Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant
Abstract
:1. Introduction
2. Cyanobacteria and Cyanobacterial Energy and Carbon Storage Compounds
2.1. Cyanobacteria–Microalgae or Not?
2.2. Cyanobacterial PHA
2.3. Cyanobacterial Glycogen
2.4. Nitrogen Chlorosis and Photosynthetic Activity
3. Different Cyanobacteria as PHA Producers
3.1. Synechocystis and Synechococcus
3.2. Arthrospira (Spirulina)
3.3. Nostoc
3.4. Other Cyanobacteria
4. CO2 and Nutrient Supply for Mass Cultivation of Cyanobacteria
4.1. CO2 Supply
4.2. Nutrient Supply
5. Three Years’ Working Experience Running a Pilot Plant for Photoautotrophic PHB Production
5.1. Location and Reactor Description
5.2. CO2 Supply of the Reactor
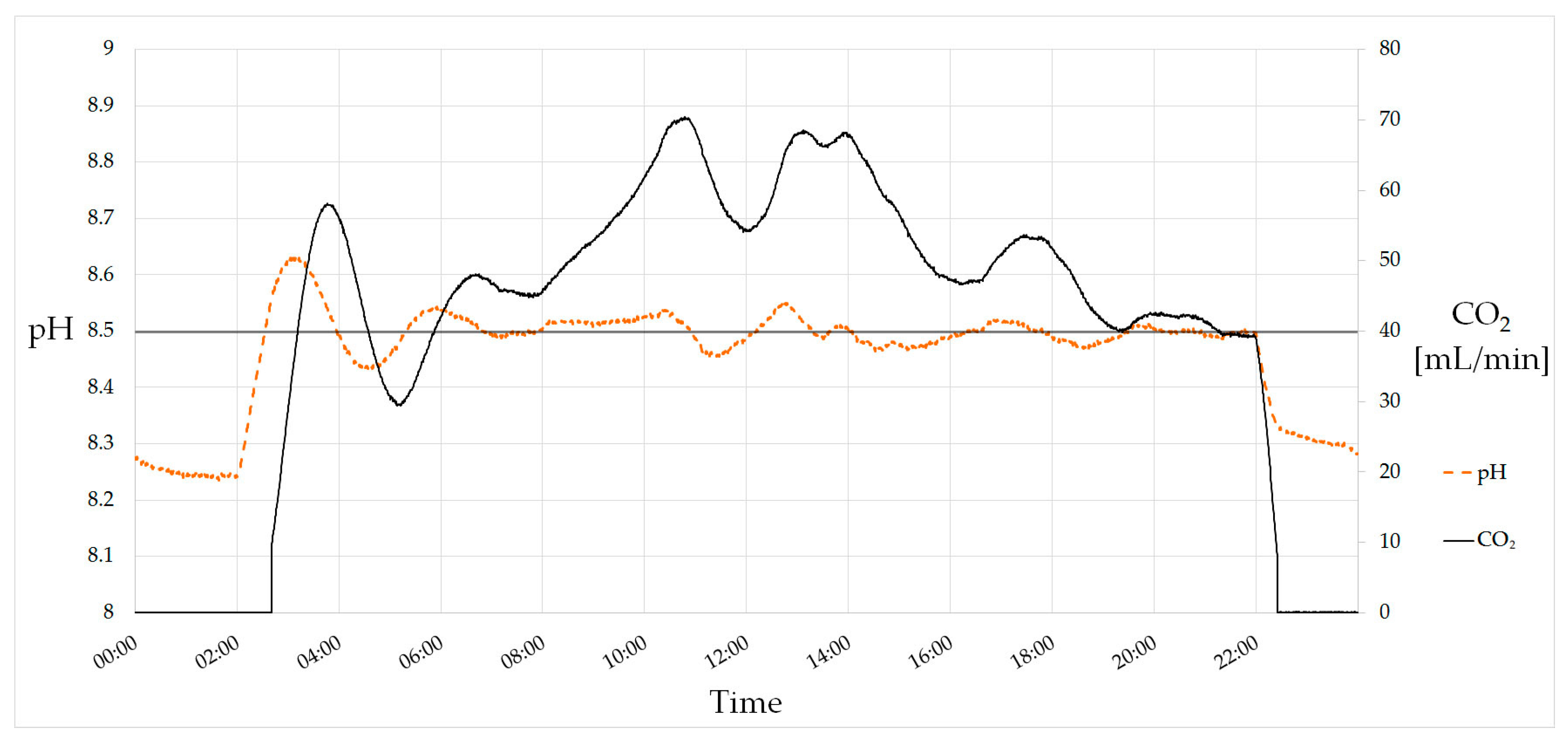
5.3. Automation and pH Control
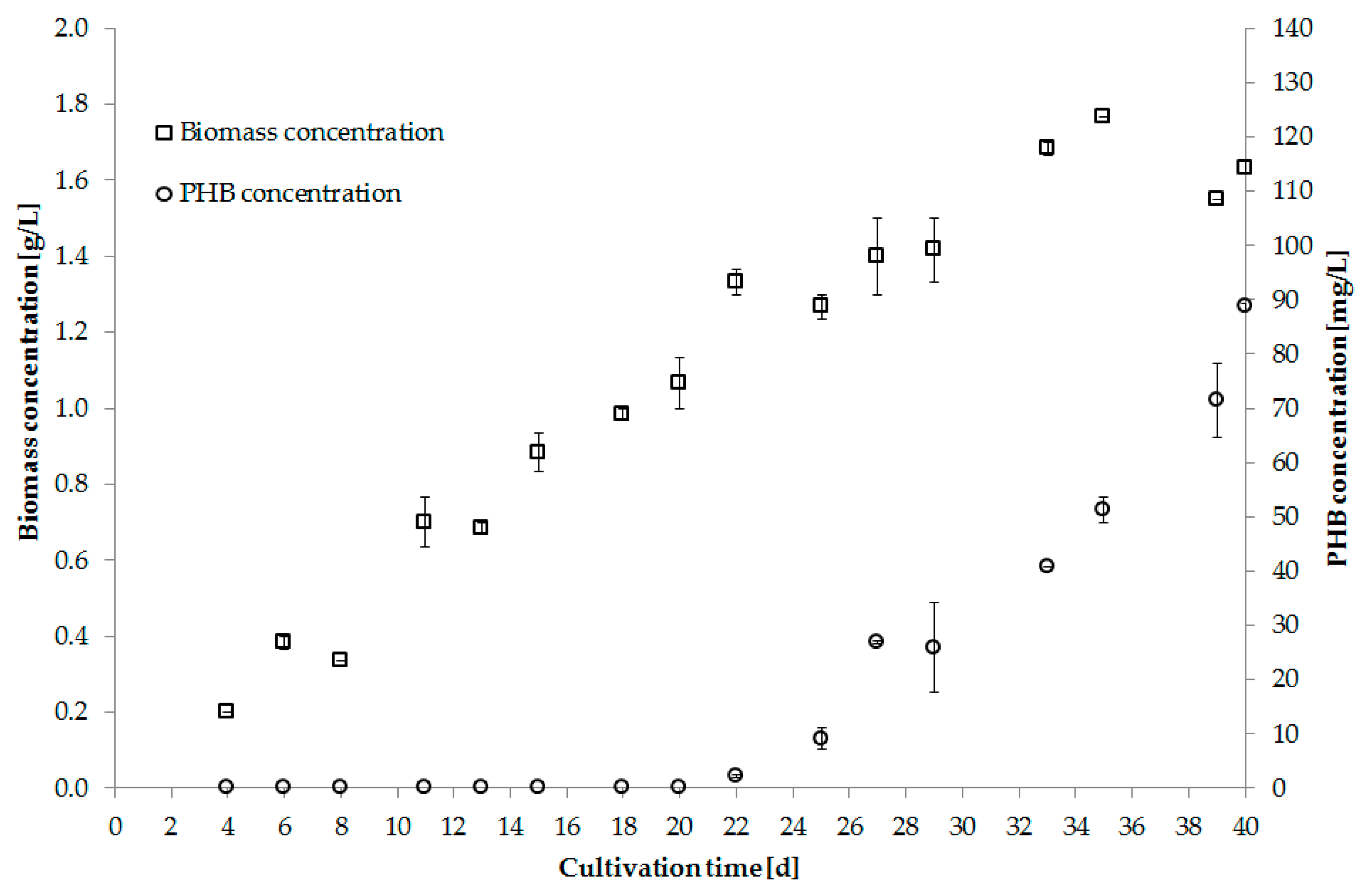
5.4. Overview of PHB Production Trials
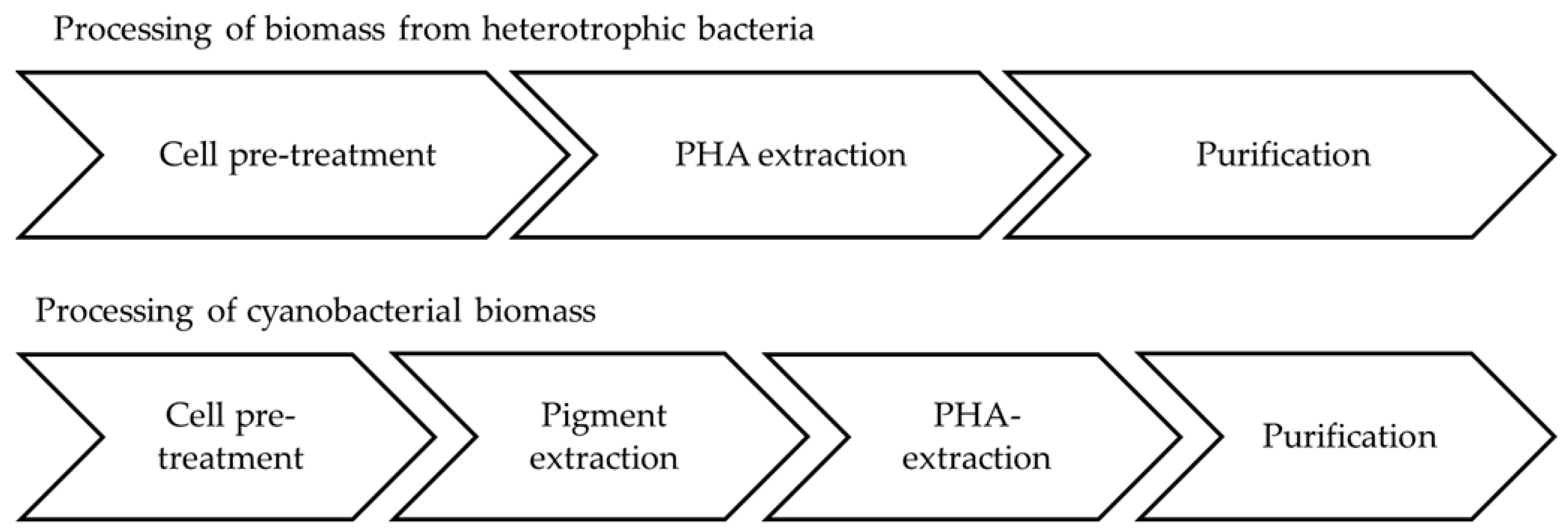
5.5. Downstream Processing of Cyanobacterial Biomass
5.6. Contaminations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khosravi-Darani, K.; Mokhtari, Z.-B.B.; Amai, T.; Tanaka, K. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Dawes, E. Polyhydroxybutyrate: An intriguing biopolymer. Biosci. Rep. 1988, 8, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Halami, P.M. Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J. Microbiol. Biotechnol. 2008, 24, 805–812. [Google Scholar] [CrossRef]
- Ting, C.S.; Rocap, G.; King, J.; Chisholm, S.W. Cyanobacterial photosynthesis in the oceans: The origins and significance of divergent light-harvesting strategies. Trends Microbiol. 2002, 10, 134–142. [Google Scholar] [CrossRef]
- IPCC. 2014: Climate Change 2014: Synthesis Report; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Stanier, R.J.; Cohen-Bazire, G. Phototrophic prokaryotes: The cyanobacteria. Ann. Rev. Microbiol. 1977, 31, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Holland, H.D.; Wang, P.-L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, B.E.; Sanchez-Baracaldo, P.; Wacey, D. Cyanobacterial evolution during the Precambrian. Int. J. Astrobiol. 2016, 15, 1–18. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Nabout, J.C.; da Silva Rocha, B.; Carneiro, F.M.; Sant’Anna, C.L. How many species of Cyanobacteria are there? Using a discovery curve to predict the species number. Biodivers. Conserv. 2013, 22, 2907–2918. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Sistrom, W.R.; Hansen, T.A.; Whitton, B.A.; Castenholz, R.W.; Pfennig, N.; Gorlenko, V.N.; Kondratieva, E.N.; Eimhjellen, K.E.; Whittenbury, R.; et al. Proposal to Place the Nomenclature of the Cyanobacteria (Blue-Green Algae) Under the Rules of the International Code of Nomenclature of Bacteria. Int. J. Syst. Bacteriol. 1978, 28, 335–336. [Google Scholar] [CrossRef]
- McNeill, J.; Barrie, F.R. International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code); Koeltz Scientific Books: Oberreifenberg, Germany, 2012. [Google Scholar]
