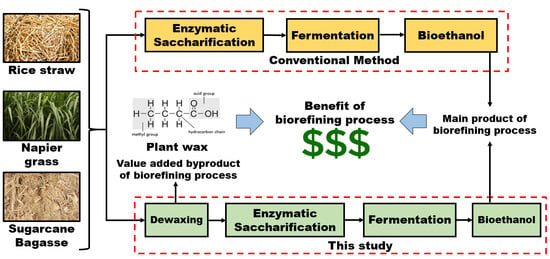
Interferences of Waxes on Enzymatic Saccharification and Ethanol Production from Lignocellulose Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lignocellulosic Biomass Substrate Collection and Characterization
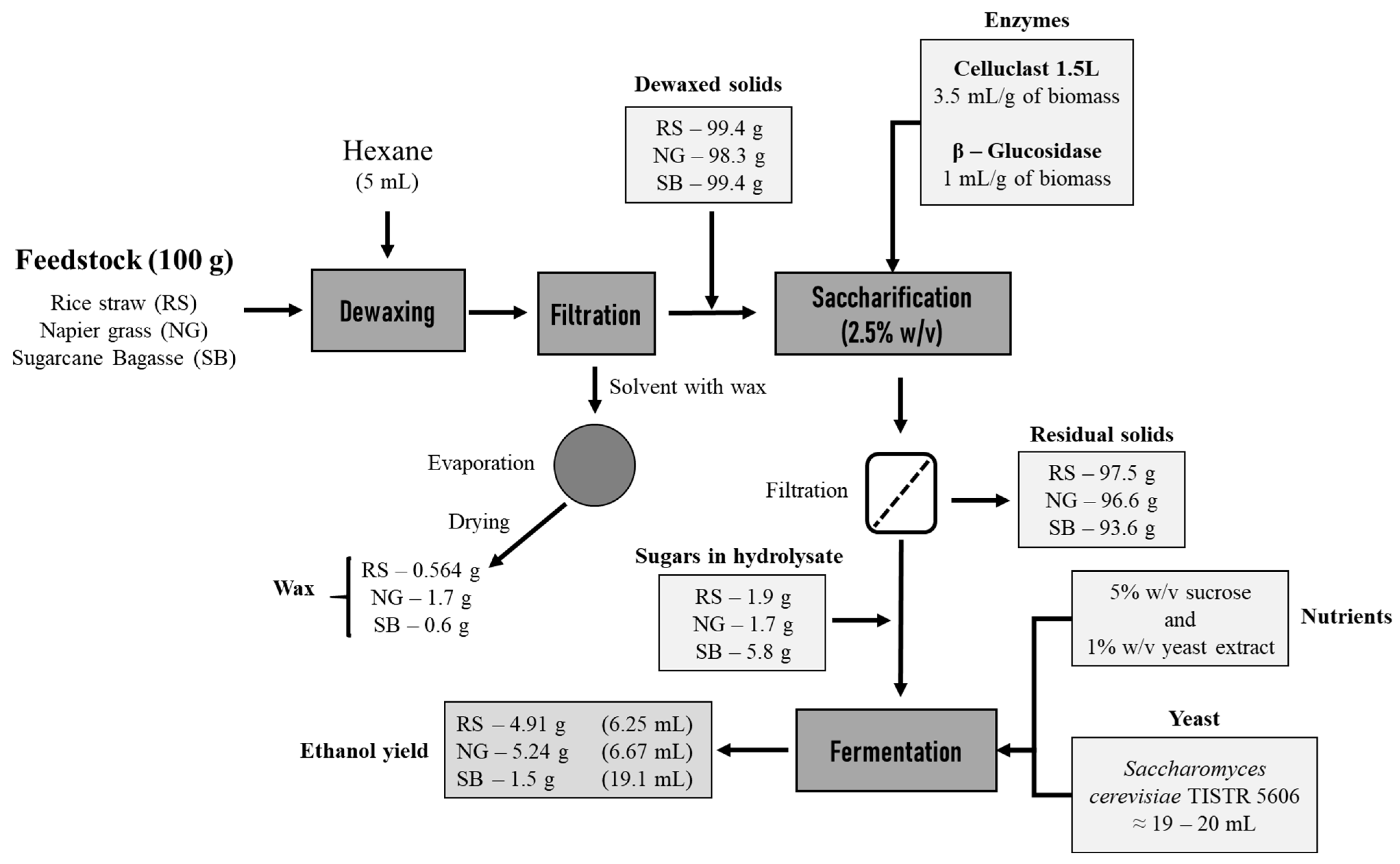
2.2. Extraction of Wax
2.3. Enzymatic Saccharification
2.4. Production of Ethanol
2.5. Scanning Electron Microscopy
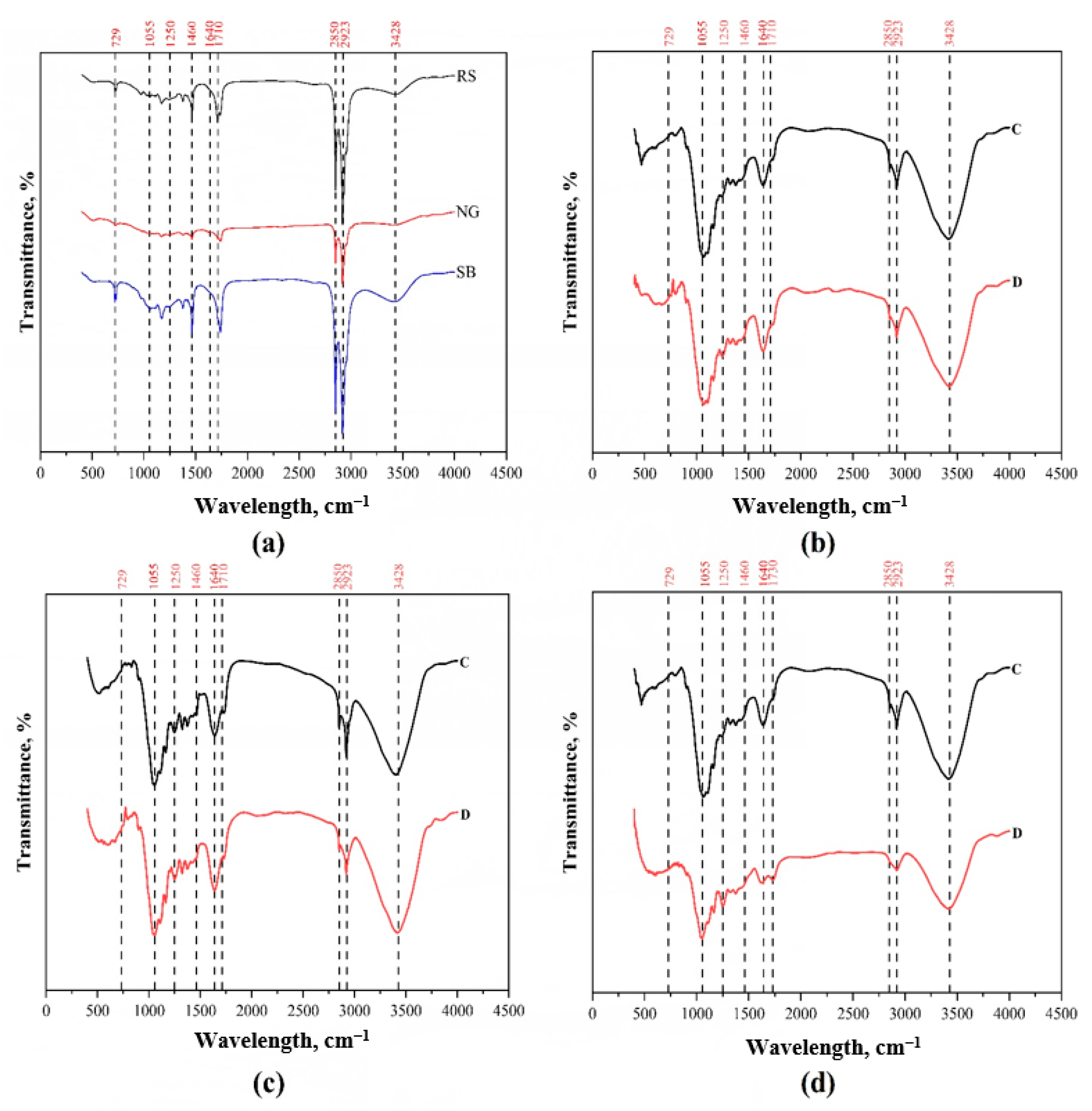
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Wax Composition Analysis by GC-MS
2.8. Ethanol Determination Using GC-MS
