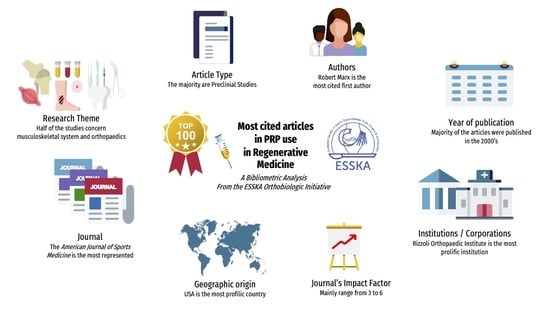
The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine—A Bibliometric Analysis—From the ESSKA Orthobiologic Initiative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Allocation of Articles
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Top 10 Most Cited Articles
3.2. Characteristics of the Top 100 Most Cited Articles
3.3. Most Recent and Promising Articles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Rank | Paper | Country (1st Author) | ACY | No. of Citations |
|---|---|---|---|---|
| 1 | Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. Doi:10.1016/s1079-2104(98)90029-4 | USA | 75.16 | 1879 |
| 2 | Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004; 62(4):489–496. Doi:10.1016/j.joms.2003.12.003 | USA | 66.05 | 1255 |
| 3 | Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. Doi:10.1160/TH03-07-0440 | Spain | 50.42 | 958 |
| 4 | Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167. Doi:10.1016/j.tibtech.2008.11.009 | Sweden | 61.36 | 859 |
| 5 | Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. Doi:10.1097/01.prs.0000138251.07040.51 | USA | 39.53 | 751 |
| 6 | Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. Doi:10.1177/0363546509349921 | USA | 52.21 | 731 |
| 7 | Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529–535. | Spain | 28.46 | 683 |
| 8 | de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. Doi:10.1001/jama.2009.1986 | Netherlands | 39.62 | 515 |
| 9 | Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31(24):6279–6308. Doi:10.1016/j.biomaterials.2010.04.053 | China | 36.38 | 473 |
| 10 | Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. Doi:10.1054/jcms.2002.0285 | Germany | 20.05 | 421 |
| 11 | Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. Doi:10.1016/j.bone.2003.12.010 | Germany | 21.68 | 412 |
| 12 | Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. Doi:10.1177/0363546512471299 | India | 40.00 | 400 |
| 13 | Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–996. Doi:10.1302/0301-620X.91B8.22546 | England | 27.43 | 384 |
| 14 | Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93–103. | USA | 18.40 | 368 |
| 15 | Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–2715. Doi:10.1007/s11999-011-1857-3 | USA | 30.17 | 362 |
| 16 | Anitua E, Andía I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23(2):281–286. Doi:10.1016/j.orthres.2004.08.015 | Spain | 19.67 | 354 |
| 17 | Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118(6):147e-159e. doi:10.1097/01.prs.0000239606.92676.cf | USA | 20.18 | 343 |
| 18 | Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):472–479. Doi:10.1007/s00167-009-0940-8 | Italy | 25.85 | 336 |
| 19 | Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. Published 2008 May 1. Doi:10.2741/2947 | France | 22.40 | 336 |
| 20 | Anitua E, Sánchez M, Orive G, Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28(31):4551–4560. Doi:10.1016/j.biomaterials.2007.06.037 | Spain | 20.75 | 332 |
| 21 | Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–301. Doi:10.1016/s0278-2391(00)90058-2 | USA | 14.39 | 331 |
| 22 | Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. Doi:10.1016/j.arthro.2011.05.011 | Italy | 27.00 | 324 |
| 23 | Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3–4):165–174. Doi:10.1007/s12178-008-9032-5 | USA | 21.33 | 320 |
| 24 | El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78(4):661–669. Doi:10.1902/jop.2007.060302 | USA | 19.88 | 318 |
| 25 | Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107(1):229–239. Doi:10.1097/00006534-200101000-00037 | USA | 14.36 | 316 |
| 26 | Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266–271. Doi:10.1177/0363546510387517 | USA | 26.17 | 314 |
| 27 | Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24(18):3095–3100. Doi:10.1016/s0142-9612(03)00114-5 | Italy | 15.50 | 310 |
| 28 | Amable POur, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. Published 2013 Jun 7. doi:10.1186/scrt218 | Brazil | 30.70 | 307 |
| 29 | Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. doi:10.1016/j.arthro.2011.10.018 | USA | 27.82 | 306 |
| 30 | Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–265. doi:10.1177/0363546510390780 | Italy | 25.50 | 306 |
