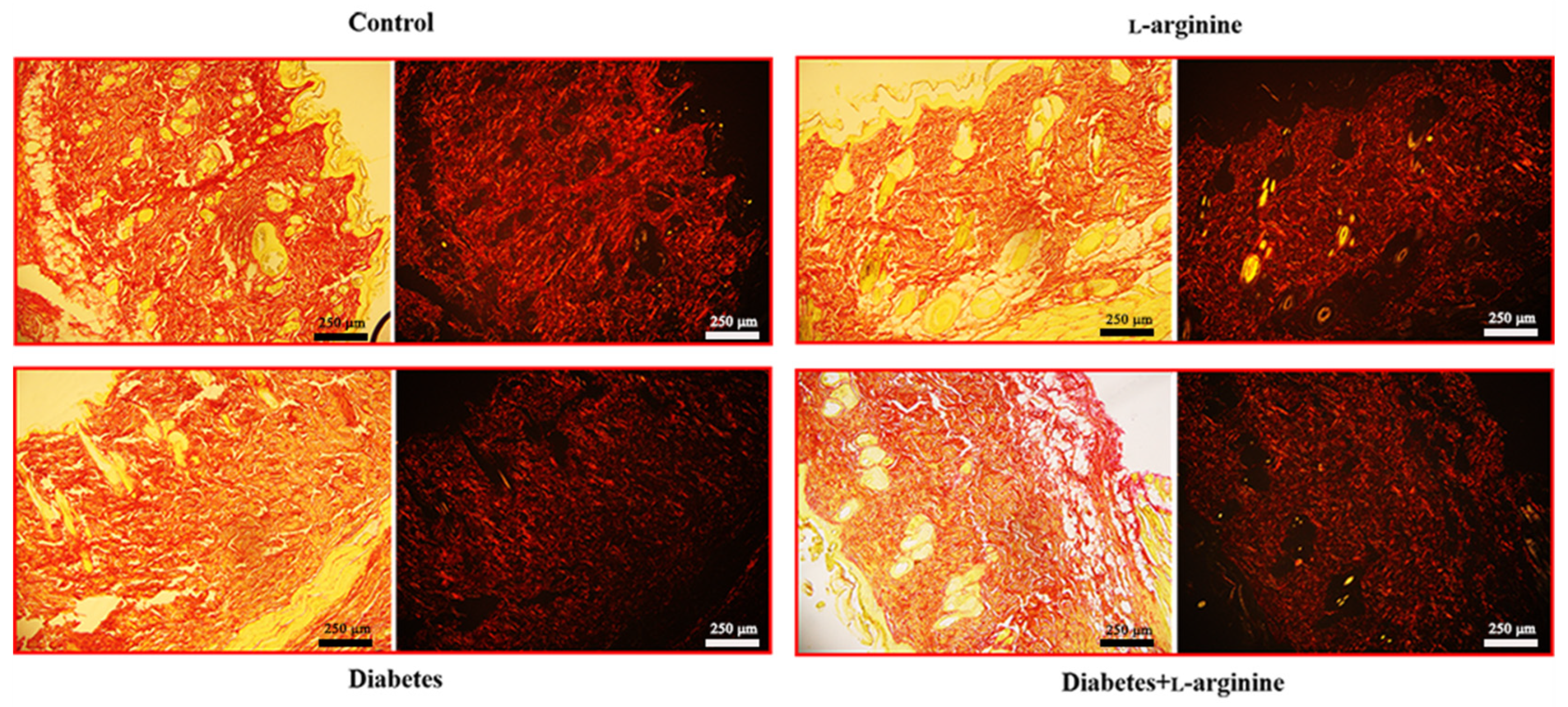
3.4. Picrosirius Red Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; IDF Scientific Committee: Brussels, Belgium, 2021. [Google Scholar]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of Disease: The Oxidative Stress Theory of Diabetic Neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef]
- Black, E.; Vibe-Petersen, J.; Jorgensen, L.N.; Madsen, S.M.; Ågren, M.S.; Holstein, P.E.; Perrild, H.; Gottrup, F. Decrease of Collagen Deposition in Wound Repair in Type 1 Diabetes Independent of Glycemic Control. Arch. Surg. 2003, 138, 34–40. [Google Scholar] [CrossRef]
- Goulding, V. The Effects of Diabetes on Collagen within Wound Healing. Diabet. Foot J. 2015, 18, 201. [Google Scholar]
- Blakytny, R.; Jude, E.B. Altered Molecular Mechanisms of Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2009, 8, 95–104. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory Factors in Refractory Diabetic Wound Healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Mahasarakham, C.P.N.; Kido, D.; Takeda, K.; Izumi, Y. High Glucose-Induced Oxidative Stress Impairs Proliferation and Migration of Human Gingival Fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef]
- Dremin, V.; Marcinkevics, Z.; Zherebtsov, E.; Popov, A.; Grabovskis, A.; Kronberga, H.; Geldnere, K.; Doronin, A.; Meglinski, I.; Bykov, A. Skin Complications of Diabetes Mellitus Revealed by Polarized Hyperspectral Imaging and Machine Learning. IEEE Trans. Med. Imaging 2021, 40, 1207–1216. [Google Scholar] [CrossRef]
- Argyropoulos, A.J.; Robichaud, P.; Balimunkwe, R.M.; Fisher, G.J.; Hammerberg, C.; Yan, Y.; Quan, T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS ONE 2016, 11, e0153806. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Ferreri, C.; Filipovic, M.; Ivanovic-Burmazovic, I.; Stancic, A.; Otasevic, V.; Korac, A.; Buzadzic, B.; Korac, B. Targeting the Superoxide/Nitric Oxide Ratio by l-arginine and SOD Mimic in Diabetic Rat Skin. Free Radic. Res. 2016, 50, S51–S63. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Forzano, I.; Avvisato, R.; Varzideh, F.; Jankauskas, S.S.; Cioppa, A.; Mone, P.; Salemme, L.; Kansakar, U.; Tesorio, T.; Trimarco, V.; et al. l-arginine in Diabetes: Clinical and Preclinical Evidence. Cardiovasc. Diabetol. 2023, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Vasilijević, A.; Buzadžić, B.; Korać, A.; Petrović, V.; Janković, A.; Korać, B. Beneficial Effects of l-arginine-Nitric Oxide-Producing Pathway in Rats Treated with Alloxan. J. Physiol. 2007, 584, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Ghasemi, A. Insulin Secretion: The Nitric Oxide Controversy. EXCLI J. 2020, 19, 1227. [Google Scholar]
