A Novel Cellulase Produced by a Newly Isolated Trichoderma virens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Media
2.2. Screening the Microbe for Cellulase and Microbe Identification
2.3. Purification of the Cellulase from the Newly Isolated Microbe Culture Broth
2.4. Evaluation of Enzymatic Properties
2.5. Cellulase Activity Assay
3. Results
3.1. Isolation of Microbe for Cellulase and Microbe Identification
3.2. Purification of the Cellulase from the Newly Isolated T. virens ZY-01 Culture Broth
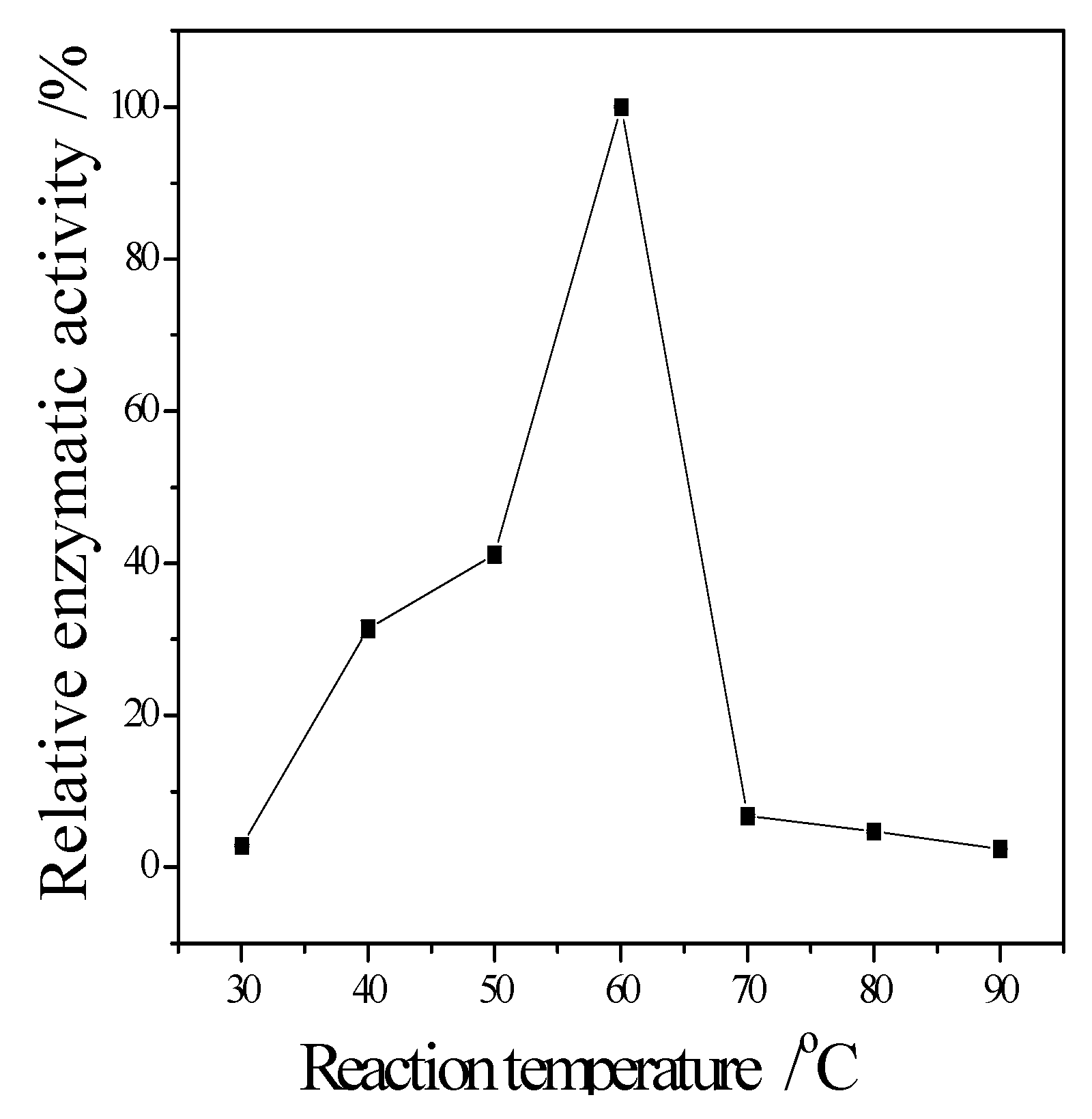
3.3. Enzymatic Properties of Cellulase Produced by T. virens ZY-01
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeya, M.; Zhang, Y.-W.; Kim, I.-W.; Lee, J.-K. Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour. Technol. 2009, 100, 5155–5161. [Google Scholar] [CrossRef] [PubMed]
- Back, S.C.; Kwon, Y.J. Optimization of the pretreatment of rice straw hemicellulosic hydrolyzates for microbial production of xulytol. Biotechnol. Bioprocess Eng. 2007, 12, 404–409. [Google Scholar] [CrossRef]
- Delmer, D.P.; Haigler, C.H. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metab. Eng. 2002, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolsates for ethanol production. Enzym. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Ramos, L.P.; Nahzad, M.; Saddler, J.N. Effect of enzymatic hydrolysis on the morphology and fine structure of pretreated cellusolic residues. Enzym. Microb. Technol. 1993, 15, 821–831. [Google Scholar] [CrossRef]
- Bothwell, M.; Daughhete, S.; Chaua, G. Binding capacilities for Thermonmonspora fusca E-3, and E-4. The E-3 binding domain and Trichoderma reesei CBHI on avicel and bacterial microcryrstallie cellulose. Bioresour. Technol. 1997, 60, 169–178. [Google Scholar] [CrossRef]
- Hii, K.-L.; Yeap, S.-P.; Mashitah, D.M. Cellulase production from palm oil mill effluent in Malaysia economical and technical perspectives. Eng. Life Sci. 2012, 12, 7–28. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, W.; Yuan, P. Screening and identification of cellulase-producing strain of Fusarium oxysporum. Procedia Environ. Sci. 2012, 12, 1213–1219. [Google Scholar]
- Assareh, R.; Shahbani, H.Z.; Akbari, K.N.; Aminzadeh, S.; Bakhshi, G.K. Characterization of the newly isolated Geobacillus sp. T1, the efficient cellulase-producer on untreated barley and wheat straws. Bioresour. Technol. 2012, 120, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yang, H.J.; Roy, B.; Park, E.Y.; Jiang, L.J.; Wang, D.; Miao, Y.G. Enhanced cellulase production of the Trichoderma viride mutated by microwave and ultraviolet. Microbiol. Res. 2010, 165, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, G.; Zhou, W.; Hou, Y.; Yang, Z. Progress of cellulase and cellulase gene research. Biotechnol. Bull. 2013, 2013, 35–40. (In Chinese) [Google Scholar]
- Fungal Identification Manual; Wei, J.C. (Ed.) Shanghai Science and Technology Press: Shanghai, China, 1979. (In Chinese)