| 31 | Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212–219. doi:10.1111/j.1600-0501.2005.01203.x | Italy | 17.41 | 296 |
| 32 | He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):707–713. doi:10.1016/j.tripleo.2009.06.044 | China | 20.79 | 291 |
| 33 | Crovetti G, Martinelli G, Issi M, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30(2):145–151. doi:10.1016/j.transci.2004.01.004 | Italy | 15.32 | 291 |
| 34 | DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi:10.1016/j.arthro.2012.04.148 | USA | 26.27 | 289 |
| 35 | Okuda K, Kawase T, Momose M, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849–857. doi:10.1902/jop.2003.74.6.849 | Japan | 14.45 | 289 |
| 36 | Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. doi:10.1177/0363546511417792 | USA | 24.00 | 288 |
| 37 | Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25(2):230–240. doi:10.1002/jor.20278 | USA | 17.88 | 286 |
| 38 | Bendinelli P, Matteucci E, Dogliotti G, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. doi:10.1002/jcp.22274 | Italy | 20.85 | 271 |
| 39 | Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. doi:10.1089/ten.tec.2008.0534 | USA | 19.36 | 271 |
| 40 | Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308–316. doi:10.2106/JBJS.K.00430 | USA | 24.45 | 269 |
| 41 | Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518–528. doi:10.1016/j.jse.2011.02.008 | Italy | 22.25 | 267 |
| 42 | Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272–1280. doi:10.1016/j.joca.2006.05.008 | USA | 15.71 | 267 |
| 43 | de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–1178. doi:10.1177/0363546508314430 | Netherlands | 17.73 | 266 |
| 44 | Driver VR, Hanft J, Fylling CP, Beriou JM; Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):. | USA | 15.59 | 265 |
| 45 | Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10(5–6):955–964. doi:10.1089/1076327041348284 | Japan | 13.89 | 264 |
| 46 | Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3–9. Published 2014 May 8. | South Korea Switzerland | 29.22 | 263 |
| 47 | Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1–9. | USA | 20.23 | 263 |
| 48 | Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113–125. doi:10.1016/j.csm.2008.08.007 | USA | 18.71 | 262 |
| 49 | Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31(5):469–484. doi:10.1054/ijom.2002.0244 | Germany | 12.48 | 262 |
| 50 | Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71(10):1654–1661. doi:10.1902/jop.2000.71.10.1654 | USA | 10.96 | 252 |
| 51 | Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434–439. doi:10.1177/154405910508400507 | Canada | 13.67 | 246 |
| 52 | Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122(5):1352–1360. doi:10.1097/PRS.0b013e3181882046 | Japan | 16.27 | 244 |
| 53 | Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J Oral Maxillofac Surg. 2002;60(10):1176–1181. doi:10.1053/joms.2002.34994 | USA | 11.48 | 241 |
| 54 | Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):528–535. doi:10.1007/s00167-010-1238-6 | Italy | 20.00 | 240 |
| 55 | Niemeyer P, Fechner K, Milz S, et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31(13):3572–3579. doi:10.1016/j.biomaterials.2010.01.085 | Germany | 18.46 | 240 |
| 56 | McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033–1042. doi:10.1002/jor.20853 | USA | 17.14 | 240 |
| 57 | Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine [published correction appears in J Am Acad Orthop Surg. 2010 Jan;18(1):17A]. J Am Acad Orthop Surg. 2009;17(10):602–608. doi:10.5435/00124635-200910000-00002 | USA | 17.07 | 239 |
| 58 | Ishida K, Kuroda R, Miwa M, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13(5):1103–1112. doi:10.1089/ten.2006.0193 | Japan | 14.75 | 236 |
| 59 | Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. doi:10.1016/j.arthro.2012.05.011 | Spain | 20.45 | 225 |
| 60 | Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi:10.1038/nrrheum.2013.141 | Spain | 22.30 | 223 |
| 61 | Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi:10.1002/jor.20282 | USA | 13.88 | 222 |
| 62 | Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–1054. doi:10.1097/01.scs.0000186454.07097.bf | USA | 12.33 | 222 |
| 63 | Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37(4):300–306. doi:10.1034/j.1600-0765.2002.01001.x | USA | 10.52 | 221 |
