1. Introduction
Recapitulation of the in vivo-like microenvironment during in vitro cell cultivation remains a challenge for researchers. Several important parameters/factors are usually neglected in conventional cell culture. These include physiological concentrations of biologicals (e.g., cytokines), energy sources (e.g., glucose and fatty acids), as well as oxygen concentrations. Moreover, cultivation in a three-dimensional (3D) microenvironment which supports cell-cell and cell-matrix interactions is still missing in most cell cultivation experiments. Several in vitro 3D cell culture systems have been developed in order to address this issue: cell aggregates, cell cultivation on natural and synthetic matrices and the encapsulation of cells in hydrogels [
1].
Ever since its first description by Van Den Bulcke et al., gelatin methacryloyl (GelMA) has proved itself as one of the most versatile hydrogels available for 3D cell culture and bioprinting [
2,
3]. GelMA is a typical semi-synthetic hydrogel, which enables the exploitation of the biological signals inherent in the gelatin molecule, while allowing control of mechanical properties [
1]. The hydrogel is obtained by the derivatization of gelatin with methacrylic anhydride, resulting in modification of lysine and hydroxyl residues with methacrylamide and methacrylate side groups [
4]. After derivatization, the parent molecule gelatin retains many of its attractive features as a biomaterial: it still displays thermoreversible physical crosslinking and retains its biological promoting properties, based on integrin-binding sequences and metalloprotease digestion sites. The GelMA hydrogel can thus provide an aqueous environment for cells and supports their adhesion, growth, and proliferation. In contrast to gelatin, however, the modification with methacryloyl side groups allows the GelMA molecule to undergo rapid polymerization in the presence of UV light and a photoinitiator (PI), resulting in covalent crosslinking through the creation of a methacryloyl backbone [
4]. This feature gives GelMA stability at physiological temperature and allows fine-tuning of mechanical properties. Moreover, the resulting material is transparent, which facilitates microscopic analysis.
GelMA is thus distinguished by its excellent biocompatibility, degradability, and low cost. Due to these features, GelMA has been extensively described for 3D cell culture applications and as a tissue engineering platform [
5]. Although many studies using GelMA have been published in the last years, intensive literature research reveals great heterogeneity in material properties, concentrations and polymerization processes used to create these hydrogels. In our opinion, what is frequently missing is a systematic approach how to adapt GelMA to a given cell type and intended application. Frequently, existing studies do not answer the question of how exactly different parameters of polymerization influence final hydrogel properties and, consequently, the cell growth which occurs inside such constructs. In this work, we present a workflow that tailors the mechanical properties of the GelMA molecule to create the desired mechanical and architectural in vitro microenvironment. We have used adipose tissue-derived mesenchymal stem cells (AD-MSCs) as a model, since these cells are mechanosensitive, possess high motility, and are frequently used in tissue engineering applications.
Micro-molding with the hydrogel of choice is usually sufficient for most straightforward 3D cell culture applications. However, in order to introduce a higher degree of complexity, spatial control and resolution within an engineered construct, biofabrication techniques like lithography and rapid prototyping are becoming increasingly commonplace. As a bioink/biomaterial, GelMA shares a drawback with many other common hydrogels, in that it displays low viscosity resulting in poor resolution of printed structures, or even complete lack of printability at low concentrations with the most commonly used extrusion-based bioprinters [
6].
To circumvent this problem, most published references print with higher concentrations of GelMA possessing high degree of functionalization (DoF) (higher crosslinking density) [
7,
8]. However, cells proliferate and migrate better when not hindered by a dense polymer network. Cell spreading and long term survival, as well as remodeling of the construct, cannot be observed in such constructs [
9]. There are some strategies to approach the problem of poor printability of GelMA. The first method, followed for example by Billiet et al., uses the inherent thermo-reversible properties of GelMA and prints at temperatures lower than 37 °C. Shape fidelity is further supported by cooling the printing platform, which supports the natural sol-gel transition of GelMA [
8].This approach is feasible, but applicable to high GelMA concentrations only (leading to hindered cell adhesion and migration as mentioned above) and limited by the fact that many low-cost extrusion bioprinters do not have temperature-controlled printing platforms.