- Parker, C.T.; Tindall, B.J.; Garrity, G.M. International Code of Nomenclature of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Handbook of Microalgal Culture, 1st ed.; Blackwell Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Tan, G.-Y.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers (Basel.) 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Nakaya, Y.; Iijima, H.; Takanobu, J.; Watanabe, A.; Hirai, M.Y.; Osanai, T. One day of nitrogen starvation reveals the effect of sigE and rre37 overexpression on the expression of genes related to carbon and nitrogen metabolism in Synechocystis sp. PCC 6803. J. Biosci. Bioeng. 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schlebusch, M.; Forchhammer, K. Requirement of the nitrogen starvation-induced protein s110783 for polyhydroxybutyrate accumulation in Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2010, 76, 6101–6107. [Google Scholar] [CrossRef] [PubMed]
- Hauf, W.; Schlebusch, M.; Hüge, J.; Kopka, J.; Hagemann, M.; Forchhammer, K. Metabolic Changes in Synechocystis PCC6803 upon Nitrogen-Starvation: Excess NADPH Sustains Polyhydroxybutyrate Accumulation. Metabolites 2013, 3, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Stal, L.J. Poly(hydroxyalkanoate) in cyanobacteria: An overview. FEMS Microbiol. Lett. 1992, 103, 169–180. [Google Scholar] [CrossRef]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Tran, H.; Steinbüchel, A. Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch. Microbiol. 1998, 170, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Taroncher-Oldenburg, G.; Nishina, K.; Stephanopoulos, G. Identification and analysis of the polyhydroxyalkanoate-specific beta-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 2000, 66, 4440–4448. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, S.; Izumi, Y.; Matsuda, F.; Hasunuma, T.; Chang, J.S.; Kondo, A. Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour. Technol. 2012, 108, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Monshupanee, T.; Incharoensakdi, A. Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 2014, 116, 830–838. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Sili, C.; Vincenzini, M. Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. J. Gen. Microbiol. 1992, 138, 1623–1628. [Google Scholar] [CrossRef]
- Deschoenmaeker, F.; Facchini, R.; Carlos, J.; Pino, C.; Bayon-Vicente, G.; Sachdeva, N.; Flammang, P.; Wattiez, R. Nitrogen depletion in Arthrospira sp. PCC 8005, an ultrastructural point of view. J. Struct. Biol. 2016, 196, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, S.; Nishida, A.; Ho, S.-H.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Glycogen production for biofuels by the euryhaline cyanobacteria Synechococcus sp. strain PCC 7002 from an oceanic environment. Biotechnol. Biofuels 2014, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Keppel, C.; Spalding, M.; Jane, J.L. Effects of growth condition on the structure of glycogen produced in cyanobacterium Synechocystis sp. PCC6803. Int. J. Biol. Macromol. 2007, 40, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ostle, A.G.; Holt, J.G. Fluorescent Stain for Poly-3-Hydroxybutyrate. Appl. Environ. Microbiol. 1982, 44, 238–241. [Google Scholar] [PubMed]