| 64 | Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–2360. doi:10.1007/s00784-016-1719-1 | Japan Switzerland | 31.43 | 220 |
| 65 | Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910–913. | Spain | 14.67 | 220 |
| 66 | van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362–2370. doi:10.1177/0363546511419278 | Netherlands | 18.25 | 219 |
| 67 | Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis–--a preliminary result of three cases. Bone. 2004;35(4):892–898. doi:10.1016/j.bone.2004.06.013 | Japan | 11.42 | 217 |
| 68 | Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. doi:10.1007/s00167-011-1837-x | Italy | 19.55 | 215 |
| 69 | Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. Published 2012 Nov 23. doi:10.1186/1471-2474-13-229 | Italy | 19.45 | 214 |
| 70 | Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi:10.1097/PHM.0b013e3182aab72 | Slovakia | 19.18 | 211 |
| 71 | Wiltfang J, Kloss FR, Kessler P, et al. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin Oral Implants Res. 2004;15(2):187–193. doi:10.1111/j.1600-0501.2004.00980.x | Germany | 11.11 | 211 |
| 72 | de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623–1629. doi:10.1177/0363546511404877 | Netherlands | 17.33 | 208 |
| 73 | Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84(2):415–421. doi:10.1002/jbm.b.30886 | Spain | 13.87 | 208 |
| 74 | Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40(6):598–603. doi:10.1016/j.injury.2008.11.026 | Italy | 14.57 | 204 |
| 75 | Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002;22(1):45–53. | USA | 9.71 | 204 |
| 76 | Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. doi:10.1016/j.joca.2013.07.017 | China | 20.3 | 203 |
| 77 | Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–7018. doi:10.1016/j.biomaterials.2012.06.058 | China | 18.45 | 203 |
| 78 | van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12(11):3067–3073. doi:10.1089/ten.2006.12.3067 | Netherlands | 11.65 | 198 |
| 79 | Carter CA, Jolly DG, Worden CE Sr, Hendren DG, Kane CJ. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74(3):244–255. doi:10.1016/s0014-4800(03)00017-0 | USA | 9.90 | 198 |
| 80 | Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–845. doi:10.1002/jcp.21368 | Japan | 13.13 | 197 |
| 81 | Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269–278. doi:10.1016/j.arthro.2009.11.015 | Canada | 15.08 | 196 |
| 82 | Sánchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–354. doi:10.2165/00007256-200939050-00002 | Spain | 14.00 | 196 |
| 83 | Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–635. doi:10.1177/0363546512472975 | Denmark | 19.20 | 192 |
| 84 | Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35–41. doi:10.1177/0363546513507766 | USA | 20.89 | 188 |
| 85 | Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. doi:10.1002/jor.20367 | USA | 11.69 | 187 |
| 86 | Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg. 2005;34(4):420–424. doi:10.1016/j.ijom.2004.10.018 | Korea | 10.33 | 186 |
| 87 | Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298–307. doi:10.2106/JBJS.K.00154 | Canada | 16.73 | 184 |
| 88 | Filardo G, Di Matteo B, Di Martino A, et al. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43(7):1575–1582. doi:10.1177/0363546515582027 | Italy | 22.88 | 183 |
| 89 | Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74(6):858–864. doi:10.1902/jop.2003.74.6.858 | Japan | 9.15 | 183 |
| 90 | Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156–1163. doi:10.1016/j.bone.2006.05.023 | Germany | 10.65 | 181 |
| 91 | Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38(7 Pt 1):1040–1046. doi:10.1111/j.1524-4725.2012.02394.x | Korea | 16.27 | 179 |
| 92 | Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77(5):806–812. doi:10.1080/17453670610013033 | Sweden | 10.47 | 178 |
| 93 | Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17(2):184–190. | Germany | 8.43 | 177 |
| 94 | Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012;13(7):1145–1152. doi:10.2174/138920112800624382 | South Korea France | 16.00 | 176 |
| 95 | Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185–1195. doi:10.2174/138920112800624283 | USA | 16.00 | 176 |
| 96 | Anitua E, Sánchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42(2):162–170. doi:10.1111/j.1365–2184.2009.00583.x | Spain | 12.57 | 176 |