- Leiss, V.; Flockerzie, K.; Novakovic, A.; Rath, M.; Schönsiegel, A.; Birnbaumer, L.; Schürmann, A.; Harteneck, C.; Nürnberg, B. Insulin Secretion Stimulated by l-arginine and Its Metabolite l-Ornithine Depends on Gαi2. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E800–E812. [Google Scholar] [CrossRef]
- Mohan, I.K.; Das, U.N. Effect of l-arginine-Nitric Oxide System on Chemical-Induced Diabetes Mellitus. Free Radic. Biol. Med. 1998, 25, 757–765. [Google Scholar] [CrossRef]
- Jankovic, A.; Saso, L.; Korac, A.; Korac, B. Relation of Redox and Structural Alterations of Rat Skin in the Function of Chronological Aging. Oxid. Med. Cell. Longev. 2019, 2019, 2471312. [Google Scholar] [CrossRef]
- Dubey, H.; Dubey, A.; Gulati, K.; Ray, A. Protective effects of l-arginine on cognitive deficits and biochemical parameters in an experimental model of type-2 diabetes mellitus induced alzheimer’s disease in rats. J. Physiol. Pharmacol. 2022, 73, 3–17. [Google Scholar] [CrossRef]
- Hristu, R.; Stanciu, S.G.; Dumitru, A.; Eftimie, L.G.; Paun, B.; Tranca, D.E.; Gheorghita, P.; Costache, M.; Stanciu, G.A. PSHG-TISS: A Collection of Polarization-Resolved Second Harmonic Generation Microscopy Images of Fixed Tissues. Sci. Data 2022, 9, 376. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.V.; Grant, C.; Alexandratou, E. Atomic Force Microscopy on Biological Materials Related to Pathological Conditions. Scanning 2019, 2019, 8452851. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A. Atomic Force Microscopy for Collagen-Based Nanobiomaterials. J. Nanomater. 2017, 2017, 9234627. [Google Scholar] [CrossRef]
- Miler, I.; Rabasovic, M.D.; Aleksic, M.; Krmpot, A.J.; Kalezic, A.; Jankovic, A.; Korac, B.; Korac, A. Polarization-Resolved SHG Imaging as a Fast Screening Method for Collagen Alterations during Aging: Comparison with Light and Electron Microscopy. J. Biophotonics 2021, 14, e202000362. [Google Scholar] [CrossRef] [PubMed]
- Beleites, C.; Sergo, V. HyperSpec: A Package to Handle Hyperspectral Data Sets in R. J. Stat. Softw. 2012. Available online: https://github.com/r-hyperspec/hyperSpec (accessed on 12 March 2024).
