Microfluidic System for In Vivo-Like Drug Permeation Studies with Dynamic Dilution Profiles
Abstract
:1. Introduction
2. Materials and Methods
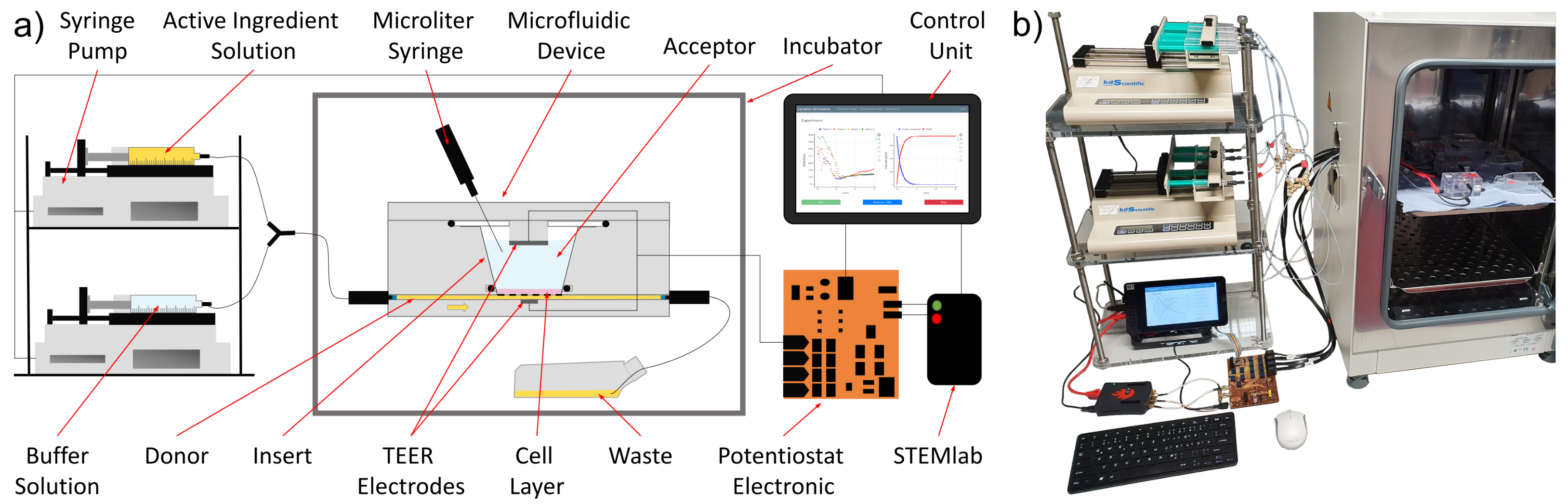
2.1. Materials and Setup
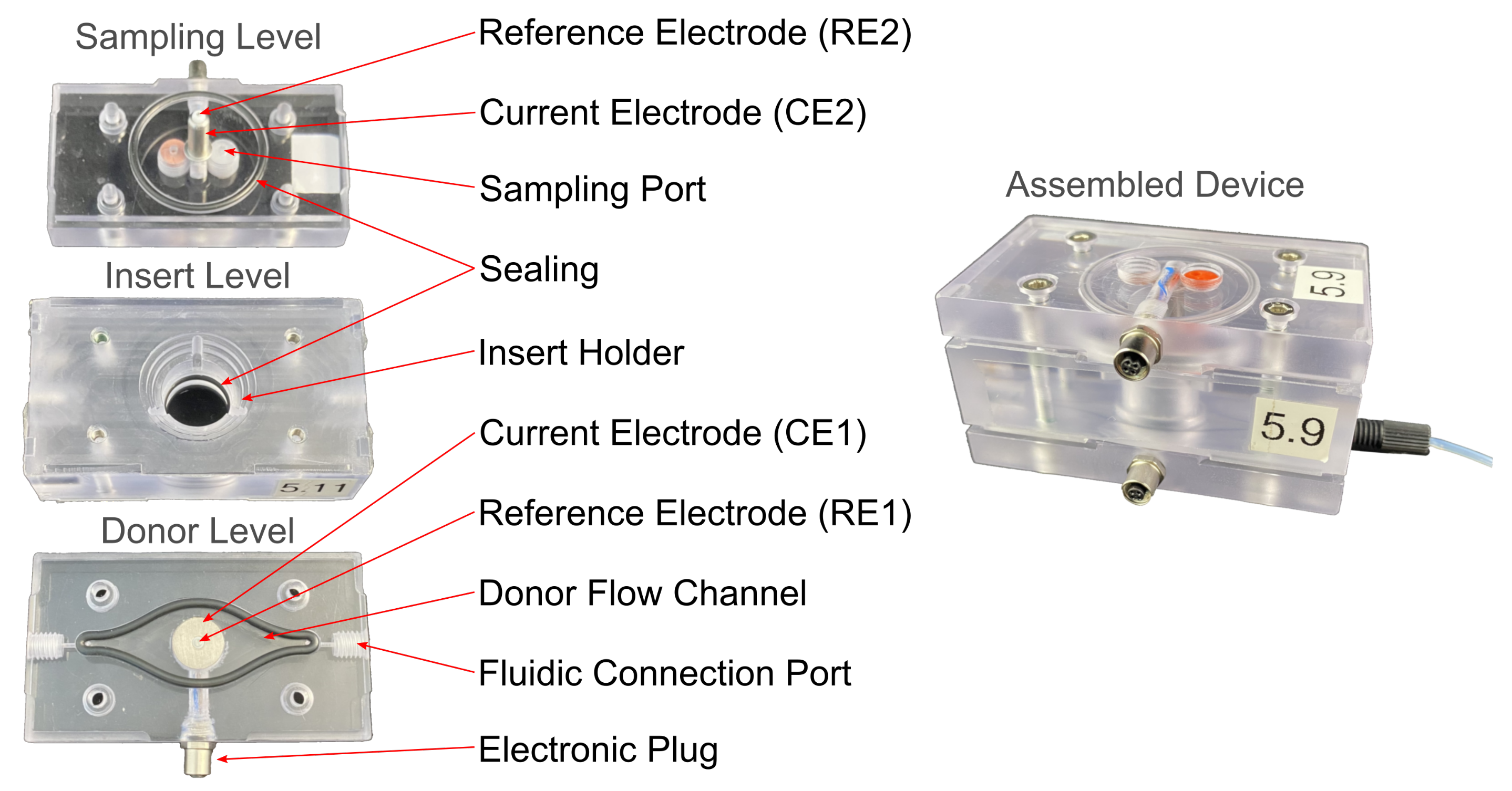
2.1.1. Microfluidic Device
2.1.2. Transepithelial Electrical Resistance Measurement
2.1.3. Automated Test Setup
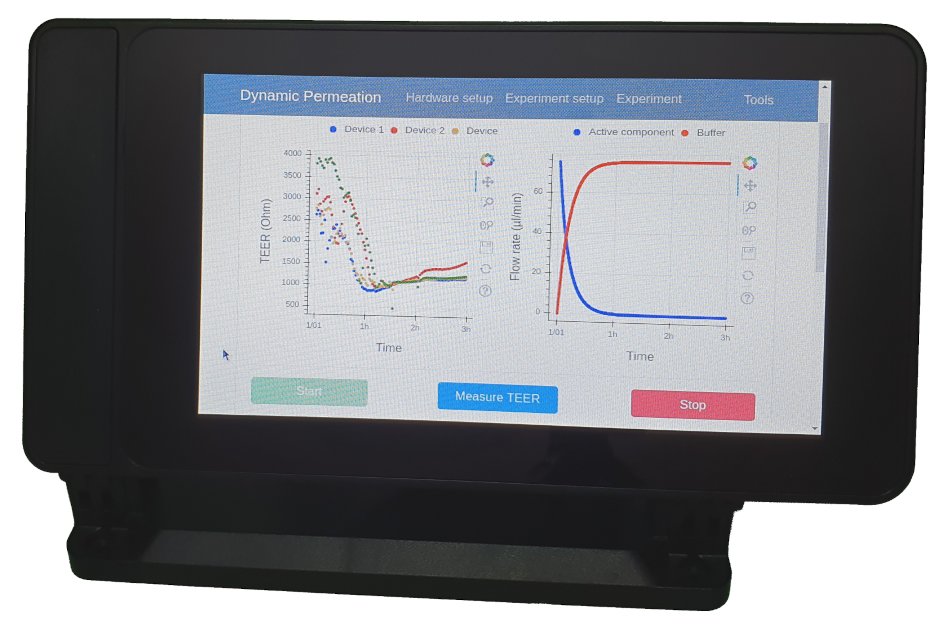
2.1.4. Control Software and Graphical User Interface
2.1.5. Chemicals
2.1.6. Materials
2.2. Methods
2.2.1. Cultivation of Madin Darby Canine Kidney Cells
2.2.2. Dynamic Concentration Verification
2.2.3. Determination of the Cellular Barrier
2.2.4. Permeation Studies
3. Results
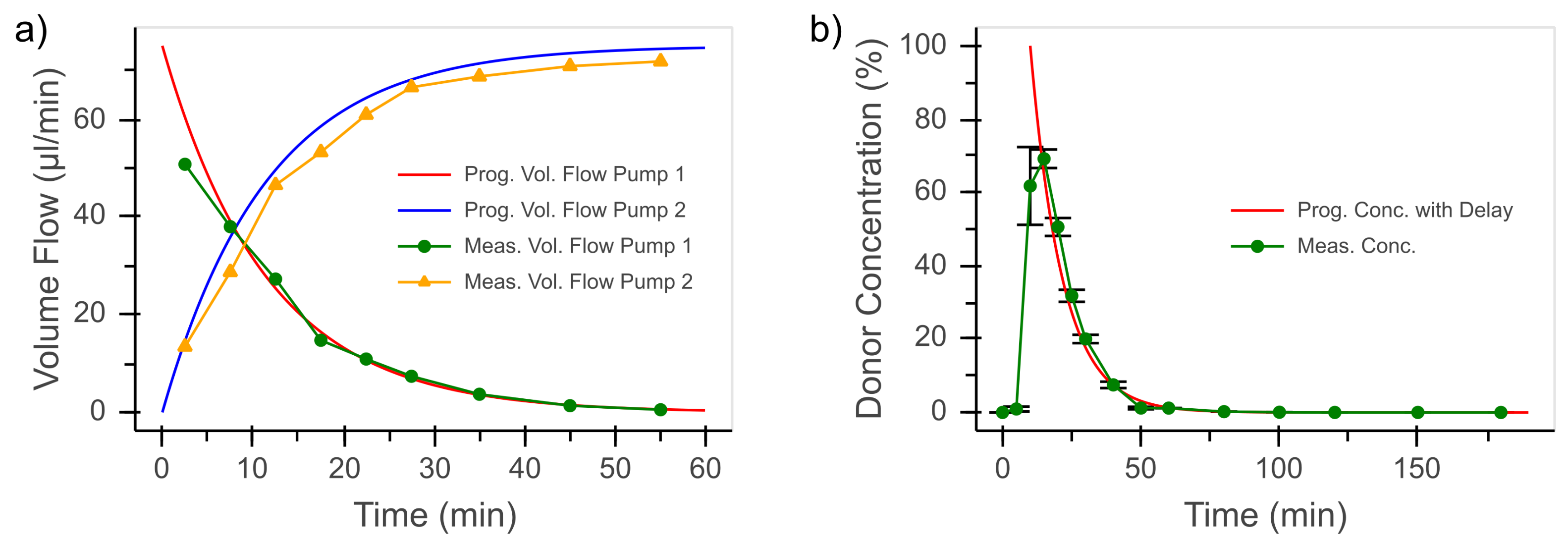
3.1. Dynamic Concentration Profile
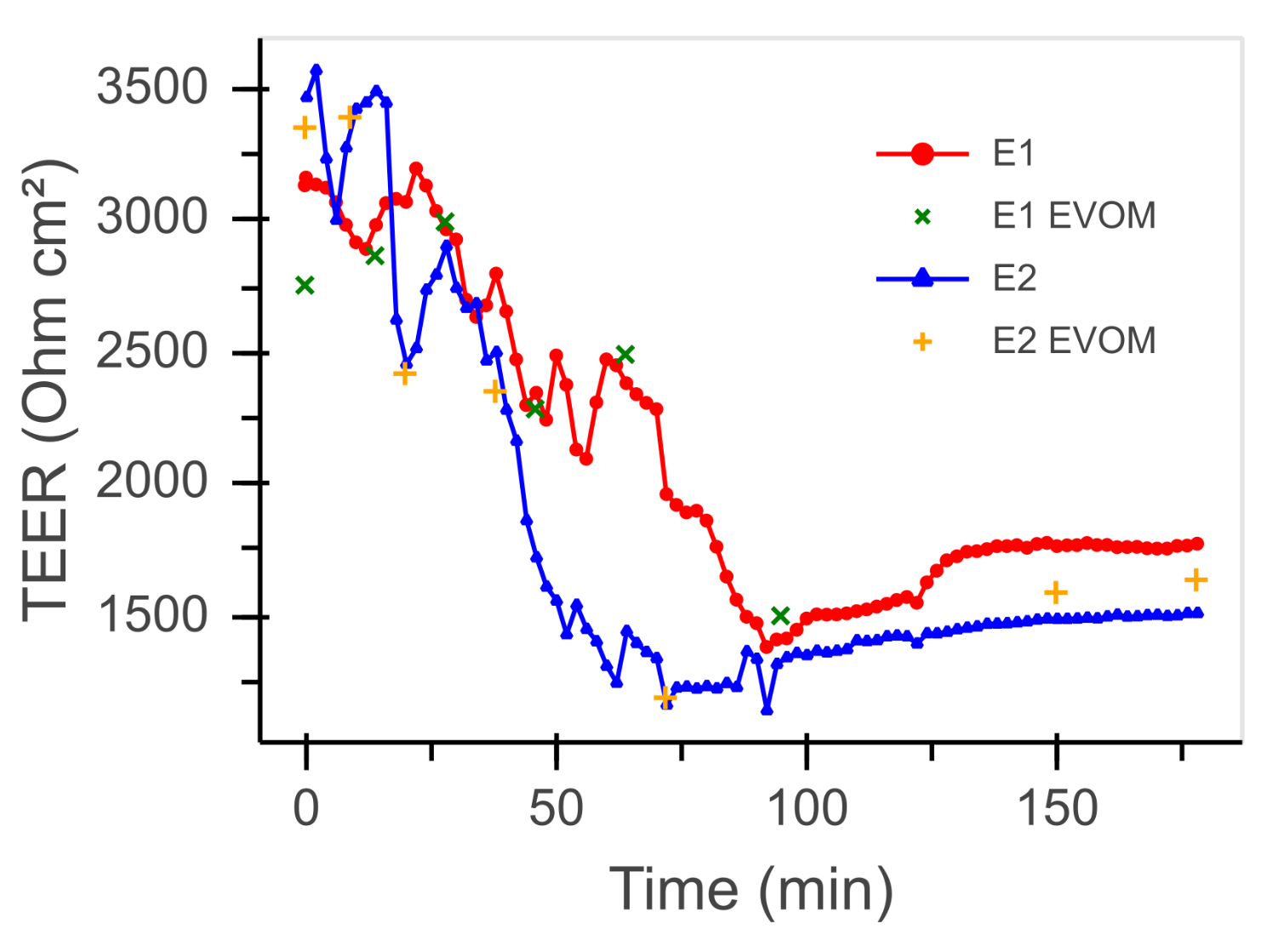
3.2. Transepithelial Electrical Resistance
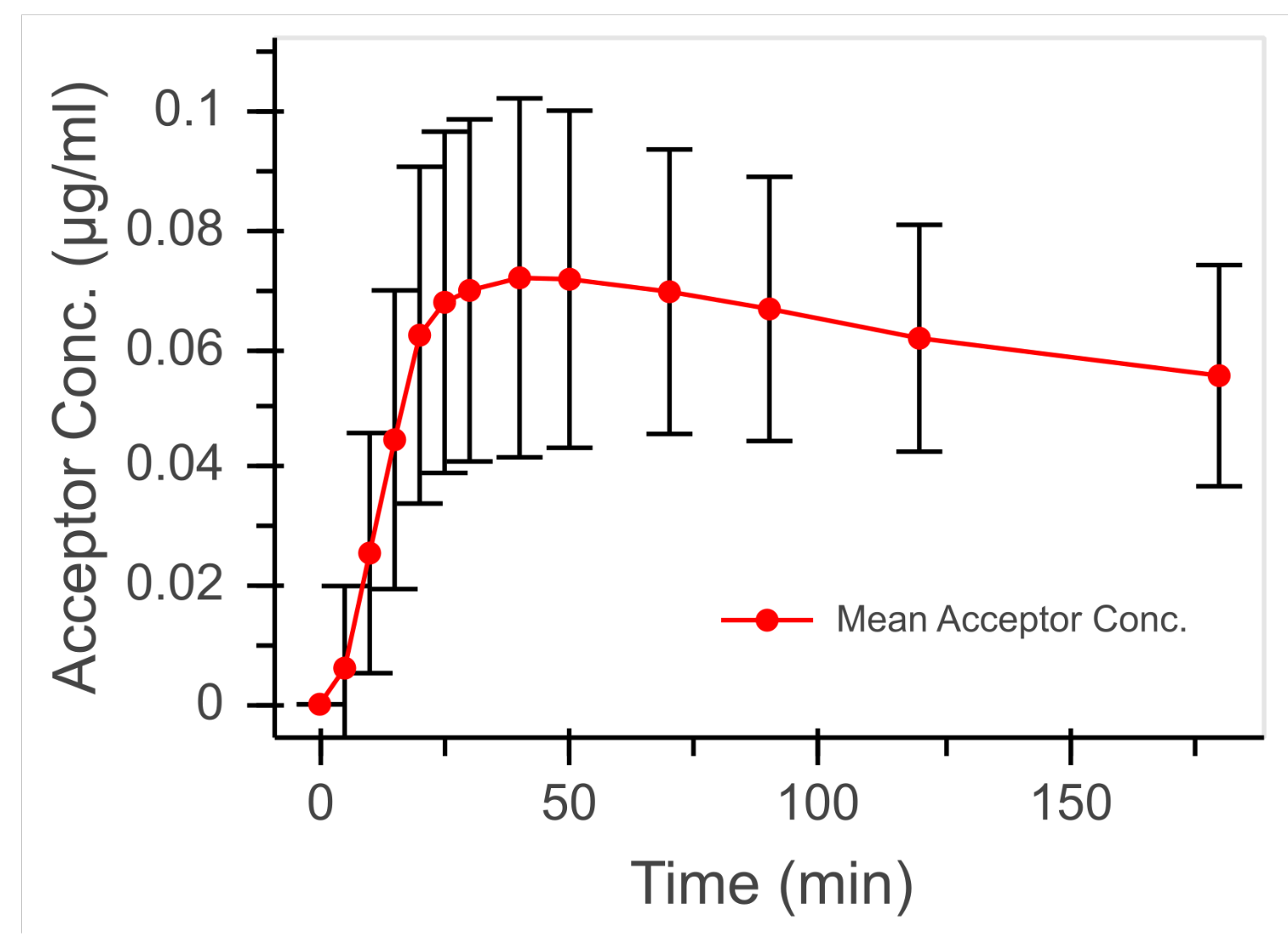
3.3. Permeation Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Current electrodes |
| CSV | Comma Separated Values |
| DA | Differential amplifier |
