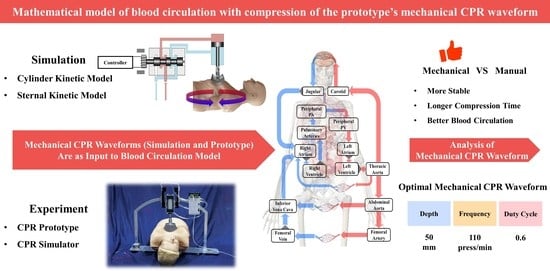
Mathematical Model of Blood Circulation with Compression of the Prototype’s Mechanical CPR Waveform
Abstract
:1. Introduction
- Development of a fast and stable novel CPR prototype, which is convenient to control. The prototype can be installed within 25 s and can operate steadily for more than 20 min;
- Verification of the superiority of mechanical waveforms over manual waveforms in CO, CPP, and CF, from both mathematical models and experimental results;
- Exploration of optimal parameters for the prototype’s mechanical waveforms during compressions. We achieved optimal blood circulation by setting the compression depth of this prototype to 50 mm, the duty cycle to 0.6, and the frequency to 110 press/min.
2. Materials and Methods
2.1. Development of a Novel CPR Prototype
2.1.1. Structural Design
- Lightweight support structure (labeled 1 in Figure 1): the thin-walled support frame and lightweight aluminum alloy base plate with a slot were designed so that the patient can maintain a flat position when placed inside the support structure;
- Quick installation mechanism (labeled 2 in Figure 1): the electromagnetic suction mechanism was designed to achieve rapid error-proof installation for users without training and to earn more CPR time for patients;
- Electrical control (labeled 3 in Figure 1): the miniaturized control module was designed to include a controller, drive circuit, solenoid valve, etc., while providing the balance of inertia during usage;
- Adaptive compression head (labeled 4 and 5 in Figure 1): the compression head was designed to fit the human chest adaptively, and the control box controls the reciprocating motion of the piston in the cylinder to achieve chest compression;
- Accessory mechanisms: a height adjuster (labeled 6 in Figure 1) to adjust the initial height of the adaptive compression module so that the compression head fits the thorax before starting CPR, and an oxygen mask (labeled 7 in Figure 1) to provide respiratory management function in conjunction with chest compression.
2.1.2. System Architecture and Control Method
2.2. Mathematical Model of the Compression Process
2.2.1. The Kinetic Model of the CPR Prototype
- (1)
- The flow at the throttle of the electro-pneumatic regulator was an adiabatic process;
- (2)
- The commutation time of the 5-port solenoid valve was ignored, and it was only considered to have a commutation function;
- (3)
- The inflating and deflating process was rapid in both chambers and the process was adiabatic.
2.2.2. Sternal Force Feedback Model
- (1)
- The action point of the external force F(t) was located directly above the spring and did not change, and its action direction was perpendicular to the chest;
- (2)
- The chest only had a vertical degree of freedom under the action of external force.
2.2.3. Human Blood Circulation Model
2.3. Experimental Design
2.4. Simulation Design
3. Results
3.1. Effects of the CPR Prototype
3.2. Comparison between Mechanical and Manual Compression
3.3. Optimal Blood Circulation Results of Mechanical Compressions
4. Discussion
4.1. Effects of the CPR Prototype
4.2. Comparison between Mechanical and Manual Compression
4.3. Optimal Blood Circulation Results of Mechanical Compressions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameters | Value |
|---|---|
| X10 | 2.445 × 10−2 m |
| X20 | 2.911 × 10−2 m |
| A1 | 1.963 × 10−3 m2 |
| A2 | 1.649 × 10−3 m2 |
| w | 1 m |
| 0.004 s | |
| 1 m/V | |
| R | 8.314 J/(mol·K) |
| k | 1.4 |
| Pa | 101,325 Pa |
| Ps | 400,000 Pa |
| T | 293 K |
| L | 50 mm |
| F1 | 15 N |
| F3 | 18.2 N |
| Name | Abbreviation | ||
|---|---|---|---|
| Right atrium | ra | 0.0095 | |
| Right ventricle | rv | 0.016 | |