- Gorenflo, V.; Steinbüchel, A.; Marose, S.; Rieseberg, M.; Scheper, T. Quantification of bacterial polyhydroxyalkanoic acids by Nile red staining. Appl. Microbiol. Biotechnol. 1999, 51, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.K.; Roberson, R.W.; Vermaas, W.F.J. Polyhydroxybutyrate particles in Synechocystis sp. PCC 6803: Facts and fiction. Photosynth. Res. 2013, 118, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Hauf, W.; Watzer, B.; Roos, N.; Klotz, A.; Forchhammer, K. Photoautotrophic Polyhydroxybutyrate Granule Formation Is Regulated by Cyanobacterial Phasin PhaP in Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2015, 81, 4411–4422. [Google Scholar] [CrossRef] [PubMed]
- Klotz, A.; Georg, J.; Bučínská, L.; Watanabe, S.; Reimann, V.; Januszewski, W.; Sobotka, R.; Jendrossek, D.; Hess, W.R.; Forchhammer, K. Awakening of a dormant cyanobacterium from nitrogen chlorosis reveals a genetically determined program. Curr. Biol. 2016, 26, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Gründel, M.; Scheunemann, R.; Lockau, W.; Zilliges, Y. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiol. (UK) 2012, 158, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Knoop, H.; Axmann, I.M.; Steuer, R. The diversity of cyanobacterial metabolism: Genome analysis of multiple phototrophic microorganisms. BMC Genom. 2012, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Fatma, T. Cyanobacterial polyhydroxybutyrate (PHB): Screening, optimization and characterization. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kaewbai-ngam, A.; Incharoensakdi, A.; Monshupanee, T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- de Jaeger, L.; Verbeek, R.E.; Draaisma, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) Mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Damrow, R.; Maldener, I.; Zilliges, Y. The multiple functions of common microbial carbon polymers, glycogen and PHB, during stress responses in the non-diazotrophic cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Smith, A.J. Nitrogen chlorosis in blue-green algae. Arch. für Mikrobiol. 1969, 69, 114–120. [Google Scholar] [CrossRef]
- Gorl, M.; Sauer, J.; Baier, T.; Forchhammer, K. Nitrogen-starvation-induced chlorosis in Synechococcus PCC 7942: Adaptation to long-term survival. Microbiology 1998, 144, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.; Schreiber, U.; Schmid, R.; Völker, U.; Forchhammer, K. Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942. Low-level photosynthesis as a mechanism of long-term survival. Plant Physiol. 2001, 126, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-beta-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Panda, B.; Mallick, N. Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klimova, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Shimamatsu, H. Mass production of Spirulina, an edible microalga. Hydrobiologia 2004, 512, 39–44. [Google Scholar] [CrossRef]
- Choi, S.-L.; Suh, I.S.; Lee, C.-G. Lumostatic operation of bubble column photobioreactors for Haematococcus pluvialis cultures using a specific light uptake rate as a control parameter. Enzyme Microb. Technol. 2003, 33, 403–409. [Google Scholar] [CrossRef]
- Lu, Y.M.; Xiang, W.Z.; Wen, Y.H. Spirulina (Arthrospira) industry in Inner Mongolia of China: Current status and prospects. J. Appl. Phycol. 2011, 23, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Stevens, S.E.; Balkwill, D.L. Accumulation of poly-beta-hydroxybutyrate in Spirulina platensis. J. Bacteriol. 1982, 149, 361–363. [Google Scholar] [PubMed]