| FBS | Fetal bovine serum |
| FPGA | Field programmable gate array |
| GLP | Good laboratory practice |
| GUI | Graphical user interface |
| SQL | Structured Query Language |
| GPIO | General purpose input/output |
| KRB | Krebs–Ringer buffer |
| MDCK | Madin–Darby canine kidney |
| NaHCO3 | Sodium hydrogen carbonate |
| OP | Operational amplifier |
| PBS | Phosphate-buffered saline |
| PC | Polycarbonate |
| PCB | Printed circuit board |
| PTFE | Polytetrafluoroethylene |
| RE | Reference electrodes |
| SCPI | Standard Commands for Programmable Instruments |
| TEER | Transepithelial electrical resistance |
References
- van Norman, G.A. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. J. Am. Coll. Cardiol. Basic Transl. Sci. 2016, 1, 170–179. [Google Scholar]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- van Norman, G.A. Phase II Trials in Drug Development and Adaptive Trial Design. J. Am. Coll. Cardiol. Basic Transl. Sci. 2019, 4, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Beißner, N.; Lorenz, T.; Reichl, S. Organ on Chip. In Microsystems for Pharmatechnology Microsystems for Pharmatechnology—Manipulation of Fluids, Particles, Droplets, and Cells; Dietzel, A., Ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 299–339. [Google Scholar]
- Cavero, I.; Gullion, J.-M.; Holzgrefe, H.H. Human organotypic bioconstructs from organ-on-chip devices for human-predictive biological insights on drug candidates. Expert Opin. Drug Saf. 2019, 18, 651–677. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Schneider, S.; Loskill, P. High-throughput organ-on-a-chip systems: Current status and remaining challenges. Curr. Opin. Biomed. Eng. 2018, 6, 33–41. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattern, K.; Beißner, N.; Reichl, S.; Dietzel, A. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 1—Engineering of microfluidic system and technical simulations. Eur. J. Pharm. Biopharm. 