| Pulmonary arteries | pa | 0.0042 | |
| Peripheral pulmonary arteries | ppa | 0.0042 | |
| Peripheral pulmonary veins | ppv | 0.00128 | |
| Left atrium | la | 0.0128 | |
| Left ventricle | lv | 0.008 | |
| Thoracic aorta | ao | 0.0008 | |
| Carotid | car | 60 | 0.0002 |
| Jugular | jug | 30 | 0.012 |
| Abdominal aorta | aa | 0.0004 | |
| Inferior vena cava | ivc | 25 | 0.0234 |
| Femoral artery | fa | 0.0002 | |
| Femoral vein | fv | 0.0047 | |
| Head | head | 5520 | |
| Pulmonary capillary bed | pc | 105 | |
| Tricuspid valve | tv | 5 | |
| Mitral valve | mv | 5 | |
| Pulmonic valve | pv | 10 | |
| Aortic valve | av | 10 | |
| Between central and peripheral pulmonary arteries | cppa | 10 | |
| Between central and peripheral pulmonary veins | cppv | 5 | |
| Coronary vessels | ht | 15780 | |
| Aorta | ald | 25 | |
| Leg vasculature | leg | 8520 | |
| Both iliac arteries | ia | 360 | |
| Both iliac veins | iv | 180 | |
| Residual systemic vasculature | sys | 1800 | |
| Airways to ventilation | airway | 1.2 | |
| Lung | lung | 0.23 |
References
- Kouwenhoven, W.B. Closed-chest cardiac massage. JAMA 1960, 173, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, B.S.; Kerber, R. Improving CPR Performance. Chest 2017, 152, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, P.S.; Kosmopoulos, M.N.; Gandhi, M.; Oshin, A.; Olson, M.D.; Ripeckyj, A.; Bahmer, L.; Bartos, J.A.; Theodorou, E.A.; Yannopoulos, D. Closed-loop machine-controlled CPR system optimises haemodynamics during prolonged CPR. Resusc. Plus 2020, 3, 100021. [Google Scholar] [CrossRef] [PubMed]
- Elola, A.; Aramendi, E.; Irusta, U.; Berve, P.O.; Wik, L. Multimodal Algorithms for the Classification of Circulation States During Out-of-Hospital Cardiac Arrest. IEEE Trans. Biomed. Eng. 2021, 68, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Johansen, J.; Pytte, M.; Tomlinson, A.-E.; Sunde, K.; Dorph, E.; Svendsen, J.V.H.; Eriksen, M.; Strømme, T.A.; Wik, L. Mechanical chest compressions with trapezoidal waveform improve haemodynamics during cardiac arrest. Resuscitation 2011, 82, 213–218. [Google Scholar] [CrossRef]
- Koeken, Y.; Aelen, P.; Noordergraaf, G.J.; Paulussen, I.; Woerlee, P.; Noordergraaf, A. The influence of nonlinear intra-thoracic vascular behaviour and compression characteristics on cardiac output during CPR. Resuscitation 2011, 82, 538–544. [Google Scholar] [CrossRef]
- Eichhorn, S.; Spindler, J.; Polski, M.; Mendoza, A.; Schreiber, U.; Heller, M.; Deutsch, M.A.; Braun, C.; Lange, R.; Krane, M. Development and validation of an improved mechanical thorax for simulating cardiopulmonary resuscitation with adjustable chest stiffness and simulated blood flow. Med. Eng. Phys. 2017, 43, 64–70. [Google Scholar] [CrossRef]
- Lampe, J.W.; Tai, Y.; Bratinov, G.; Weiland, T.R.; Kaufman, C.L.; Berg, R.A.; Becker, L.B. Developing a kinematic understanding of chest compressions: The impact of depth and release time on blood flow during cardiopulmonary resuscitation. Biomed. Eng. OnLine 2015, 14, 102. [Google Scholar] [CrossRef] [Green Version]
- Lampe, J.W.; Yin, T.; Bratinov, G.; Kaufman, C.L.; Berg, R.A.; Venema, A.; Becker, L.B. Effect of compression waveform and resuscitation duration on blood flow and pressure in swine: One waveform does not optimally serve. Resuscitation 2018, 131, 55–62. [Google Scholar] [CrossRef]
- Remino, C.; Baronio, M.; Pellegrini, N.; Aggogeri, F.; Adamini, R. Automatic and manual devices for cardiopulmonary resuscitation: A review. Adv. Mech. Eng. 2018, 10, 168781401774874. [Google Scholar] [CrossRef]
- 2020 American Heart Association Guidelines for CPR and ECC. Available online: https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines (accessed on 29 November 2022).