Two additional methods for improving GelMA printability were demonstrated by the group of Schuurman et al. and include thermoplastic co-deposition or the mixing of hyaluronic acid into the bioink [
10]. Many groups follow the latter approach and employ various rheological modifiers for enhancing GelMA deposition. Rheological modifiers are also sometimes called rheological additives and include components which enhance the rheology of the resulting bioink, especially with regard to viscosity and yield stress. Currently, there is no consensus for which rheological modifier works best for GelMA bioprinting. Good results have been obtained with materials as diverse as gellan gum [
11] and nanoparticles [
12]. The modifier may be selected based solely on its viscosity-enhancing properties (e.g., alginate) or depending on the final application (e.g., calcium phosphate for bone tissue engineering). In any case, fine-tuning is mandatory in order to close down on the optimal biofabrication window [
9]. While viscosity can be increased by the use of rheological modifiers, care should be taken that the resulting hydrogel mixture can still support cell adhesion, spreading, and proliferation.
In this work, we share our experiences on how GelMA can be established as a general all-round platform for 3D cell culture and bioprinting. Using accessible bioprinting equipment and cost-efficient protocols, it is our conviction that any lab can establish an economic and versatile platform for 3D cell cultures of varying complexity based on GelMA. We will cover how GelMAs of specific DoF can be produced and characterized. These materials should then be screened together with the cell type of interest in order to determine which material is best suited to the intended cell type. Moreover, the subsequent analysis of the total cell population inside the hydrogel construct may require cell release. Therefore, we will also cover the enzymatic degradation of GelMA. Finally, we describe additives which are applicable for extrusion-based bioprinting techniques, and discuss their advantages and limitations.
2. Methods
2.1. GelMA Synthesis
Gelatin methacryloyl of various DoFs was synthesized by the method of Shirahama and Lee [
13]. The A100 high DoF material was synthesized via the one-pot method [
13], while the lower DoF materials were obtained by the sequential pH adjustment method [
14]. Briefly, a 10% (w/v) gelatin solution of type A (porcine, Bloom strength 300, Merck KGaA, Darmstadt, Germany) or type B (bovine, Bloom strength 225, Merck KGaA, Darmstadt, Germany) was dissolved under stirring in a pH 9, 0.1 M carbonate-bicarbonate buffer (CB buffer, Merck KGaA, Darmstadt, Germany) at 60 °C. The reaction was started by the addition of methacrylic anhydride (MAA, Merck KGaA, Darmstadt, Germany) at 50 °C and with rigorous stirring at 500 rpm. MAA was added with sequential steps, giving a total MAA amount equal to the defined ratio in each recipe (x mL MAA/g gel, see
Table 1, Results). The pH was adjusted back to 9 after each addition. The reaction was carried out for 60 min and terminated by pH adjustment to 7.4. The A100 material was synthesized in a 0.25 M CB buffer with a 0.1 MAA/g Gel ratio, by performing a single MAA addition and pH adjustment. After completion, the reaction mixture was filtered using standard paper filters (Whatman™, 90 mm diameter, GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK) and dialyzed with a 14 kDa molecular-weight-cutoff (MWCO) membrane (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) at 40 °C for 36 h against ultrapure water. Water was changed every 4 h to ensure complete removal of low molecular weight gelatins and methacrylic reaction byproducts. GelMA solution after dialysis was frozen and lyophilized. The resulting GelMA product was stored in the dark at 4 °C.
2.2. DoF Determination
The degree of functionalization (DoF) was determined by the trinitrobenzenesulfonic (TNBS) acid method based on Habeeb [
15] and modified by Lee and Shirahama [
13]. Briefly, samples and underivatized gelatin A & B were dissolved in 0.1 M CB buffer (90 mg/100 mL). A 500 µL sample was mixed with 500 µL reagent (0.01% TNBS in 0.1 M CB buffer) and incubated for 2 h at 37 °C. The reaction was stopped by the addition of 250 µL HCl (1 M) and 500 µL SDS (10%). The resulting UV absorption was measured at 335 nm with a Multiskan GO spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA) and quantified by using a glycine calibration curve.