2.9. Statistical Analysis
3. Results and Discussion
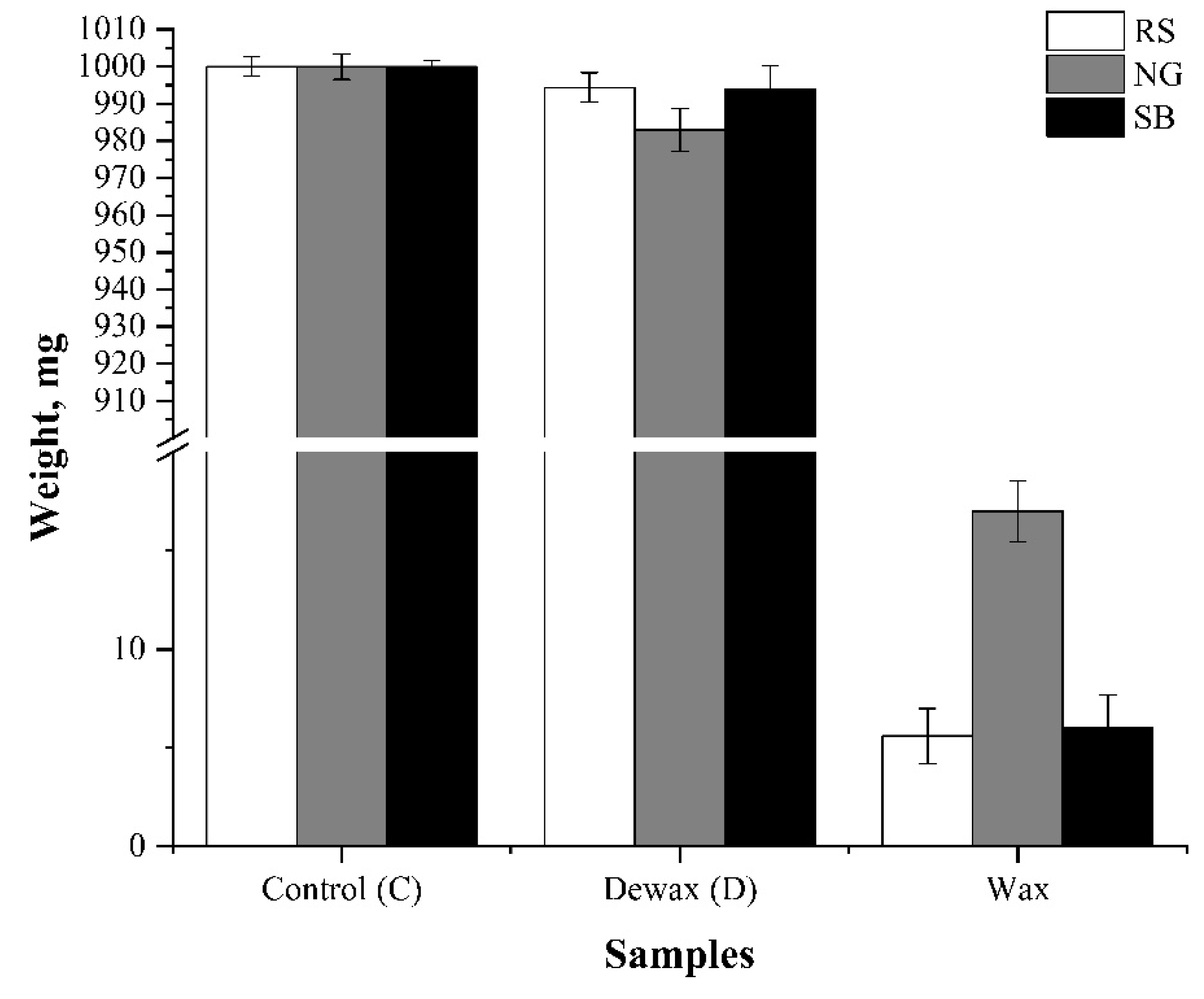
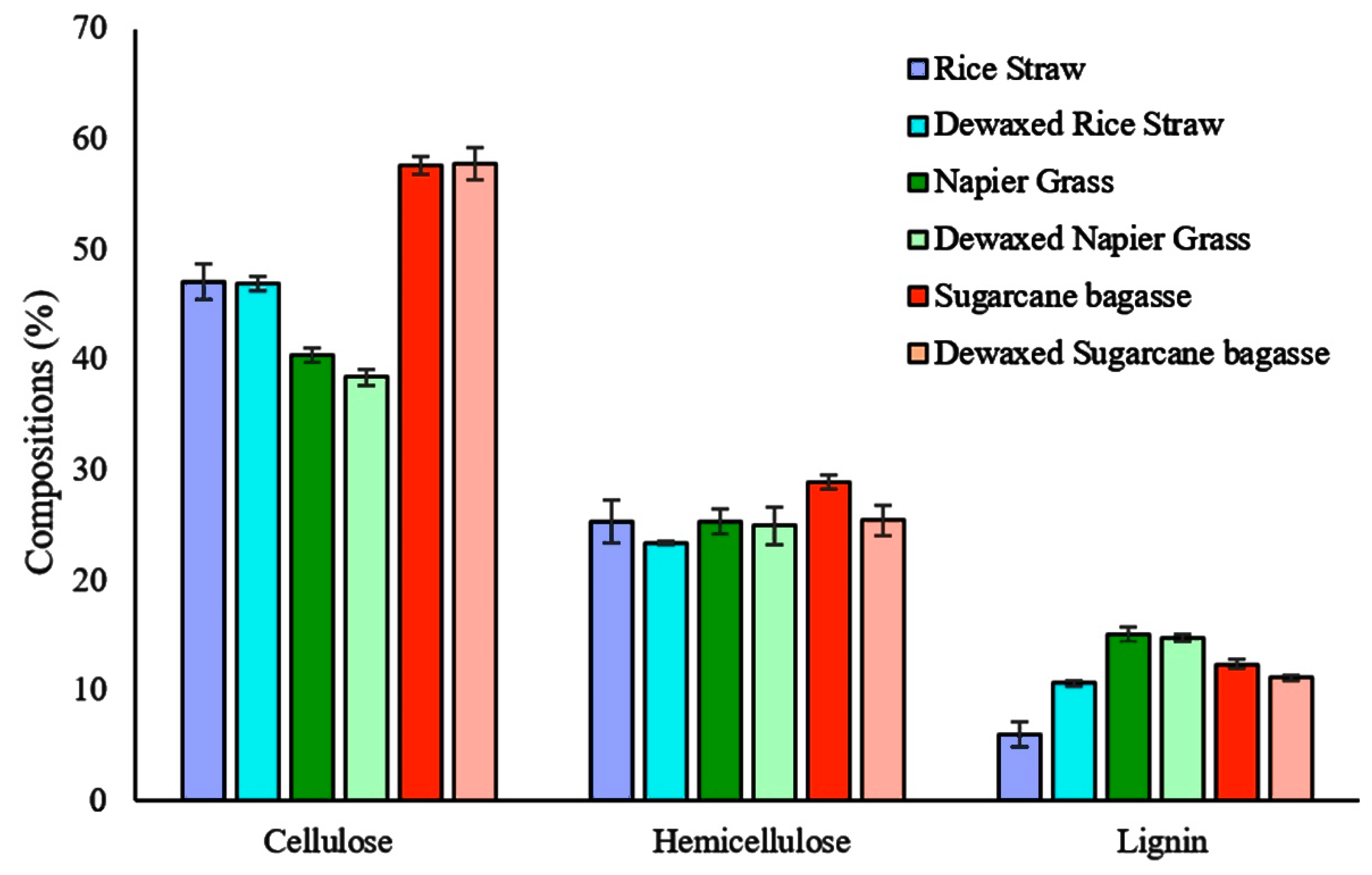
3.1. Wax Yield and Lignocellulosic Biomass Composition
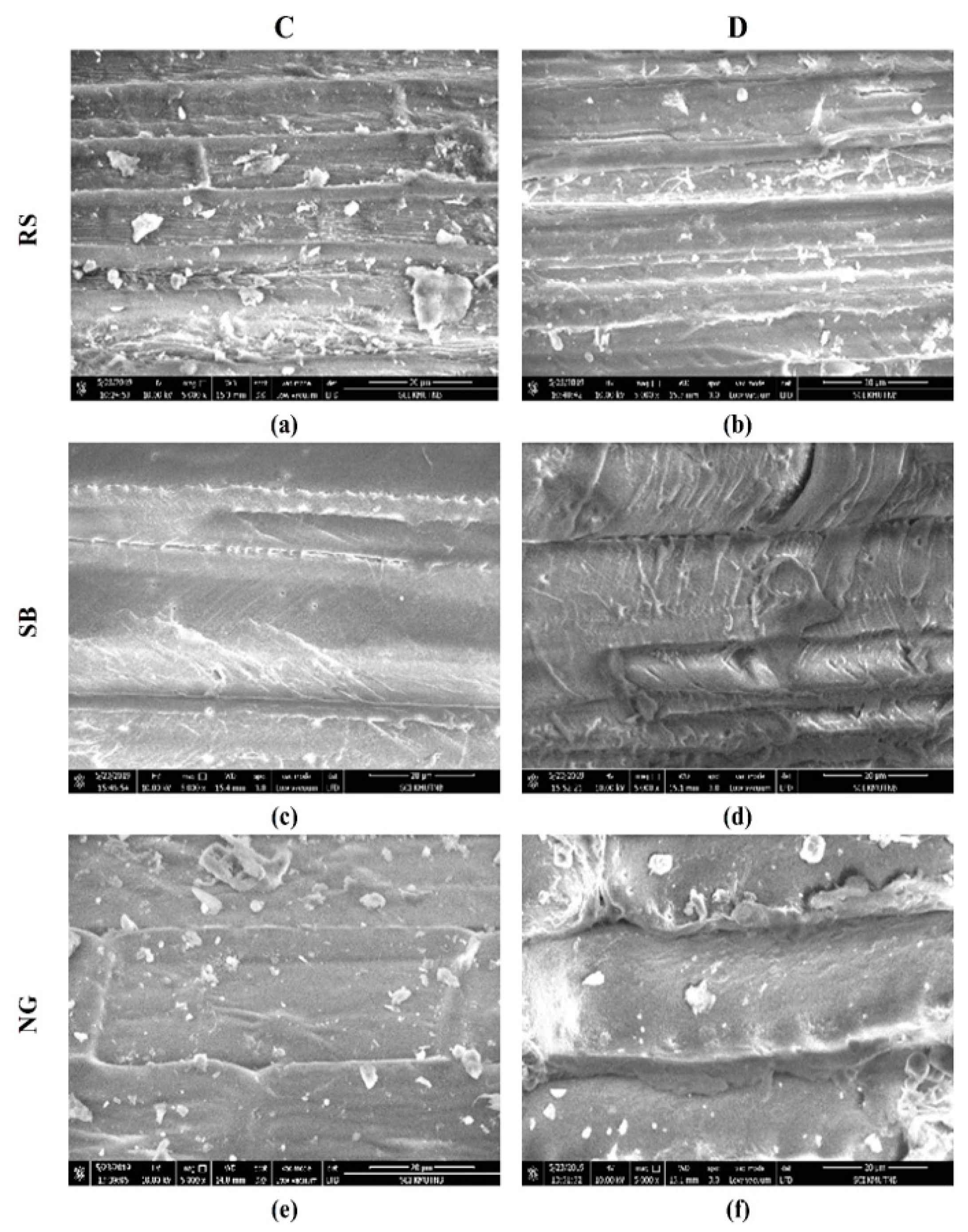
3.2. SEM Image Analysis
3.3. FTIR Analysis
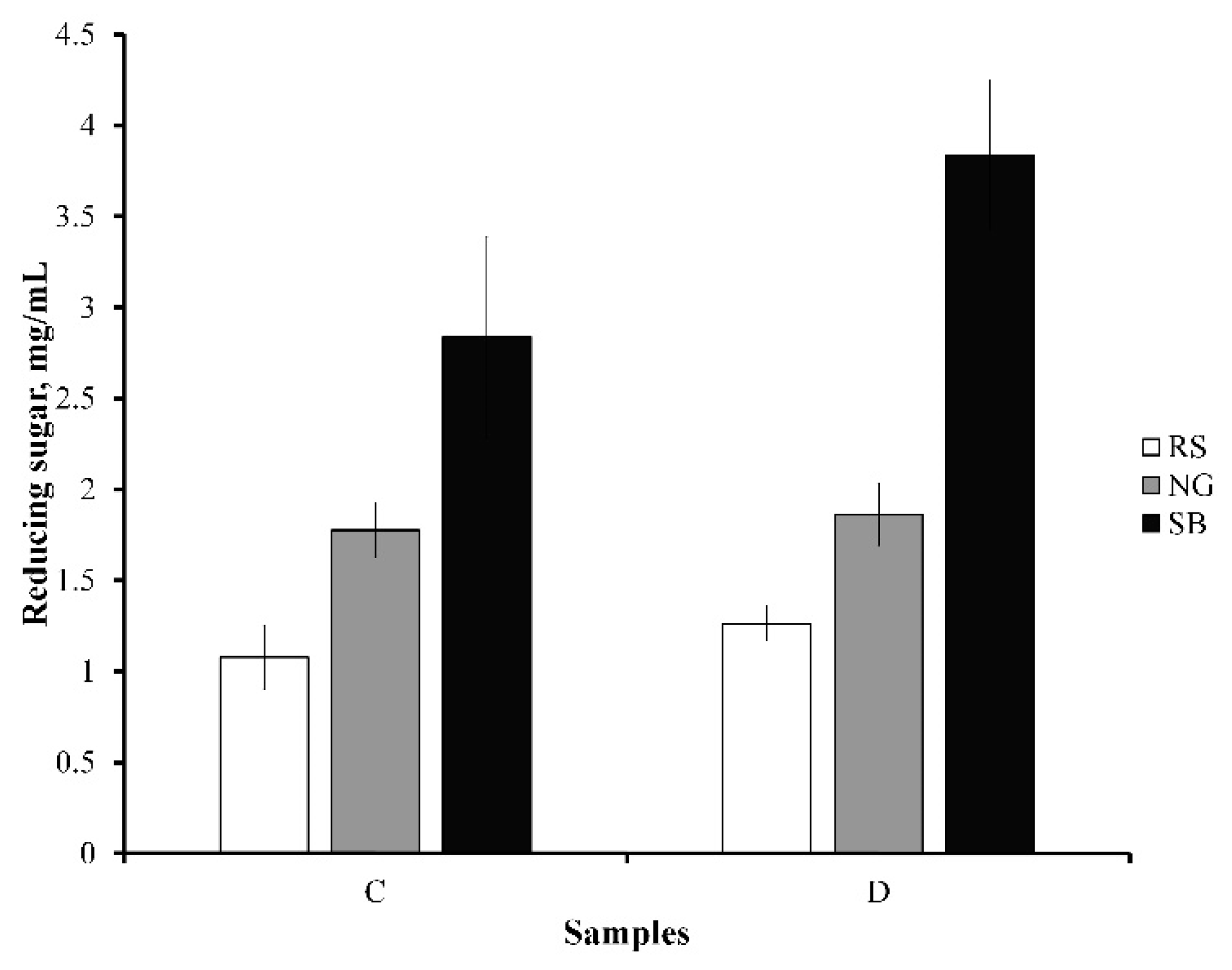
3.4. Enzymatic Saccharification
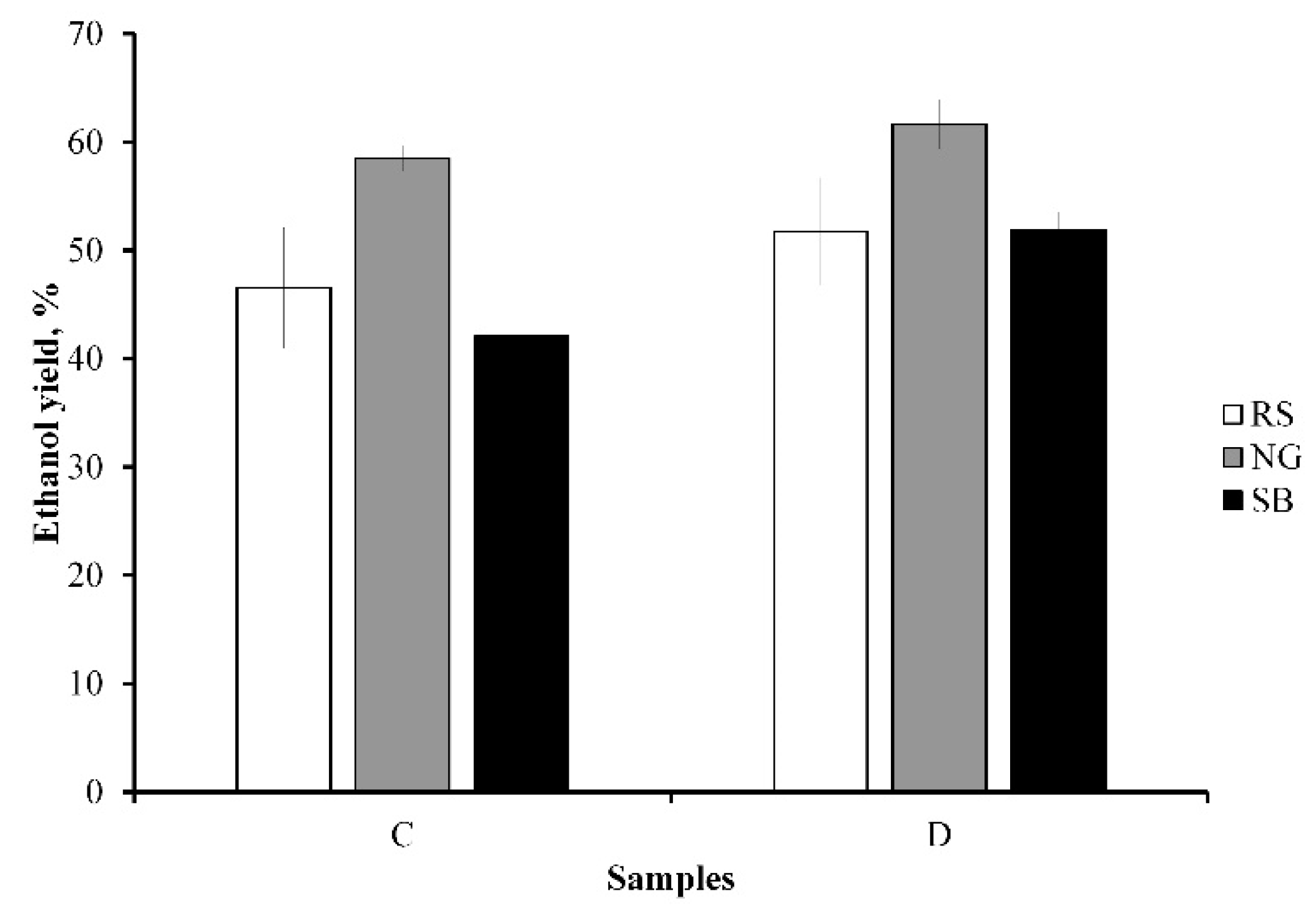
3.5. Ethanol Production
3.6. Wax Compositional Analysis by GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen Production from Organic Wastes by Dark Fermentation. Biohydrogen 2013, 103–144. [Google Scholar] [CrossRef]