- Vincenzini, M.; Sili, C.; de Philippis, R.; Ena, A.; Materassi, R. Occurrence of poly-beta-hydroxybutyrate in Spirulina species. J. Bacteriol. 1990, 172, 2791–2792. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Sharma, L.; Mallick, N. Poly-β-hydroxybutyrate accumulation in Nostoc muscorum and Spirulina platensis under phosphate limitation. J. Plant Physiol. 2005, 162, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Stillings, C.; Roland, D.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Extraction of poly(3-hydroxybutyrate) from Spirulina LEB 18 for developing nanofibers. Polímeros 2015, 25, 161–167. [Google Scholar] [CrossRef]
- De Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Biofunctionalized nanofibers using Arthrospira (spirulina) biomass and biopolymer. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. Review the Ecology of Nostoc. J. Phycol. 1995, 18, 2–18. [Google Scholar] [CrossRef]
- Qiu, B.; Liu, J.; Liu, Z.; Liu, S. Distribution and ecology of the edible cyanobacterium Ge-Xian-Mi (Nostoc) in rice fields of Hefeng County in China. J. Appl. Phycol. 2002, 14, 423–429. [Google Scholar] [CrossRef]
- Sharma, L.; Mallick, N. Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: Regulation by pH, light-dark cycles, N and P status and carbon sources. Bioresour. Technol. 2005, 96, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Mallick, N. Enhancement of poly-β-hydroxybutyrate accumulation in Nostoc muscorum under mixotrophy, chemoheterotrophy and limitations of gas-exchange. Biotechnol. Lett. 2005, 27, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Mallick, N. Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by a N 2-fixing cyanobacterium, Nostoc muscorum Agardh. J. Chem. Technol. Biotechnol. 2012, 87, 505–512. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: A sustainable approach. J. Appl. Phycol. 2016, 28, 161–168. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Lama, L.; Nicolaus, B.; Calandrelli, V.; Manca, M.C.; Romana, I.; Gambacorta, A. Effect of growth conditions on endo- and exopolymer biosynthesis in Anabaena cylindrical 10 C. Phytochemistry 1996, 42, 655–659. [Google Scholar] [CrossRef]
- Gopi, K.; Balaji, S.; Muthuvelan, B. Isolation Purification and Screening of Biodegradable Polymer PHB Producing Cyanobacteria from Marine and Fresh Water Resources. Iran. J. Energy Environ. 2014, 5, 94–100. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae for biofuels and animal feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Uma, V.S.; Mathimani, T.; Deviram, G.; Arul Ananth, D.; Prabaharan, D.; Uma, L. On-site concurrent carbon dioxide sequestration from flue gas and calcite formation in ossein effluent by a marine cyanobacterium Phormidium valderianum BDU 20041. Energy Convers. Manag. 2016, in press. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Lin, C.Y.; Wang, E.I.C.; Ho, C.L.; Huang, K.M.; Yu, C.C.; Yang, F.L.; et al. Application of power plant flue gas in a photobioreactor to grow Spirulina algae, and a bioactivity analysis of the algal water-soluble polysaccharides. Bioresour. Technol. 2012, 120, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, A.; Pathak, A.K.; Guria, C. Carbon dioxide assisted Spirulina platensis cultivation using NPK-10:26:26 complex fertilizer in sintered disk chromatographic glass bubble column. J. CO2 Util. 2014, 8, 49–59. [Google Scholar] [CrossRef]