2.3. Hydrogel Preparation
GelMA solutions were prepared at the indicated concentrations by weighing an appropriate amount of GelMA and dissolving it in phosphate-buffered-saline (PBS, pH 7.4). The GelMA solution was incubated in a water bath until complete dissolution of the GelMA solid and disappearance of foam at 37 °C. All GelMA solutions contained 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959) at a final concentration of 0.1% (w/v). Prior to cell experiments, warm GelMA solutions were sterile-filtered using 0.45 µm polyethersulfone (PES) filters (10 mm diameter) in a laminar-flow cabinet.
2.4. Rheological Characterization
The viscosity of GelMA solutions was determined at 25 °C by rotational viscosimetry using a MCR 302 Modular Rheometer (Anton Paar, Graz, Austria) equipped with plate-plate geometry (40 mm plate diameter) and a gap size of 0.4 mm. The shear rate was varied from 0.01 to 1000 s−1, which is a typical shear rate region associated with pneumonic extrusion bioprinting.
The UV polymerization of GelMA solutions was investigated at 25 °C using a MCR 302 Modular Rheometer (Anton Paar, Graz, Austria) equipped with a plate-plate geometry (20 mm diameter, 0.3 mm gap size) and by performing in situ UV crosslinking by irradiation with a UV lamp (Delolux 80, Delo, Windach, Germany) from below. The crosslinking was recorded with a time sweep oscillatory test under constant strain amplitude of 1% and at a constant frequency of 1 Hz, which is within the linear viscoelastic (LVE) region. The sample volume was 100 µL, and the UV dosage was varied as indicated in the results.
2.5. Cell Culture
Human adipose tissue-derived mesenchymal stem cells (hAD-MSCs were isolated from the adipose tissue of four donors after abdominoplasty. All patients have given their informed consent, as approved by the Institutional Review Board (Hannover Medical School). The isolated cell populations have been extensively characterized as mesenchymal stem cells by surface marker analysis and functional properties [
16]. Briefly, cells were characterized by flow cytometry for specific immunotypic MSCs markers and were positive for CD44, CD73, CD90, and CD105 and negative for hematopoetic (CD34 and CD45) and endothelial (CD31) markers. Furthermore, hAD-MSCs were able to differentiate under controlled conditions toward adipocytes, chondrocytes, and osteocytes (confirmed by BODIPY, Alcian Blue and Alizarin red stainings respectively). AD-MSCs were expanded in alpha-MEM medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 1 g/L glucose, 2 mM
l-glutamine, 10% human serum (CC-pro, Oberdorla, Germany) and 50 µg/mL gentamicin (Merck KGaA, Darmstadt, Germany), harvested by accutase treatment (Merck KGaA, Darmstadt, Germany) and cryopreserved at passage two until the start of the experiment. Experiments were performed with cells of passages two to eight.
2.6. 3D Cell Culture in GelMA Hydrogels
For 3D cell culture experiments, 1.5 × 106 cells/mL hydrogel were encapsulated in 50 µL disks (6 mm diameter) in silicon molds with the help of a cross linker (BLX-365 BIO-LINK, 365 nm, Vilber Lourmat Deutschland GmbH). UV intensity varied between 1.2 J/cm2 and 2.4 J/cm2. Polymerized hydrogel constructs were incubated in 24-well plates in 400 µL alpha-MEM medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 1 g/L glucose, 2 mM l-glutamine, 10% human serum (CC-pro, Oberdorla, Germany), and 50 µg/mL gentamicin (Merck, Darmstadt, Germany).