| 97 | Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2017;33(3):659–670.e1. doi:10.1016/j.arthro.2016.09.024 | China | 29.17 | 175 |
| 98 | Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am J Sports Med. 2016;44(3):792–800. doi:10.1177/0363546515580787 | USA | 25.00 | 175 |
| 99 | Anitua E, Sánchez M, Nurden AT, et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford). 2007;46(12):1769–1772. doi:10.1093/rheumatology/kem234 | Spain | 10.88 | 174 |
| 100 | Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010;28(2):211–217. doi:10.1002/jor.20980 | Netherlands | 13.31 | 173 |
| Rank | No. of Citations | ACY | Publication Year | Paper | First Author | Article Type | Theme | Topics and Conclusions |
|---|---|---|---|---|---|---|---|---|
| 1 | 1879 | 75 | 1998 | Platelet-rich plasma-Growth factor enhancement for bone grafts | Marx | Clinical study | Dentistry/ Maxillofacial surgery | Randomized Controlled Trial demonstrating that PRP additions to cancellous cellular bone graft reconstructions of mandibular continuity defects enhanced radiographic maturation and bone density. |
| 2 | 1255 | 66 | 2004 | Platelet-rich plasma: Evidence to support its use | Marx | Review | Dentistry/ Maxillofacial surgery | Review about the mechanism of action, platelet dose, therapeutical indications and safety of PRP. |
| 4 | 958 | 50 | 2004 | Autologous platelets as a source of proteins for healing and tissue regeneration | Anitua | Review | Multiple | Review about the effects of autologous platelets in different clinical situations requiring accelerated healing and tissue regeneration. |
| 3 | 859 | 61 | 2009 | Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) | Ehrenfest | Classification | Biology | The review aimed to clarify the different medical devices for the production of PRP and to categorize them under 3 parameters: fibrin density, leucocyte dose and degree of standardization of the procedure. |
| 5 | 751 | 40 | 2004 | Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing | Eppley | Preclinical study | Cosmetic/Plastic sur-gery | Results from this preclinical study demonstrated that platelets can be separated and concentrated 8-fold from whole blood without activating the platelets and providing increased levels of different growth factors, particularly for PDGF-BB, TGF-β1, VEGF and EGF. |
| 6 | 731 | 52 | 2009 | Platelet-Rich Plasma From Basic Science to Clinical Applications | Foster | Review | Musculoskeletal system/ Orthopaedics | This article examines the basic science of PRP, and evaluates the human studies that have been published in the ortho-paedic sur-gery and sports medi-cine litera-ture. The use of PRP in amateur and professional sports is reviewed, and the regulation of PRP by anti-doping agen-cies is dis-cussed. |
| 7 | 683 | 28 | 1999 | Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants | Anitua | Clinical study | Dentistry/Maxillofacial surgery | This clinical study compared epithelialization of areas treated or not with plasma rich in growth factors after dental extraction. Wound healing and bone formation was better in PRGF group. |
| 8 | 515 | 40 | 2010 | Platelet-Rich Plasma Injection for Chronic Achilles Tendinopathy A Randomized Controlled Trial | de Vos | Clinical study | Musculoskeletal system/ Orthopaedics | This randomized controlled trial compared eccentric exercises with either a PRP injection or saline injection among patients with chronic Achilles tendinopathy. No benefit on pain and function was observed in PRP group. |
| 9 | 473 | 36 | 2010 | Toward delivery of multiple growth factors in tissue engineering | Chen | Review | Multiple | This article summarizes the concept of delivery of multiple growth factors, as well as current efforts to develop sophisticated delivery platforms that allow for controlled spatial presentation and release kinetics of key biological cues. |
| 10 | 421 | 20 | 2002 | Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count | Weibrich | Preclinical study | Biology | Major-age and gender-specific differences in individual growth factor levels were not found. Prediction of resulting growth factor levels based on the thrombocyte counts of whole blood or PRP are not reliable. |
References