- He, L.; Subramanian, V.R.; Tang, Y.J. Experimental analysis and model-based optimization of microalgae growth in photo-bioreactors using flue gas. Biomass Bioenergy 2012, 41, 131–138. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; Converti, A.; Sato, S.; Carvalho, J.C.M. Arthrospira (spirulina) platensis cultivation in tubular photobioreactor: Use of no-cost CO2 from ethanol fermentation. Appl. Energy 2012, 92, 379–385. [Google Scholar] [CrossRef]
- Sumardiono, S.; Syaichurrozi, I.; Budi Sasongko, S. Utilization of Biogas as Carbon Dioxide Provider for Spirulina platensis Culture. Curr. Res. J. Biol. Sci. 2014, 6, 53–59. [Google Scholar]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2016. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Siangdung, W.; Paithoonrangsarid, K.; Bunnag, B. Cultivation of spirulina platensis using pig wastewater in a semi-continuous process. J. Microbiol. Biotechnol. 2010, 20, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Cicci, A.; Bravi, M. Production of the freshwater microalgae scenedesmus dimorphus and arthrospira platensis by using cattle digestate. Chem. Eng. Trans. 2014, 38, 85–90. [Google Scholar] [CrossRef]
- Fouilland, E.; Vasseur, C.; Leboulanger, C.; Le Floc’h, E.; Carré, C.; Marty, B.; Steyer, J.-P.P.; Sialve, B. Coupling algal biomass production and anaerobic digestion: Production assessment of some native temperate and tropical microalgae. Biomass Bioenergy 2014, 70, 564–569. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour. Technol. 2012, 112, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Kumar, P.; Malik, A.; Vijay, V.K. Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process. Bioresour. Technol. 2014, 158, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Daelman, M.R.J.; Sorokin, D.; Kruse, O.; van Loosdrecht, M.C.M.; Strous, M. Haloalkaline Bioconversions for Methane Production from Microalgae Grown on Sunlight. Trends Biotechnol. 2016, 34, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Nolla-Ardevol, V.; Strous, M.; Tegetmeyer, H.E.E. Anaerobic digestion of the microalga Spirulina at extreme alkaline conditions: Biogas production, metagenome and metatranscriptome. Front. Microbiol. 2015, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Schideman, L.; Yu, G.; Zhang, Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 2013, 6, 3765. [Google Scholar] [CrossRef]
- Zheng, M.; Schideman, L.C.; Tommaso, G.; Chen, W.-T.; Zhou, Y.; Nair, K.; Qian, W.; Zhang, Y.; Wang, K. Anaerobic digestion of wastewater generated from the hydrothermal liquefaction of Spirulina: Toxicity assessment and minimization. Energy Convers. Manag. 2016, in press. [Google Scholar] [CrossRef]
- Depraetere, O.; Pierre, G.; Noppe, W.; Vandamme, D.; Foubert, I.; Michaud, P.; Muylaert, K. Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res. 2015, 10, 48–54. [Google Scholar] [CrossRef]