- Jorgenson, A.K.; Fiske, S.; Hubacek, K.; Li, J.; McGovern, T.; Rick, T.; Schor, J.B.; Solecki, W.; York, R.; Zycherman, A. Social science perspectives on drivers of and responses to global climate change. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- González-García, S.; Luo, L.; Moreira, M.T.; Feijoo, G.; Huppes, G. Life cycle assessment of hemp hurds use in second generation ethanol production. Biomass Bioenergy 2012, 36, 268–279. [Google Scholar] [CrossRef]
- Das, P.K.; Das, B.P.; Dash, P. Potentials of postharvest rice crop residues as a source of biofuel. Refin. Biomass Residues Sustain. Energy Bioprod. Technol. Adv. Life Cycle Assess. Econ. 2019, 275–301. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymer 2019, 11, 751. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamizawa, K.; Ishikawa, E.; Nakamura, K.; Futamura, T. Bioethanol production from enzymatically saccharified lawn clippings from a golf course. J. Mater. Cycles Waste Manag. 2013, 15, 16–24. [Google Scholar] [CrossRef]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Kitagami, J.T.; Salatino, A.; Guerreiro-Filho, O.; Faria Salatino, M.L. Foliar cuticular waxes of cultivated species and varieties of Coffea. Biochem. Syst. Ecol. 2013, 46, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Kothari, S.L.; Rathore, M.S.; Gour, V.S. Properties, variations, roles, and potential applications of epicuticular wax: A review. Turk. J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Ur-Rahman, M.; Ali, Q.; Awan, S.I. Plant cuticular waxes: A review on functions, composition, biosyntheses mechanism and transportation. Life Sci. J. 2015, 12, 60–67. [Google Scholar] [CrossRef]
- Krstić, G.; Aljančić, I.; Stanković, J.; Cvetković, M.; Marin, P.; Janaćković, P.; Tešević, V. Leaf epicuticular waxes of eleven Euphorbia species (Euphorbiaceae) from the central Balkans: Impact on chemotaxonomy. Arch. Biol. Sci. 2019, 71, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Thushari, I.; Vicheanteab, J.; Janjaroen, D. Material flow analysis and life cycle assessment of solid waste management in urban green areas, Thailand. Sustain. Environ. Res. 2020, 30. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970.