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Wright, J.F. Quality Control Testing, Characterization and Critical Quality Attributes of Adeno-Associated Virus Vectors Used for Human Gene Therapy. Biotechnol. J. 2021, 16, e2000022. [Google Scholar] [CrossRef] [PubMed]
- Lavon, N.; Zimerman, M.; Itskovitz-Eldor, J. Scalable Expansion of Pluripotent Stem Cells. Adv. Biochem. Eng./Biotechnol. 2018, 163, 23–37. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.; Frueh, F.S.; McLuckie, M.; Sanchez-Macedo, N.; Wolint, P.; Lindenblatt, N.; Plock, J.A.; Calcagni, M.; Buschmann, J. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat-a review. Cytotherapy 2020, 22, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Born, S.; Dörfel, M.J.; Hartjen, P.; Haschemi Yekani, S.A.; Luecke, J.; Meutsch, J.K.; Westphal, J.K.; Birkelbach, M.; Köhnke, R.; Smeets, R.; et al. A short-term plastic adherence incubation of the stromal vascular fraction leads to a predictable GMP-compliant cell-product. BioImpacts BI 2019, 9, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Dragoo, J.L.; Guzman, R.A. Evaluation of the Consistency and Composition of Commercially Available Bone Marrow Aspirate Concentrate Systems. Orthop. J. Sports Med. 2020, 8, 2325967119893634. [Google Scholar] [CrossRef]
- Pachito, D.V.; Bagattini, Â.M.; de Almeida, A.M.; Mendrone-Júnior, A.; Riera, R. Technical Procedures for Preparation and Administration of Platelet-Rich Plasma and Related Products: A Scoping Review. Front. Cell Dev. Biol. 2020, 8, 598816. [Google Scholar] [CrossRef]
- Del Amo, C.; Perez-Valle, A.; Atilano, L.; Andia, I. Unraveling the Signaling Secretome of Platelet-Rich Plasma: Towards a Better Understanding of Its Therapeutic Potential in Knee Osteoarthritis. J. Clin. Med. 2022, 11, 473. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports medicine applications of platelet rich plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Lana, J.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L., Jr.; Malheiros Luzo, Â.C.; Belangero, W.D.; et al. Contributions for classification of platelet rich plasma—Proposal of a new classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Rachul, C.; Rasko, J.; Caulfield, T. Implicit hype? Representations of platelet rich plasma in the news media. PLoS ONE 2017, 12, e0182496. [Google Scholar] [CrossRef] [Green Version]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2021, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; van Erp, A.; DeSimone, A.; Cohen, D.S.; Gardner, R.D. Platelet Rich Plasma in Orthopedic Surgical Medicine. Platelets 2021, 32, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; LaPrade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J. Am. Acad. Orthop. Surg. 2019, 27, e50–e63. [Google Scholar] [CrossRef] [PubMed]
- Finnoff, J.T.; Awan, T.M.; Borg-Stein, J.; Harmon, K.G.; Herman, D.C.; Malanga, G.A.; Master, Z.; Mautner, K.R.; Shapiro, S.A. American Medical Society for Sports Medicine Position Statement: Principles for the Responsible Use of Regenerative Medicine in Sports Medicine. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2021, 31, 530–541. [Google Scholar] [CrossRef] [PubMed]
- European Society of Sports Traumatology, Knee Surgery & Arthroscopy. ESSKA Consensus Project—Injectable Orthobiologics in Knee OA—Part 1, PRP 1 The Use of Injectable Orthobiologics for Knee Osteoarthritis: A Formal ESSKA Consensus. Part 1—Blood-Derived Products (PRP). Regen Lab. 2022. Available online: https://cdn.ymaws.com/www.esska.org/resource/resmgr/docs/consensus_projects/orbit_consensus_complete2022.pdf (accessed on 1 September 2022).
- Mautner, K.; Malanga, G.A.; Smith, J.; Shiple, B.; Ibrahim, V.; Sampson, S.; Bowen, J.E. A call for a standard classification system for future biologic research: The rationale for new PRP nomenclature. PM&R 2015, 7 (Suppl. 4), S53–S59. [Google Scholar] [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence; Oxford Centre for Evidence-Based Medicine: Oxford, UK; OCEBM Table of Evidence Working Group = Jeremy Howick, Iain Chalmers (James Lind Library), Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, Hazel Thornton, Olive Goddard and Mary Hodgkinson. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 9 January 2022).