- Xue, S.J.; Yue, T.L.; Yuan, Y.H.; Gao, Z.P. An improved method for extracting fungal DNA. Food Res. Dev. 2006, 27, 39–40. (In Chinese) [Google Scholar]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Muthuvelayudham, R.; Viruthagiri, T. Application of statistical design for the production of cellulase by Trichoderma reesei using mango peel. Enzym. Res. 2012, 2012, 157643. [Google Scholar]
- Tang, B.; Pan, H.; Tang, W.; Zhang, Q.; Dinga, L.; Zhang, F. Fermentation and purification of cellulase from a novel strain Rhizopus stolonifer var. reflexus TP-02. Biomass Bioenergy 2012, 36, 366–372. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Toubarro, D.; Teixeira, M.; Simõs, N. Purification and biochemical characterization of a novel thermo-stable carboxymethyl cellulase from azorean isolate Bacillus mycoides S122C. Appl. Biochem. Biotechnol. 2012, 168, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, W.; Pei, Y.; Lu, F. Screening and Identification of Cellulase-Producing Strain of Fusarium Oxysporum. Procedia Environ. Sci. 2011, 12, 1213–1219. [Google Scholar] [CrossRef]
- Ketna, M.; Digantkumar, C.; Jyoti, D.; Anand, N.; Datta, M. Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int. Biodeterior. Biodegrad. 2013, 78, 24–33. [Google Scholar]







| Strain 1 | Colony Diameter/cm | Hydrolysised Circle Diameter/cm | Colony Color | Colony Morphology |
|---|---|---|---|---|
| ZY-01 | 2.30 ± 0.22 | 4.32 ± 0.26 | Early phase white, Anaphase green | Edge irregular dentatus, Surface filiform |
| ZY-02 | 1.87 ± 0.41 | 2.82 ± 0.38 | white | Edge irregular dentatus, Surface filiform |
| ZY-03 | 2.12 ± 0.49 | 3.02 ± 0.37 | Front milk white, Back yellow | Edge irregular dentatus, Surface filiform |
| ZY-04 | 3.27 ± 0.43 | fuzzy | Front yellow-white, Back red | Edge irregular dentatus, Surface smooth |
| Procedure | Protein Content/mg | Enzyme Activity/IU | Specific Activity IU/mg | Yield % | Purification Fold |
|---|---|---|---|---|---|
| Crude enzyme | 197.3 | 174.1 | 0.88 | – | – |
| Fractional precipitation | 21.6 | 135.3 | 6.26 | 77.7 | 7.11 |
| Anion exchange chromatography | 15.8 | 106.4 | 6.73 | 61.1 | 7.65 |
| Gel chromatography | 2.6 | 81.9 | 31.5 | 47.04 | 35.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, R.; Yin, X.-Y.; Ruan, T.; Hu, Q.; Hou, Y.-L.; Zuo, Z.-Y.; Huang, H.; Yang, Z.-H. A Novel Cellulase Produced by a Newly Isolated Trichoderma virens. Bioengineering 2016, 3, 13. https://doi.org/10.3390/bioengineering3020013
Zeng R, Yin X-Y, Ruan T, Hu Q, Hou Y-L, Zuo Z-Y, Huang H, Yang Z-H. A Novel Cellulase Produced by a Newly Isolated Trichoderma virens. Bioengineering. 2016; 3(2):13. https://doi.org/10.3390/bioengineering3020013
Chicago/Turabian StyleZeng, Rong, Xiao-Yan Yin, Tao Ruan, Qiao Hu, Ya-Li Hou, Zhen-Yu Zuo, Hao Huang, and Zhong-Hua Yang. 2016. "A Novel Cellulase Produced by a Newly Isolated Trichoderma virens" Bioengineering 3, no. 2: 13. https://doi.org/10.3390/bioengineering3020013
APA StyleZeng, R., Yin, X.-Y., Ruan, T., Hu, Q., Hou, Y.-L., Zuo, Z.-Y., Huang, H., & Yang, Z.-H. (2016). A Novel Cellulase Produced by a Newly Isolated Trichoderma virens. Bioengineering, 3(2), 13. https://doi.org/10.3390/bioengineering3020013