2.7. Enzymatic Digestion of GelMA with and without Cells
To measure the enzymatic digestion of GelMA with various DoFs, 50 µL disks (6 mm in diameter) were made using a silicon mold in a Petri dish under sterile conditions. GelMA hydrogel solution was dispensed into the mold and subsequently polymerized with a cross linker (BLX-365 BIO-LINK, 365 nm, Vilber Lourmat Deutschland GmbH, Eberhardzell, Germany). After polymerization, the hydrogel discs were transferred to 24-well-plates and allowed to swell overnight in 500 µL of alpha-MEM medium supplemented with 10% human serum (CC-pro, Oberdorla, Germany) and 50 µg/mL gentamicin (Merck, Darmstadt, Germany). After swelling of the constructs, cell culture medium was removed and 500 µL of collagenase-CLS I with 20 or 30 U/mL (Merck, Darmstadt, Germany) in Hanks Balances Salt Solution (Sigma Aldrich (now Merck KGaA, Darmstadt, Germany) supplemented with 3 mM CaCl2 were added to each well. Hydrogels in collagenase-CLS I solution were placed in an incubator equipped with a rotary shaker and incubated for over 5 h (130 rpm, 37 °C). At the given time point, constructs were removed from the enzymatic solution and excessive liquid was carefully removed by blotting dry with a delicate tissue wipe (Kimtech Science, Kimberly-Clark, Irving, TX, USA). The degree of degradation was estimated by the measurement of hydrogel weight loss in comparison to the original wet weight after swelling. Four separate constructs per time point and digestion condition were used in this experiment.
2.8. Cell Viability and Proliferation
The viability and proliferation of AD-MSCs in the GelMA hydrogels was measured indirectly by the Cell Titer-Blue® (CTB) cell viability assay (Promega, Mannheim, Germany). Briefly, cells were encapsulated in the hydrogels by polymerization in a UV-cross linker (BLX-365 BIO-LINK, 365 nm, Vilber Lourmat Deutschland GmbH, Eberhardzell, Germany) at different UV intensities. After polymerization, the cell-laden constructs were placed in 24-well-plates and 400 µL alpha-MEM medium supplemented with 10% human serum (CC-pro, Oberdorla, Germany) and 50 µg/mL gentamicin (Merck, Darmstadt, Germany) was added to each well. On day 1 (24 h), day 3 (72 h), and day 7 (168 h) of cultivation, cell culture medium was removed and 400 µL CTB working solution (1:10 (v/v) in basal alpha-MEM) were added to each well. After 5 h of incubation, CTB fluorescence was measured at an extinction wavelength of 544 nm and an emission wavelength of 590 nm using a microplate reader (Fluoroskan Ascent, Thermo Fisher Scientific Inc., Waltham, MA, USA). Values are presented as a direct CTB fluorescence signal after blank subtraction (400 µL CTB working solution (1:10 (v/v) in basal alpha-MEM) after 5 h incubation in the absence of cells and hydrogel). At least four constructs per material were measured for a single time point.
2.9. Live/Dead Staining
For representative live/dead staining, as well as for morphological examination, cell-laden hydrogels (one construct per time point and per material) were incubated in 3 µm Calcein-AM (Merck, Darmstadt, Germany) and 2.5 µm propidium iodide (Merck, Darmstadt, Germany) solution in basal alpha-MEM for 15 min at 37 °C and analyzed with a fluorescent microscope (Olympus, IX50, Olympus Corporation, Tokyo, Japan), equipped with a camera (Olympus SC30, IX-TVAD, Olympus Corporation, Tokyo, Japan) and the CellSens Software (CellSens Standard 1.7.1, Olympus Corporation, Tokyo, Japan).
2.10. Bioprinting
GelMA hydrogel was prepared and mixed with additives at the desired concentration. The hydrogel was filled in a sterile disposable syringe and inserted into an extrusion bioprinter (BioBot 1, Allevi, Philadelphia, PA, USA). The printing needle diameter (Nordson EFD, Oberhaching, Germany) was 0.40 mm, the extrusion air pressure ranged between 2.8 and 3.8 psi, and printing was performed at 30 °C or 37 °C with a deposition speed of 260 mm/min. A diagonal grid pattern of 2.5 cm × 2 cm dimensions was printed with and without curing by a UV cross linker (BLX-365, BIO-LINK 365 nm, Vilber Lourmat Deutschland GmbH, Eberhardzell, Germany). Micrographs of the printed constructs were taken with a Stereo zoom microscope BMS 133, (BMS microscopes b.v., Capelle aan den Ijssel, the Netherlands). Alginate-2-Hydroxyethyl Methacrylate (AlgHEMA) was provided by Petersburg State University, Saint Petersburg, Russia. Silicate nanoparticles (SiNP), trade name Laponite XLG, were provided by BYK Additives Ltd., Widnes, UK.