- Coleman, B.D.; Khan, K.M.; Maffulli, N.; Cook, J.L.; Wark, J.D. Studies of surgical outcome after patellar tendinopathy: Clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand. J. Med. Sci. Sports 2000, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.; Faloppa, F.; Belloti, J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014, 2014, CD010071. [Google Scholar] [CrossRef]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. EFORT Open Rev. 2021, 6, 225–235. [Google Scholar] [CrossRef]
- Sharara, F.I.; Lelea, L.L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous Platelet-Rich Plasma Efficacy in the Field of Regenerative Medicine: Product and Quality Control. BioMed Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. PRP Therapy. Adv. Exp. Med. Biol. 2018, 1059, 241–253. [Google Scholar] [CrossRef]
- Bossard, N.; Boissel, F.H.; Boissel, J.P. Level of evidence and therapeutic evaluation: Need for more thoughts. Fundamental & Clin. Pharmacol. 2004, 18, 365–372. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmann, E.; Feldman, M.; Hunt, T.J.; Cote, M.P.; Brand, J.C. Research Pearls: How Do We Establish the Level of Evidence? Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Boffa, A.; Andriolo, L.; Di Martino, A.; Zaffagnini, S.; Filardo, G. The 50 most-cited clinical articles in cartilage surgery research: A bibliometric analysis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2022, 30, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- ELAndaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Alsousou, J. Studies on platelet rich plasma—New editorial policy for “Platelets”. Platelets 2020, 31, 281–282. [Google Scholar] [CrossRef] [Green Version]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. Am. Vol. 2017, 99, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Alves, R.; Cole, J.P.; Andjelkov, K.; Van Helmelryck, T.; Fernandez, J.; Trivisonno, A.; Guillaume, L.; Verpaele, A.; Tonnard, P.; et al. AIRMESS—Academy of International Regenerative Medicine & Surgery Societies: Recommendations in the use of platelet-rich plasma (PRP), autologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Expert Opin. Biol. Ther. 2021, 21, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Evangelopoulos, D.S.; Abbasian, M.; Röder, C.; Kohl, S. The hundred most-cited publications in orthopaedic knee research. J. Bone Jt. Surg. Am. Vol. 2014, 96, e190. [Google Scholar] [CrossRef]
- Patel, S.; Kumar, V.; Sharma, S.; Sharma, R.; Kaur, R. Trends of the publications of platelet-rich plasma use in osteoarthritis knee—A PubMed and Scopus bibliometric analysis. J. Arthrosc. Surg. Sports Med. 2022, 3, 101–110. [Google Scholar] [CrossRef]









| Part A—Only One Score to Be Given for Each of the Sections | ||
|---|---|---|
| Score | ||
| 1. Study Size—number of injected sites | >60 | 10 |
| 41–60 | 7 | |
| 20–40 | 4 | |
| <20, not stated | 0 | |
| 2. Mean follow-up (mths) | >24 | 5 |
| 12–24 | 2 | |
| <12, not stated or unclear | 0 | |
| 3. Platelet-Rich Plasma characterization | Complete | 10 |
| Incomplete | 7 | |
| No data | 0 | |
| 4. Type of study | Randomized controlled trial | 15 |
| Cohort study | 10 | |
| Case-series | 0 | |
| 5. Diagnosis certainty (use of preoperative ultrasound, magnetic resonance imaging or post-operative histopathology to confirm diagnosis) | In all | 5 |
| In > 80% | 3 | |
| In < 80%, or NS or unclear | 0 | |
| Part B—Scores may be given for each option in each of the three sections if applicable | ||
| 1. Outcome criteria | Outcome measures clearly defined | 2 |
| Timing of outcome assessment clearly stated (e.g., at best outcome after injection or at follow-up) | 2 | |
| 2. Procedures for assessing outcomes | Subjects recruited (results not taken from surgeons’ files) | 3 |
| Investigator independent of surgeon/injector | 3 | |
| Written assessment | 3 | |
| 3. Description of subject selection process | Selection criteria reported and unbiased | 3 |
| Recruitment rate reported: >80% | 4 | |
| Recruitment rate reported: <80% | 3 | |
| Author | No. of Articles | Institution(s)/Corporation(s) | Rank of Articles | Total No. of Citations |
|---|---|---|---|---|