- Athukorala, Y.; Mazza, G. Supercritical carbon dioxide and hexane extraction of wax from triticale straw: Content, composition and thermal properties. Ind. Crop. Prod. 2010, 31, 550–556. [Google Scholar] [CrossRef]
- Tantayotai, P.; Rattanaporn, K.; Tepaamorndech, S.; Cheenkachorn, K.; Sriariyanun, M. Analysis of an Ionic Liquid and Salt Tolerant Microbial Consortium Which Is Useful for Enhancement of Enzymatic Hydrolysis and Biogas Production. Waste Biomass Valorization 2019, 10, 1481–1491. [Google Scholar] [CrossRef]
- Rattanaporn, K.; Tantayotai, P.; Phusantisampan, T.; Pornwongthong, P.; Sriariyanun, M. Organic acid pretreatment of oil palm trunk: Effect on enzymatic saccharification and ethanol production. Bioprocess Biosyst. Eng. 2018, 41, 467–477. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Amnuaycheewa, P.; Rodiahwati, W.; Sanvarinda, P.; Cheenkachorn, K.; Tawai, A. Effect of Organic Acid Pretreatment on Napier Grass (Pennisetum purpureum) Straw Biomass Conversion. J. King Mongkut’s Univ. Technol. North Bangk. 2017. [Google Scholar] [CrossRef]
- Paulraj Gundupalli, M.; Cheng, Y.-S.; Chuetor, S.; Bhattacharyya, D.; Sriariyanun, M. Effect of dewaxing on saccharification and ethanol production from different lignocellulosic biomass. Bioresour. Technol. 2021, 339, 125596. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic acid: A promising and sustainable platform chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar] [CrossRef]
- Yanagida, T.; Shimizu, N.; Kimura, T. Extraction of Wax from Banana Leaves as an Alternative Way of Utilizing Agricultural Residues. Jpn. J. Food Eng. 2005, 6, 29–35. [Google Scholar] [CrossRef]
- Uddin, M.N.; Marshall, D.R. Variation in epicuticular wax content in wheat. Euphytica 1988, 38, 3–9. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Hoffman, L.L. Composition of wax from seed flax straw. J. Am. Oil Chem. Soc. 1977, 54, 587–588. [Google Scholar] [CrossRef]
- Morrison, W.H.; Holser, R.; Akin, D.E. Cuticular wax from flax processing waste with hexane and super critical carbon dioxide extractions. Ind. Crop. Prod. 2006, 24, 119–122. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Li, Z.; Lin, Y.; Liu, L.; Zhang, X.; Deng, H. Supercritical carbon dioxide and hexane extraction of wax from apple peel pomace: Content, composition, and thermal properties. Sep. Sci. Technol. 2015, 50, 2230–2237. [Google Scholar] [CrossRef]
- Eiman, M.A.M.; Hatil, H.E.-K.; Salah, A.A.; Mohammed, Y.A.-A.; Marwa, E.M.; Safa, O.B.; Safia, M.S. Antibacterial properties and phytochemical screening of Tamarix nilotica leaves from Sudan. GSC Biol. Pharm. Sci. 2018, 3, 006–010. [Google Scholar] [CrossRef]
- Qi, G.; Peng, F.; Xiong, L.; Lin, X.; Huang, C.; Li, H.; Chen, X.; Chen, X. Extraction and characterization of wax from sugarcane bagasse and the enzymatic hydrolysis of dewaxed sugarcane bagasse. Prep. Biochem. Biotechnol. 2017, 47, 276–281. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, A.; Sharma, P.; Singh, S.; Das, D.; Chawla, G.; Singha, A.; Nain, L. Valorization of jute (Corchorus sp.) biomass for bioethanol production. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef]
- Jenks, M.A.; Ashworth, E.N. Plant Epicuticular Waxes: Function, Production, and Genetics. In Horticultural Reviews; John Wiley & Sons, Inc.: Oxford, UK, 2010; pp. 1–68. [Google Scholar]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Ewurum, A.; Ankem, A.; Georgiev, G.; Borchman, D. A spectroscopic study of the composition and conformation of cholesteryl and wax esters purified from meibum. Chem. Phys. Lipids 2021, 238. [Google Scholar] [CrossRef]
- Farber, C.; Li, J.; Hager, E.; Chemelewski, R.; Mullet, J.; Rogachev, A.Y.; Kurouski, D. Complementarity of Raman and Infrared Spectroscopy for Structural Characterization of Plant Epicuticular Waxes. ACS Omega 2019, 4, 3700–3707. [Google Scholar] [CrossRef] [Green Version]
- Bucio, A.; Moreno-tovar, R.; Bucio, L.; Espinosa-dávila, J.; Anguebes-franceschi, F. Characterization of beeswax, candelilla wax and paraffin wax for coating cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Lozhechnikova, A.; Bellanger, H.; Michen, B.; Burgert, I.; Österberg, M. Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl. Surf. Sci. 2017, 396, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural changes of oak wood main components caused by thermal modification. Polymer 2020, 12, 485. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Al-Swaidan, H.M. Optimization and characterization of sliced activated carbon prepared from date palm tree fronds by physical activation. Biomass Bioenergy 2015, 73, 124–134. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Xu, J.; Xu, H. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. [Google Scholar] [CrossRef]