4. Discussion and Conclusions
The attempt to approach physiological conditions in in vitro experiments plays an important role for the better understanding of cell physiology, cell-matrix interactions, and intercellular communication. Moreover, 3D cell models allow better evaluation of drug candidates, which helps with prediction of treatment outcomes before starting animal trials, thus saving costs and reducing the number of animal experiments required. Numerous original studies and reviews have shown great differences in cell reactions between two-dimensional (2D) and 3D cell cultures, and the importance of creating a more physiological in vitro cell microenvironment [
17,
18,
19]. Additive manufacturing technologies (bioprinting) represent an advanced technique of 3D cell culture. Bioprinting brings 3D cell culture to the next level by allowing spatial control of construct architecture. Thus, it is possible to print different materials (e.g., with variable mechanical stiffness or pore size) with different cells for heightening the complexity of the cell models of interest
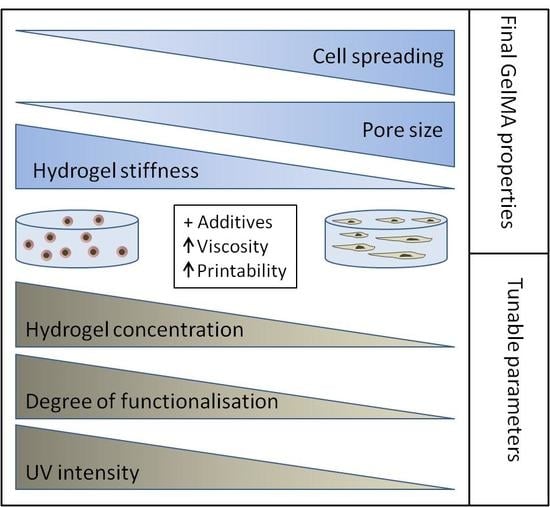
The possibility of precisely tuning and adapting hydrogels to the intended application provides researchers with a valuable tool for the creation of specific in vitro microenvironments. From this point of view, GelMA provides a perfect cultivation platform: (1) it can be easily synthesized in the lab for a low price, (2) it is transparent (convenient cell monitoring), (3) it has RGD motifs for cell adhesion, (4) its concentration can be varied in order to achieve a desired stiffness, (5) its DoF can be also adapted to create hydrogels with particular stiffness and pore size and (6) it can be digested in a controllable manner if cell analysis is required after cultivation.
Using the GelMA toolbox, researchers can either identify optimal hydrogel conditions for encapsulation of the cells of interest, or may manipulate the scaffold for the study of the influence of microenvironment on cell fate. Since different tissues have different mechanical properties, combination of specific concentrations, DoFs and UV polymerization dosages can be used to approach the stiffness of every soft tissue (
Figure 9).
In this work, we aimed to adapt GelMA as a 3D cell culture platform and as a bioink for the cultivation of hAD-MSCs. The encapsulation of cells in restrictive GelMA microenvironments of high stiffness for the purpose of bioprinting [
7,
8,
10,
20], microtissues [
21], or tissue engineering [
10] is well-described in the literature. In contrast, reports of the adaptation of GelMA to constructs that do not restrict cell spreading and migration are more rare (for example, the 3D GelMA cancer models of Kaemmerer et al. [
22]). We showed that a low polymer concentration of GelMA and a low UV dosage are essential for creating a cell promoting microenvironment for MSCs. Encapsulated cells displayed excellent viability under these conditions.