| Anitua | 7 | Biotechnology Institute Corporation Private Practice in Implantology and Oral Rehabilitation | 3, 7, 16, 20, 73, 96, 99 | 2885 |
| Filardo | 4 | Rizzoli Orthopedic Institute | 54, 68, 69, 88 | 852 |
| Sanchez, Mikel | 3 | Arthroscopic Surgery Unit—Hospital Vithas Vitoria | 59, 65, 82 | 641 |
| Ehrenfest | 3 | The Sahlgrenska Academy at University of Gothenburg Chonnam National University University of Geneva | 4, 46, 94 | 1298 |
| Kon | 3 | Rizzoli Orthopedic Institute | 18, 22, 74 | 864 |
| Mishra | 3 | Stanford University | 39, 48, 95 | 709 |
| Weibrich | 3 | Johannes Gutenberg University of Mainz | 10, 11, 93 | 1010 |
| Eppley | 2 | Indiana University University of Illinois | 5, 17 | 1094 |
| Marx | 2 | University of Miami School of Medicine | 1,2 | 3134 |
| Murray | 2 | Harvard University | 61, 85 | 409 |
| Sundman | 2 | Cornell University | 36, 84 | 476 |
| Journal | Country | Impact Factor (2020) | No. of Articles | Total Citations |
|---|---|---|---|---|
| American Journal of Sports Medicine | USA | 6.2 | 12 | 2739 |
| Arthroscopy-The Journal of Arthroscopic and Related Surgery | UK | 4.8 | 6 | 1515 |
| Journal of Orthopedic Research | USA | 3.5 | 5 | 1462 |
| Biomaterials | Netherlands | 12.5 | 5 | 1558 |
| Journal of Periodontology | USA | 2.3 | 4 | 1042 |
| Plastic and Reconstructive Surgery | USA | 4.7 | 4 | 1654 |
| Bone | USA | 4.4 | 3 | 810 |
| International Journal of Oral and Maxillofacial Implants | USA | 2.8 | 3 | 1228 |
| Journal of Oral and Maxillofacial Surgery | USA | 2.8 | 3 | 1827 |
| Knee Surgery Sports Traumatology Arthroscopy | Germany | 4.3 | 3 | 791 |
| Tissue Engineering | USA | 2.6 (2019) | 3 | 698 |
| Clinical Oral Implants Research | Denmark | 6.0 | 2 | 507 |
| Current Pharmaceutical Biotechnology | United Arab Emirates | 2.8 | 2 | 352 |
| International Journal of Oral and Maxillofacial Surgery | USA | 2.8 | 2 | 448 |
| Journal of Bone and Joint Surgery-American Volume | USA | 5.3 | 2 | 453 |
| Journal of Cellular Physiology | USA | 6.4 | 2 | 468 |
| Osteoarthritis and Cartilage | UK | 6.6 | 2 | 470 |
| Acta Orthopaedica | UK | 3.7 | 1 | 178 |
| American Journal of Physical Medicine and Rehabilitation | USA | 2.2 | 1 | 211 |
| BMC Musculoskeletal Disorders | UK | 2.4 | 1 | 214 |
| Cell Proliferation | China | 6.8 | 1 | 176 |
| Clinical and Experimental Rheumatology | Italy | 4.5 | 1 | 220 |
| Clinical Oral Investigations | Germany | 3.6 | 1 | 220 |
| Clinical Orthopedics and Related Research | USA | 4.3 | 1 | 362 |
| Clinics in Sports Medicine | USA | 2.2 | 1 | 262 |
| Current Reviews in Musculoskeletal Medicine | USA | Not available | 1 | 320 |
| Dermatologic Surgery | USA | 3.4 | 1 | 179 |
| Experimental and Molecular Pathology | USA | 3.4 | 1 | 198 |
| Frontiers in Bioscience-Landmark | USA | 4.0 | 1 | 336 |
| Injury-International Journal of the Care of the Injured | UK | 2.6 | 1 | 204 |
| International Journal of Periodontics and Restorative Dentistry | USA | 1.8 | 1 | 204 |
| Jama-Journal of the American Medical Association | USA | 56.3 | 1 | 515 |
| Journal of Biomedical Materials Research Part B-Applied Biomaterials | USA | 3.4 | 1 | 208 |
| Journal of Bone and Joint Surgery-British Volume | UK | 3.3 | 1 | 384 |
| Journal of Cranio-Maxillofacial Surgery | UK | 2.1 | 1 | 421 |
| Journal of Craniofacial Surgery | USA | 1.0 | 1 | 222 |
| Journal of Dental Research | USA | 6.1 | 1 | 246 |
| Journal of Periodontal Research | Denmark | 4.4 | 1 | 221 |
| Journal of Shoulder and Elbow Surgery | USA | 3.0 | 1 | 267 |
| Journal of the American Academy of Orthopedic Surgeons | USA | 3.0 | 1 | 239 |
| Muscles, Ligaments and Tendons Journal | Italy | 0.4 | 1 | 263 |
| Nature Reviews Rheumatology | USA | 20.5 | 1 | 223 |
| Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics | USA | 1.2 (2006) | 1 | 1879 |
| Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology | USA | 1.5 (2011) | 1 | 291 |
| Ostomy Wound Management | USA | 2.6 | 1 | 265 |
| Rheumatology | UK | 7.6 | 1 | 174 |
| Sports Medicine | New Zealand | 11.1 | 1 | 196 |
| Stem Cell Research and Therapy | UK | 6.8 | 1 | 307 |
| The Yale Journal of Biology and Medicine | USA | 3.0 | 1 | 263 |
| Thrombosis and Haemostasis | Germany | 5.7 | 1 | 958 |
| Tissue Engineering Part C-Methods | USA | 3.1 | 1 | 271 |