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using Ft-Ir And Ft-Raman vibrational spectroscopy. Maderas Cienc. Y Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Martín, C.; Puls, J.; Saake, B.; Schreiber, A. Effect of glycerol preatreatment on component recovery and enzymatic hydrolysis of sugarcane bagasse. Cellul. Chem. Technol. 2011, 45, 487–494. [Google Scholar]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; O’Connor, J.P.; Hums, M.E. Integrated process for extraction of wax as a value-added co-product and improved ethanol production by converting both starch and cellulosic components in sorghum grains. Fermentation 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Kádár, Z.; Schultz-Jensen, N.; Jensen, J.S.; Hansen, M.A.T.; Leipold, F.; Bjerre, A.B. Enhanced ethanol production by removal of cutin and epicuticular waxes of wheat straw by plasma assisted pretreatment. Biomass Bioenergy 2015, 81, 26–30. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef]
- Liu, D.C.; Zeng, Q.; Ji, Q.X.; Liu, C.F.; Liu, S.B.; Liu, Y. A comparison of the ultrastructure and composition of fruits’ cuticular wax from the wild-type “Newhall” navel orange (Citrus sinensis [L.] Osbeck cv. Newhall) and its glossy mutant. Plant Cell Rep. 2012, 31, 2239–2246. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Byju, K.; Vasundhara, G.; Anuradha, V.; Nair, S.M.; Kumar, N.C. Presence of Phytol, a Precursor of Vitamin E in Chaetomorpha Antinnina. Mapana J. Sci. 2013, 12, 57–65. [Google Scholar] [CrossRef]
- Ko, G.A.; Cho, S.K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem. Biol. Interact. 2018, 286, 132–140. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Wang, C.; Mu, C.; Huang, X. High-strength charcoal briquette preparation from hydrothermal pretreated biomass wastes. Fuel Process. Technol. 2018, 171, 293–300. [Google Scholar] [CrossRef]

| Lignocellulosic Biomass | Wax Yield, % | References |
|---|---|---|
| M. liukiuensis | 0.58 | [23] |
| M. acuminate | 1.05 | |
| M. chiliocarpa | 1.41 | |
| Wheat leaves | 0.41 | [24] |
| Flax straw | 0.20 | [25] |
| Flax processing waste | 4.00 | [26] |
| Apple peel pomace | 3.98 | [27] |
| Tamarix nilotica leaves | 0.70 | [28] |
| Sugarcane bagasse | 1.20 | [29] |
| Rice straw (RS) | 0.57 ± 0.080 | Present study |
| Napier grass (NG) | 0.61 ± 0.035 | |
| Sugarcane bagasse (SB) | 1.69 ± 0.092 |
| Lignocellulosic Biomass | FAME, % | FA, % | Alcohol, % | Alkane, % | Ketone, % | Other, % | References |
|---|---|---|---|---|---|---|---|
| Carnauba | 54 | na | 12 | 1 | na | 23 | [47] |
| Sugarcane bagasse | 66.26 | 4.58 | 0.22 | 28.83 | na | 0.11 | [48] |
| Wheat straw | 11 | 25 | 20 | na | na | na | [49] |
| Pyrus pyrifolia | 3.45 | 7.09 | 40.72 | 24.47 | na | 43.67 | [50] |
| Blueberry | na | 2.8 | 3.2 | 1.3 | 16.4 | 7.8 | [51] |
| Citrus sinensis | na | 7.8 | 0.16 | 11.5 | na | na | [52] |
| Rice straw (RS) | 64.68 | 12.03 | 6.43 | 16.85 | 1.93 | 0.00 | Present study |
| Napier grass (NG) | 82.46 | 3.48 | 9.60 | 3.05 | 1.34 | 1.41 | |
| Sugarcane bagasse (SB) | 88.65 | nd | 1.47 | 9.88 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundupalli, M.P.; Chuetor, S.; Cheenkachorn, K.; Rattanaporn, K.; Show, P.-L.; Cheng, Y.-S.; Sriariyanun, M. Interferences of Waxes on Enzymatic Saccharification and Ethanol Production from Lignocellulose Biomass. Bioengineering 2021, 8, 171. https://doi.org/10.3390/bioengineering8110171
Gundupalli MP, Chuetor S, Cheenkachorn K, Rattanaporn K, Show P-L, Cheng Y-S, Sriariyanun M. Interferences of Waxes on Enzymatic Saccharification and Ethanol Production from Lignocellulose Biomass. Bioengineering. 2021; 8(11):171. https://doi.org/10.3390/bioengineering8110171
Chicago/Turabian StyleGundupalli, Marttin Paulraj, Santi Chuetor, Kraipat Cheenkachorn, Kittipong Rattanaporn, Pau-Loke Show, Yu-Shen Cheng, and Malinee Sriariyanun. 2021. "Interferences of Waxes on Enzymatic Saccharification and Ethanol Production from Lignocellulose Biomass" Bioengineering 8, no. 11: 171. https://doi.org/10.3390/bioengineering8110171