There are two general ways in which cell physiology can be affected in the resulting 3D GelMA construct. First, the UV polymerization conditions themselves (such as UV light intensity and PI) may affect cell viability. Second, the architecture of the construct, especially pore size, may affect oxygen and nutrient diffusion, as well as inhibiting migration and intercellular interactions. In this work, cells were studied 24 h after encapsulation and after three days and seven days of cultivation. Higher UV intensity (2.4 J/cm
2) resulted in lower cell viability in comparison to cells encapsulated in the same material at 1.2 J/cm
2. The lower cell viability after higher UV dosage exposure might not necessary be the result of direct cytotoxicity. Earlier, Fedorovich et al. demonstrated that UV polymerization can be toxic to MSCs in 2D cultures, but has no effect on cells encapsulated in hydrogels [
23]. This can be explained by the fact that the radicals produced in the reaction are usually captured by unreacted methacrylate groups, which exert a protective effect on encapsulated cells. On the other hand, UV irradiation doses of 2.7 J/cm
2 have been linked with decreased viability in encapsulated cells in comparison to lower UV intensities. Billiet et al. showed that this decreased viability is probably related to both- the denser network properties and the higher amount of generated free radicals under this illumination conditions [
8].
We could also show that a higher UV dosage leads to a dramatic increase of stiffness in high-DoF materials. The increase was not as significant in constructs of 70% DoF. Here, additional effects such as final pore size may play a role in the explanation as to why cell spreading does not occur at this crosslinking intensity. For example, Chen et al. demonstrated that the pore size in 49.8% DoF materials is significantly larger than in 73.2% DoF materials. This group could also show the generation of 3D vascular networks with GelMA and also employed low polymer concentration and UV intensity to achieve cell spreading (5% w/v GelMA and 0.134 J/cm
2) [
24]. Further studies, however, must be performed in order to understand how material stiffness and pore size work together to create promoting cell microenvironments. To our knowledge, there are no reliable methods available to estimate the pore size of wet hydrogels.
Cells encapsulated in A50 and B50 showed good spreading. However, higher viability was observed in A50 after seven days of cultivation. No spreading was detected in materials of DoF 70% and above. The reason could be a smaller pore size in high DoF GelMA constructs, which disturbs migration and cell-cell interaction. However, cell viability increased in all GelMA constructs during the course of a week. One reason for this observation could be cell growth on the surface of the constructs with high DoF. It is interesting to note, that hAD-MSCs proliferated much faster on the surface of materials with 70% DoF and higher. Indeed, it was demonstrated earlier for 2D cell cultures growing on the surface of hydrogels with different stiffness, that a soft matrix had a negative effect on cell proliferation in comparison to a stiffer matrix [
25].
Although this work provides a detailed systematic approach for establishing a GelMA-based 3D cell culture, a deeper biological investigation into the cell biology after encapsulation must still be performed. The influence of hydrogel stiffness and architecture on the focal adhesion, Yes-associated protein expression, and cell migration in the hydrogels can be explored further. Another important issue would be a study of MSCs differentiation in hydrogels of different architecture, since stiffness has a great influence on mechanosensitive MSCs [
26,
27]. Our work, however, provides a fundament for further studies involving the creation of in-vitro platforms with variable stiffness, such as hydrogels with stiffness gradients.
Excellent studies describing various additives for GelMA bioprinting have been published in recent years, such as the use of gellan gum [
11], nanosilicates [
12], hyaluronic acid [
10], and alginate [
28]. There is no consensus for which additive works best, and considerations are focused on the intended application (like adding methacrylated hyaluronic acid (HAMA) as an extracellular matrix (ECM) component for cartilage tissue engineering [
29,
30]) or are limited by the availability of hardware specifics of the bioprinter equipment (like a coaxial extrusion system based on a microfluidic system [
28]). Both unmodified and methacrylated biopolymers can be used for viscosity increase of the GelMA bioink. Here as well, further studies are needed in the field on order to evaluate whether covalent crosslinking between the polymers is necessary for long-term stability, or whether a simple inter-penetrating polymer network of GelMA and the polymer additive is sufficient for the fabrication purpose. To adapt GelMA as a bioink without compromising the cell promoting environment necessitates a careful formulation of additives to increase the viscosity, without impacting cell spreading and proliferation to a great extent. Our experience with different additives shows that SiNPs and the novel alginate-HEMA are good candidates for achieving this task.