- Meixner, K.; Fritz, I.; Daffert, C.; Markl, K.; Fuchs, W.; Drosg, B. Processing recommendations for using low-solids digestate as nutrient solution for production with Synechocystis salina. J. Biotechnol. 2016, 240, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly-b-hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.R.; Costa, J.A.V. Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture 2007, 264, 130–134. [Google Scholar] [CrossRef]
- Phang, S.M.; Miah, M.S.; Yeoh, B.G.; Hashim, M.A. Spirulina cultivation in digested sago starch factory wastewater. J. Appl. Phycol. 2000, 12, 395–400. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Castaldo, L.; Martello, A.; Paduano, A.; Sacchi, R.; Fogliano, V. Evaluation of anaerobic digestates from different feedstocks as growth media for Tetradesmus obliquus, Botryococcus braunii, Phaeodactylum tricornutum and Arthrospira maxima. New Biotechnol. 2017, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge, X.; Park, S.Y.; Li, Y. Comparison of Synechocystis sp. PCC6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresour. Technol. 2013, 144, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Sánchez Pérez, J.A.; Molina Grima, E.; Chisti, Y. Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chem. Eng. Sci. 2001, 56, 2721–2732. [Google Scholar] [CrossRef]
- Rabensteiner, M.; Kinger, G.; Koller, M.; Gronald, G.; Hochenauer, C. Pilot plant study of ethylenediamine as a solvent for post combustion carbon dioxide capture and comparison to monoethanolamine. Int. J. Greenh. Gas Control 2014, 27, 1–14. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Drosg, B.; Fritz, I.; Gattermayr, F.; Silvestrini, L. Photo-autotrophic Production of Poly(hydroxyalkanoates) in Cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Michels, M.H.A.; van der Goot, A.J.; Vermuë, M.H.; Wijffels, R.H. Cultivation of shear stress sensitive and tolerant microalgal species in a tubular photobioreactor equipped with a centrifugal pump. J. Appl. Phycol. 2016, 28, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Heinrich, D.; Madkour, M.H.; Al-Ghamdi, M.; Shabbaj, I.I.; Steinbüchel, A. Large scale extraction of poly(3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2012, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Acién, F.G.; Fernández-Sevilla, J.M.; González, C.V.; Bermejo, R. Development of a process for large-scale purification of C-phycocyanin from Synechocystis aquatilis using expanded bed adsorption chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2015, 100, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
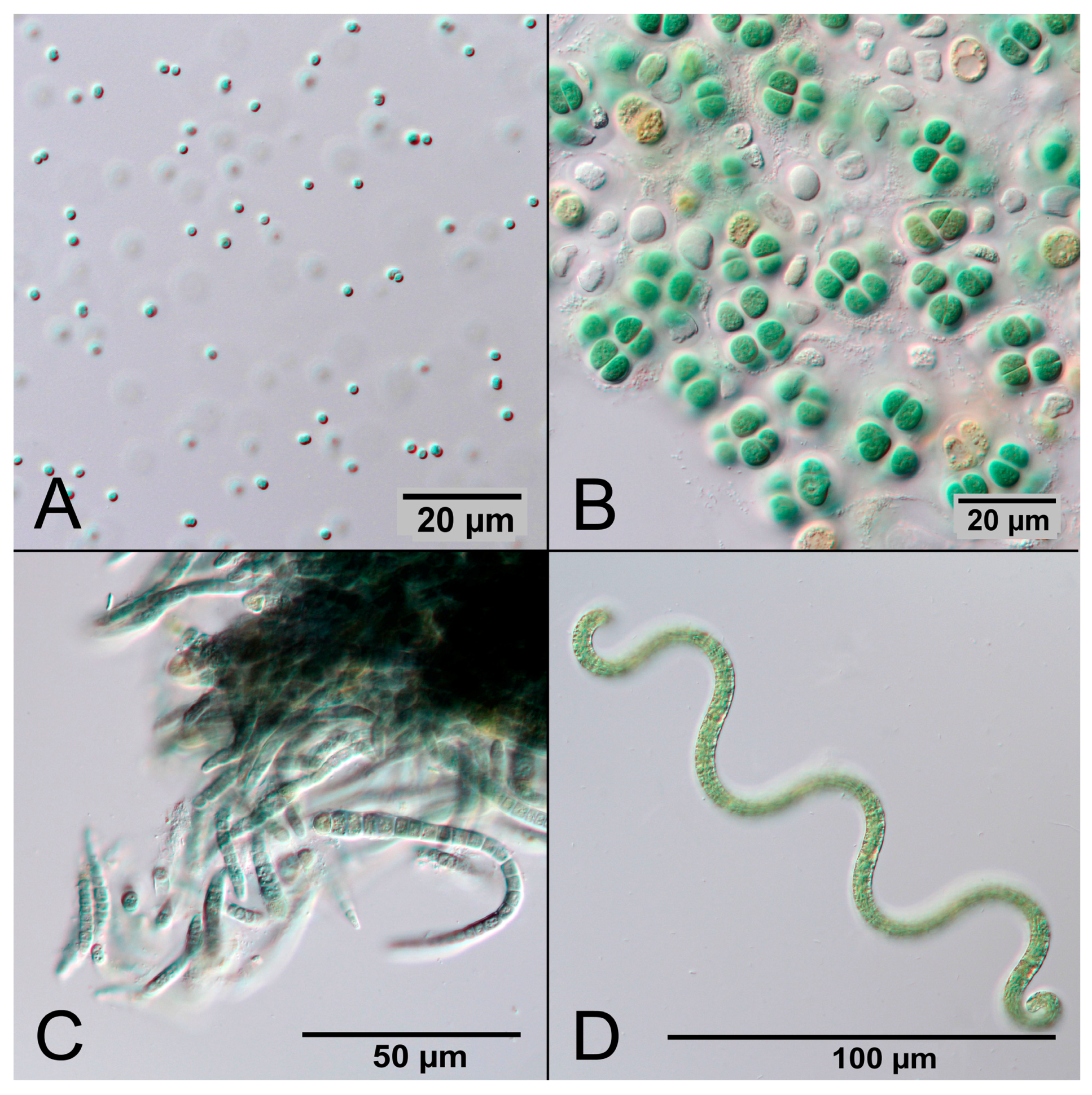

| Carbon Source | Cyanobacterium | Culture Condition | %PHA of cdw | PHA Composition | Total cdw | Reference |
|---|---|---|---|---|---|---|
| Photoautotrophic | Synechocystis PCC6803 | Photoautotrophic, nitrogen lim. | 4.1% | PHB | 0.65 g/L | [45] |
| Synechocystis PCC6803 | Photoautotrophic, nitrogen lim. | 9.5% | PHB | n.r. | [46] | |
| Synechocystis PCC6803 | Photoautotrophic, phosphate lim. | 11.2% | PHB | n.r. | [46] | |
| Synechocystis PCC6803 (recombinant) | Photoautotrophic, nitrogen lim. | 26% | PHB | n.r. | [47] | |
| Synechococcus MA19 | Photoautotrophic, phosphate lim., 50 °C | 55% | PHB | 4.4 g/L | [48] | |