- Stylianou, A.; Gkretsi, V.; Patrickios, C.S.; Stylianopoulos, T. Exploring the Nano-Surface of Collagenous and Other Fibrotic Tissues with AFM. In Fibrosis; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1627. [Google Scholar]
- Hermanowicz, P.; Sarna, M.; Burda, K.; Gabryś, H. AtomicJ: An Open Source Software for Analysis of Force Curves. Rev. Sci. Instrum. 2014, 85, 063703. [Google Scholar] [CrossRef]
- Stylianou, A.; Mpekris, F.; Voutouri, C.; Papoui, A.; Constantinidou, A.; Kitiris, E.; Kailides, M.; Stylianopoulos, T. Nanomechanical Properties of Solid Tumors as Treatment Monitoring Biomarkers. Acta Biomater. 2022, 154, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Voutouri, C.; Mpekris, F.; Stylianopoulos, T. Pancreatic Cancer Collagen-Based Optical Signatures. In Polarized Light and Optical Angular Momentum for Biomedical Diagnostics; SPIE: Bellingham, WA, USA, 2021. [Google Scholar]
- Gad, M.Z. Anti-Aging Effects of l-arginine. J. Adv. Res. 2010, 1, 169–177. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. l-arginine Stimulates Fibroblast Proliferation through the GPRC6A-ERK1/2 and PI3K/Akt Pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef]
- Frank, S.; Kämpfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric Oxide Drives Skin Repair: Novel Functions of an Established Mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine Supplementation and Wound Healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Wittmann, F.; Prix, N.; Mayr, S.; Angele, P.; Wichmann, M.W.; Van Den Engel, N.K.; Hernandez-Richter, T.; Chaudry, I.H.; Jauch, K.W.; Angele, M.K. l-arginine Improves Wound Healing after Trauma-Hemorrhage by Increasing Collagen Synthesis. J. Trauma Inj. Infect. Crit. Care 2005, 59, 162–168. [Google Scholar] [CrossRef]
- Martinez, M.G.; Bullock, A.J.; MacNeil, S.; Rehman, I.U. Characterisation of Structural Changes in Collagen with Raman Spectroscopy. Appl. Spectrosc. Rev. 2019, 54, 509–542. [Google Scholar] [CrossRef]
- Van Gulick, L.; Saby, C.; Morjani, H.; Beljebbar, A. Age-Related Changes in Molecular Organization of Type I Collagen in Tendon as Probed by Polarized SHG and Raman Microspectroscopy. Sci. Rep. 2019, 9, 7280. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman Spectroscopy for Identification of Epithelial Cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Kyriakidou, M.; Malesiou, E.; Rallis, M.; Theophanides, T. Infrared and Raman Spectroscopic Studies of Molecular Disorders in Skin Cancer. In Vivo 2019, 33, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman Lines of Phenylalanine Analyzed by a Multiconformational Approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Hernández, B.; Coïc, Y.M.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. All Characteristic Raman Markers of Tyrosine and Tyrosinate Originate from Phenol Ring Fundamental Vibrations. J. Raman Spectrosc. 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gobinet, C.; Feru, J.; Brassart-Pasco, S.; Manfait, M.; Piot, O. Characterization of Type i and IV Collagens by Raman Microspectroscopy: Identification of Spectral Markers of the Dermo-Epidermal Junction. Spectroscopy 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Spanheimer, R.G.; Umpierrez, G.E.; Stumpf, V. Decreased Collagen Production in Diabetic Rats. Diabetes 1988, 37, 371–376. [Google Scholar] [CrossRef]