| Transfusion and Apheresis Science | UK | 1.8 | 1 | 291 |
| Trends in Biotechnology | Netherlands | 19.5 | 1 | 859 |
| Investigated PRP Subjects | No. of Preclinical Studies |
|---|---|
| In vitro | |
| Cell proliferation test * | 13 |
| Tendon and/or ligament gene expression | 2 |
| Inflammation | 2 |
| Osteoarthritis | 3 |
| Quantification of platelets and/or growth factors | 11 |
| Extracellular matrix production of periodontal ligament and osteoblasts | 1 |
| In vitro and in vivo | |
| Cartilage regeneration | 1 |
| Hair growth | 1 |
| Meniscus defects | 1 |
| In vivo | |
| Bone regeneration | 5 |
| Cutaneous wound healing | 1 |
| Ligament wound | 2 |
| Tendon healing | 3 |
| Investigated PRP Indications | No. of Clinical Studies |
|---|---|
| Bleeding capillary bed of surgical flaps | 1 |
| Cutaneous chronic ulcers | 3 |
| Degenerative cartilage lesion or Osteoarthritis | 10 |
| Distraction osteogenesis | 1 |
| Maxillary sinus or mandibulary grafts | 5 |
| Tendinopathy | 4 |
| Arthroscopic rotator cuff repair | 2 |
| Rank | Article | No. of Citations in the First 5 Years (Total Citations) | Type | Modified Coleman Methodology Score | Journal Impact Factor (2020) |
|---|---|---|---|---|---|
| 1 | Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81–96. Published 2017 Jan 1. doi:10.7150/thno.16803 | 160 (160) | Preclinical study | NA | 11.6 |
| 2 | Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3):733–750. Published 2017 Jan 15. doi:10.7150/thno.17450 | 126 (127) | Preclinical study | NA | 11.6 |
| 3 | Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016;4:16036. Published 2016 Dec 13. doi:10.1038/boneres.2016.36 | 110 (125) | Review | NA | 13.6 |
| 4 | Smith PA. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-blind, Placebo-controlled Clinical Trial. Am J Sports Med. 2016;44(4):884–891. doi:10.1177/0363546515624678 | 108 (139) | Clinical study | 26 | 6.2 |
| 5 | Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;(5):CD006899. Published 2016 May 25. doi:10.1002/14651858.CD006899.pub3 | 107 (124) | Meta-analysis | NA | 9.3 |
| 6 | Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. Published 2015 Jan 7. doi:10.4137/CMAMD.S17894 | 103 (166) | Clinical study | 39 | 0.5 |
| 7 | Masuki H, Okudera T, Watanebe T, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent. 2016;2(1):19. doi:10.1186/s40729-016-0052-4 | 101 (141) | Preclinical study | NA | 2.4 |
| 8 | Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42–49. doi:10.1177/0363546513507566 | 87 (119) | Preclinical study | NA | 6.2 |
| 9 | Campbell KA, Saltzman BM, Mascarenhas R, et al. Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared with Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthroscopy. 2015;31(11):2213–2221. doi:10.1016/j.arthro.2015.03.041 | 85 (129) | Meta-analysis | NA | 4.8 |
| 10 | Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013;39(1):138–144. doi:10.1016/j.joen.2012.09.015 | 83 (142) | Clinical study | 17 | 4.2 |
| 11 | Chang KV, Hung CY, Aliwarga F, Wang TG, Han DS, Chen WS. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(3):562–575. doi:10.1016/j.apmr.2013.11.006 | 81 (147) | Meta-analysis | NA | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulange Zavarro, A.; De Girolamo, L.; Laver, L.; Sánchez, M.; Tischer, T.; Filardo, G.; Sabatier, F.; Magalon, J. The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine—A Bibliometric Analysis—From the ESSKA Orthobiologic Initiative. Bioengineering 2022, 9, 580. https://doi.org/10.3390/bioengineering9100580
Coulange Zavarro A, De Girolamo L, Laver L, Sánchez M, Tischer T, Filardo G, Sabatier F, Magalon J. The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine—A Bibliometric Analysis—From the ESSKA Orthobiologic Initiative. Bioengineering. 2022; 9(10):580. https://doi.org/10.3390/bioengineering9100580
Chicago/Turabian StyleCoulange Zavarro, Anouck, Laura De Girolamo, Lior Laver, Mikel Sánchez, Thomas Tischer, Giuseppe Filardo, Florence Sabatier, and Jérémy Magalon. 2022. "The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine—A Bibliometric Analysis—From the ESSKA Orthobiologic Initiative" Bioengineering 9, no. 10: 580. https://doi.org/10.3390/bioengineering9100580