| Heterotrophic | Synechocystis PCC6803 | Acetate + Fructose supplementation | 38% | PHB | n.r. | [46] |
| Synechocystis PCC6803 (recombinant) | Acetate supplementation | 35% | PHB | n.r. | [47] |
| Carbon Source | Cyanobacterium | Culture Condition | %PHA of cdw | PHA Composition | Total cdw | Reference |
|---|---|---|---|---|---|---|
| Photoautotrophic | Arthrospira platensis | Photoautotrophic | 6% | PHB | n.r. | [54] |
| Arthrospira sp. | Photoautotrophic | <1% | PHB | n.r. | [55] | |
| Arthrospira platensis | Photoautotrophic, phosphate lim. | 3.5% | PHB | 0.3 g/L | [56] | |
| Arthrospira subsalsa | Photoautotrophic, nitrogen lim. | 14.7% | PHB | 1.97 g/L | [57] | |
| Arthrospira platensis | n.r. | 22% | PHB | n.r. | [59] | |
| Heterotrophic | Arthrospira maxima | Acetate + CO2 | 5% | PHB | 1.4 g/L | [26] |
| Arthrospira sp. | Acetate + CO2 | 2.5% | PHB | n.r. | [55] |
| Carbon Source | Cyanobacterium | Culture Condition | %PHA of cdw | PHA Composition | Total cdw | Reference |
|---|---|---|---|---|---|---|
| Photoautotrophic | Nostoc muscorum | Photoautotrophic, nitrogen and phosphorous lim. | 8.7% | PHB | n.r. | [62] |
| Nostoc muscorum agardh | Photoautotrophic, 10% CO2 | 22% | PHB | 1.1 g/L | [66] | |
| Nostoc muscorum | Photoautotrophic, nitrogen and phosphorous lim. | 22% | PHB | 0.13 g/L | [56] | |
| Heterotrophic | Nostoc muscorum agardh | Acetate, valerate, nitrogen lim. | 58% | P[3HB-co-3HV] | 0.29 g/L | [64] |
| Nostoc muscorum | Acetate, limited gas exchange | 40% | PHB | n.r. | [63] | |
| Nostoc muscorum agardh | Acetate, glucose, valerate, 10% CO2 | 70% | P[3HB-co-3HV] | 0.98 g/L | [66] | |
| Nostoc muscorum agardh | Acetate, glucose, valerate, nitrogen lim. | 78% | P[3HB-co-3HV] | 0.56 g/L | [65] | |
| Nostoc muscorum | Acetate, dark incubation, nitrogen and phosphorous lim. | 35% | PHB | n.r. | [62] |
| Carbon Source | Cyanobacterium | Culture Condition | %PHA of cdw | PHA Composition | Total cdw | Reference |
|---|---|---|---|---|---|---|
| Photoautotrophic | Phormidium sp. TISTR 8462 | Photoautotrophic, nitrogen lim. | 14.8% | PHB | n.r. | [38] |
| Oscillatoria jasorvensis TISTR 8980 | Photoautotrophic, nitrogen lim. | 15.7% | PHB | n.r. | [38] | |
| Calothrix scytonemicola TISTR 8095 | Photoautotrophic, nitrogen lim. | 25.2% | PHB | n.r. | [38] | |
| Anabaena sp. | Photoautotrophic | 2.3% | PHB | n.r. | [69] | |
| Aulosira fertilissima | Photoautotrophic, phosphorous lim. | 10% | PHB | n.r. | [67] | |
| Heterotrophic | Aulosira fertilissima | Acetate, phosphorous lim. | 77% | PHB | n.r. | [67] |
| Aulosira fertilissima | Maltose, balanced | 15.9% | PHB | 2.3 g/L | [67] |
| Type of Gas | Cyanobacterium | CO2 Source | Reference |
|---|---|---|---|
| Flue gases | Phormidium valderianum | Coal combustion flue gas | [71] |
| Atrhrospira platensis | Coal combustion flue gas | [72] | |
| Arthrospira sp. | Synthetic flue gas | [73] | |
| Synechocystis sp. | Flue gas from natural gas combustion | [74] | |
| CO2 rich fermentation gases | Arthrospira platensis | CO2-offgas from ethanol fermentation | [75] |
| Arthrospira platensis | Biogas | [76] |
| Nutrient Source | Cyanobacterium | Total cdw/Growth Rate | Product/Purpose | Reference | |
|---|---|---|---|---|---|