- Contorno, S.; Darienzo, R.E.; Tannenbaum, R. Evaluation of Aromatic Amino Acids as Potential Biomarkers in Breast Cancer by Raman Spectroscopy Analysis. Sci. Rep. 2021, 11, 1698. [Google Scholar] [CrossRef]
- Cheng, W.T.; Liu, M.T.; Liu, H.N.; Lin, S.Y. Micro-Raman Spectroscopy Used to Identify and Grade Human Skin Pilomatrixoma. Microsc. Res. Tech. 2005, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Prokopi, N.; Andrikopoulos, K.S.; Beobide, A.S.; Voyiatzis, G.A.; Papachristou, D.J. Collagen Orientation Probed by Polarized Raman Spectra Can Serve as Differential Diagnosis Indicator between Different Grades of Meniscus Degeneration. Sci. Rep. 2021, 11, 20299. [Google Scholar] [CrossRef] [PubMed]
- Su, J.W.; Wang, Q.; Tian, Y.; Madden, L.; Ling Teo, E.M.; Becker, D.L.; Liu, Q. Depth-Sensitive Raman Spectroscopy for Skin Wound Evaluation in Rodents. Biomed. Opt. Express 2019, 10, 6114. [Google Scholar] [CrossRef] [PubMed]
- Joodaki, F.; Martin, L.M.; Greenfield, M.L. Planarity and Out-of-Plane Vibrational Modes of Tryptophan and Tyrosine in Biomolecular Modeling. Phys. Chem. Chem. Phys. 2019, 21, 23943–23965. [Google Scholar] [CrossRef]
- Cárcamo, J.J.; Aliaga, A.E.; Clavijo, R.E.; Brañes, M.R.; Campos-Vallette, M.M. Raman Study of the Shockwave Effect on Collagens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Moraes, V.R.; Melo, M.O.; Maia Campos, P.M.B.G. Evaluation of Morphological and Structural Skin Alterations on Diabetic Subjects by Biophysical and Imaging Techniques. Life 2023, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kyriakides, T.R. The Role of Extracellular Matrix in the Pathophysiology of Diabetic Wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef] [PubMed]
- de Souza, Á.d.P.B.; de Oliveira, M.M.R.; de Andrade, R.R.; de Amorim, R.F.B.; Bocca, A.L.; Borin, M.d.F. The in Vivo Effect of l-arginine on Skin Elasticity in Mice. Braz. J. Pharm. Sci. 2017, 53, e00045. [Google Scholar] [CrossRef]
- Saini, R.; Badole, S.L.; Zanwar, A.A. Arginine Derived Nitric Oxide: Key to Healthy Skin. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Humana Press: Totowa, NJ, USA, 2013. [Google Scholar]
- Kotova, S.L.; Timashev, P.S.; Guller, A.E.; Shekhter, A.B.; Misurkin, P.I.; Bagratashvili, V.N.; Solovieva, A.B. Collagen Structure Deterioration in the Skin of Patients with Pelvic Organ Prolapse Determined by Atomic Force Microscopy. Microsc. Microanal. 2015, 21, 324–333. [Google Scholar] [CrossRef]
- Stancic, A.; Jankovic, A.; Korac, A.; Buzadzic, B.; Otasevic, V.; Korac, B. The Role of Nitric Oxide in Diabetic Skin (Patho)Physiology. Mech. Ageing Dev. 2018, 172, 21–29. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miler, I.; Rabasovic, M.D.; Askrabic, S.; Stylianou, A.; Korac, B.; Korac, A. Short-Term l-arginine Treatment Mitigates Early Damage of Dermal Collagen Induced by Diabetes. Bioengineering 2024, 11, 407. https://doi.org/10.3390/bioengineering11040407
Miler I, Rabasovic MD, Askrabic S, Stylianou A, Korac B, Korac A. Short-Term l-arginine Treatment Mitigates Early Damage of Dermal Collagen Induced by Diabetes. Bioengineering. 2024; 11(4):407. https://doi.org/10.3390/bioengineering11040407
Chicago/Turabian StyleMiler, Irena, Mihailo D. Rabasovic, Sonja Askrabic, Andreas Stylianou, Bato Korac, and Aleksandra Korac. 2024. "Short-Term l-arginine Treatment Mitigates Early Damage of Dermal Collagen Induced by Diabetes" Bioengineering 11, no. 4: 407. https://doi.org/10.3390/bioengineering11040407