2018, 126, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. CMGH Cell Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beißner, N.; Bolea, A.A.; Füller, J.; Kellner, T.; Lauterboeck, L.; Liang, J.; Böl, M.; Glasmacher, B.; Müller-Goymann, C.C.; Reichl, S. Improved in vitro models for preclinical drug and formulation screening focusing on 2D and 3D skin and cornea constructs. Eur. J. Pharm. Biopharm. 2018, 126, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, M.; Shin, H.-W.; Chung, S. In vitro nasal mucosa gland-like structure formation on a chip. Lab Chip 2017, 17, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- McLean, I.C.; Schwerdtfeger, L.A.; Tobet, S.A.; Henry, C.S. Powering ex vivo tissue models in microfluidic systems. Lab Chip 2018, 18, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Beißner, N.; Mattern, K.; Dietzel, A.; Reichl, S. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 2—Ocular DynaMiTES for drug absorption studies of the anterior eye. Eur. J. Pharm. Biopharm. 2018, 126, 166–176. [Google Scholar]
- Hinkel, S.; Mattern, K.; Dietzel, A.; Reichl, S.; Müller-Goymann, C.C. Parametric investigation of static and dynamic cell culture conditions and their impact on hCMEC/D3 barrier properties. Int. J. Pharm. 2019, 566, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.; Figaszewski, Z. A four-electrode potentiostat-galvanostat for studies of bilayer lipid membranes. Meas. Sci. Technol. 1995, 6, 1050–1055. [Google Scholar] [CrossRef]
- Red Pitaya STEMlab Board. Available online: https://www.redpitaya.com/f130/STEMlab-board (accessed on 21 March 2021).
- Sørensen, T.; Jensen, F.T. Tear flow in normal human eyes. Determination by means of radioisotope and gamma camera. Acta Ophthalmol. 1979, 57, 564–581. [Google Scholar] [CrossRef] [PubMed]
- The Pallets Project. Available online: https://palletsprojects.com/p/flask/ (accessed on 21 March 2021).
- Hahne, M.; Zorn-Kruppa, M.; Guzman, G.; Brandner, J.M.; Haltner-Ukomado, E.; Wätzig, H.; Reichl, S. Prevalidation of a human cornea construct as an alternative to animal corneas for in vitro drug absorption studies. J. Pharm. Sci. 2012, 101, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Cramer, S.; Galla, H.-J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douville, N.J.; Tung, Y.-C.; Li, R.; Wang, J.D.; El-Sayed, M.E.H.; Takayama, S. Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal. Chem. 2010, 82, 2505–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenz, T.; Kirschke, M.; Ledwig, V.; Reichl, S.; Dietzel, A. Microfluidic System for In Vivo-Like Drug Permeation Studies with Dynamic Dilution Profiles. Bioengineering 2021, 8, 58. https://doi.org/10.3390/bioengineering8050058
Lorenz T, Kirschke M, Ledwig V, Reichl S, Dietzel A. Microfluidic System for In Vivo-Like Drug Permeation Studies with Dynamic Dilution Profiles. Bioengineering. 2021; 8(5):58. https://doi.org/10.3390/bioengineering8050058
Chicago/Turabian StyleLorenz, Thomas, Mona Kirschke, Verena Ledwig, Stephan Reichl, and Andreas Dietzel. 2021. "Microfluidic System for In Vivo-Like Drug Permeation Studies with Dynamic Dilution Profiles" Bioengineering 8, no. 5: 58. https://doi.org/10.3390/bioengineering8050058