| Agro-industrial effluents and waste waters | Raw cow manure | Arthrospira maxima | 3.15 g/L | Biomass production | [80] |
| Molasses | Arthrospira platensis | 2.9 g/L | Biomass production | [95] | |
| Olive-oil mill wastewater | Arthrospira platensis | 1.69 g/L | Nutrient removal | [84] | |
| Poultry litter | Nostoc muscorum agardh | 0.62 g/L | PHA production | [66] | |
| Anaerobic digestate | Waste from pig farm | Arthrospira platensis | 20 g/m2/d | Nutrient removal | [81] |
| Digested sago effluent | Arthrospira platensis | 0.52–0.61 g/L | Nutrient removal | [96] | |
| Digestate from municipal solid waste | Arthrospira platensis | Growth rate 0.04 d−1 | Nutrient removal | [97] | |
| Digestate from vegetable waste | Arthrospira platensis | Growth rate 0.20 d−1 | Nutrient removal | [97] | |
| Waste from pig farm | Arthrospira sp. | 15 g/m2/d | Nutrient removal | [85] | |
| Algal digestate | Chroococcus sp. | 0.79 g/L | Nutrient removal | [86] | |
| Digestate sludges | Lyngbya aestuarii | 0.28 g/L | Biomass production | [83] | |
| Digestates of Scenedesmus spp. | Lyngbya aestuarii | 0.11 g/L | Biomass production | [83] | |
| Thin stillage digestate | Synechocystis cf. salina Wislouch | 1.6 g/L | PHB production | [92] | |
| Anaerobic digester effluent | Synechocystis sp. | 0.15 g/L | Lipid production | [98] | |
| Trial | Strain | Nutrient Solution | Cultivation Time | Final Biomass Concentration | Final PHB-Concentration of cdw |
|---|---|---|---|---|---|
| 1. Mineral medium | Synechocystis salina CCALA192 | Optimized BG11 | June 21 days | 2.0 ± 0.12 g/L | 6.6% ± 0.5% |
| 2. Acetate addition | Synechocystis salina CCALA192 | Optimized BG11, 20 mM acetate | July 26 days | 1.9 ± 0.02 g/L | 6.0% ± 0.1% |
| 3. Acetate addition | Synechocystis salina CCALA192 | Optimized BG11, 60 mM acetate | September 24 days | Trial cancelled, due to contaminations with fungi | |
| 4. 24 h illumination | Synechocystis salina CCALA192 | Optimized BG11 | October 27 days | 1.8 ± 0.02 g/L | 4.8% ± 0.0% |
| 5. Alternative nutrient source | Synechocystis salina CCALA192 | Digestate supernatant | November–December 40 days | 1.6 ± 0.02 g/L | 5.5% ± 0.3% |
| 6. Mineral medium | Synechocystis salina CCALA192 | Optimized BG11 | December–January 30 days | 2.1 ± 0.03 g/L | 6.0% ± 0.02% |
| 7. Optimal degassing | Synechocystis salina CCALA192 | Optimized BG11 | May 7 days | 0.9 ± 0.03 g/L (Trial prematurely cancelled due to ciliates) | 9% ± 0.1% (Trial prematurely cancelled due to ciliates) |
| 8. Chlorogloeopsis fritschii CCALA39 | Chlorogloeopsis fritschii CCALA39 | Optimized BG11 | February 11 days | Trial cancelled, due to lack of growth | |
| 9. Arthrospira | Arthrospira sp. | Spirulina Medium | October 7 days | Trial cancelled, due to lack of growth | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. https://doi.org/10.3390/bioengineering4020026
Troschl C, Meixner K, Drosg B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering. 2017; 4(2):26. https://doi.org/10.3390/bioengineering4020026
Chicago/Turabian StyleTroschl, Clemens, Katharina Meixner, and Bernhard Drosg. 2017. "Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant" Bioengineering 4, no. 2: 26. https://doi.org/10.3390/bioengineering4020026