- Overbeek, R.; Schmitz, J.; Rehnberg, L.; Benyoucef, Y.; Dusse, F.; Russomano, T.; Hinkelbein, J. Effectiveness of CPR in Hypogravity Conditions—A Systematic Review. Life 2022, 12, 1958. [Google Scholar] [CrossRef]
- Małysz, M.; Smereka, J.; Jaguszewski, M.; Dąbrowski, M.; Nadolny, K.; Ruetzler, K.; Ładny, J.R.; Sterliński, M.; Filipiak, K.J.; Szarpak, Ł. An optimal chest compression technique using personal protective equipment during resuscitation in the COVID-19 pandemic: A randomized crossover simulation study. Kardiol. Pol. (Pol. Heart J.) 2020, 78, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, U.; Reiser, D.; Wenzel, V.; Knapp, J.; Tissi, M.; Theiler, L.; Rauch, S.; Meuli, L.; Albrecht, R. Mechanical chest compression devices in the helicopter emergency medical service in Switzerland. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 71. [Google Scholar] [CrossRef] [PubMed]
- Drinhaus, H.; Nüsgen, S.; Adams, N.; Wetsch, W.A.; Annecke, T. Rescue under ongoing CPR from an upper floor: Evaluation of three different evacuation routes and mechanical and manual chest compressions: A manikin trial. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliocca, A.; Olivari, D.; De Giorgio, D.; Zani, D.; Manfredi, M.; Boccardo, A.; Cucino, A.; Sala, G.; Babini, G.; Ruggeri, L.; et al. LUCAS Versus Manual Chest Compression During Ambulance Transport: A Hemodynamic Study in a Porcine Model of Cardiac Arrest. J. Am. Heart Assoc. 2019, 8, e011189. [Google Scholar] [CrossRef] [Green Version]
- Poole, K.; Couper, K.; Smyth, M.A.; Yeung, J.; Perkins, G.D. Mechanical CPR: Who? When? How? Crit. Care 2018, 22, 140. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.U.; Lone, A.N.; Talluri, S.; Khan, M.Z.; Khan, M.U.; Kaluski, E. Efficacy and safety of mechanical versus manual compression in cardiac arrest—A Bayesian network meta-analysis. Resuscitation 2018, 130, 182–188. [Google Scholar] [CrossRef]
- McIntosh, B.; Miller, K.; Carmack, S.; Abraham, S. A Comparison Review of Mechanical Cardiopulmonary Resuscitation Vs Manual Cardiopulmonary Resuscitation. Int. J. Sci. Res. Methodol. 2022, 20, 184–196. [Google Scholar] [CrossRef]
- Friberg, N.; Schmidbauer, S.; Walther, C.; Englund, E. Skeletal and soft tissue injuries after manual and mechanical chest compressions. Eur. Heart J.—Qual. Care Clin. Outcomes 2019, 5, 259–265. [Google Scholar] [CrossRef]
- Koster, R.W.; Beenen, L.F.; van der Boom, E.B.; Spijkerboer, A.M.; Tepaske, R.; van der Wal, A.C.; Beesems, S.G.; Tijssen, J.G. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: A randomized clinical trial for non-inferiority. Eur. Heart J. 2017, 38, 3006–3013. [Google Scholar] [CrossRef]
- Milling, L.; Astrup, B.S.; Mikkelsen, S. Prehospital cardiopulmonary resuscitation with manual or mechanical chest compression: A study of compression-induced injuries. Acta Anaesthesiol. Scand. 2019, 63, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Alam, M.M. Research and Development of a Low-Cost Smart Cardio-Pulmonary Resuscitation (CPR) Device Using Locally Available Raw Materials for Cardiac Arrest Patients. In Proceedings of the 1st International Electronic Conference on Applied Sciences, Online, 10–30 November 2020; p. 10. [Google Scholar]
- Dziekan, M.; Jubaer, S.; Sell, V.; Manda, S.; Aboelzahab, A.; Romero-Velasteguí, S.; Mejía-Viana, J.C.; Uquillas, J.A. Design of a low-cost, portable, and automated cardiopulmonary resuscitation device for emergency scenarios in Ecuador. In Proceedings of the 2017 IEEE Second Ecuador Technical Chapters Meeting (ETCM), Salinas, Ecuador, 16–20 October 2017; pp. 1–6. [Google Scholar]
- Riess, J.D.; Balzer, C.; Riess, M.L.; Cleveland, W.J. Electronic Depth Adjustment in an Automatic Chest Compression Device for Rodents—Design and Implementation of a LabVIEW-based Control Unit. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Sung, C.-W.; Wang, H.-C.; Shieh, J.-S.; Jaw, F.-S. A novel mechanical chest compressor with rapid deployment in all population cardiopulmonary resuscitation. Sci. Rep. 2020, 10, 6099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaran, S.; Pradeep Kumar, M.; Geethaanandhi, C. An Automatic Cardio Pulmonary Resuscitator—A Device for People Affected with Cardiac Arrest. In Proceedings of the Soft Computing: Theories and Applications; Sharma, T.K., Ahn, C.W., Verma, O.P., Panigrahi, B.K., Eds.; Springer: Singapore, 2021; pp. 445–453. [Google Scholar]
- Inzamam Ul Haque, M.F.; Sabina, S.; Khan, M.M.; Nanda, I. Arduino based Smart Design of a Cheaper and Portable Automated Cardiopulmonary Resuscitation (CPR) Device. In Proceedings of the 2022 6th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 29–31 March 2022; pp. 1098–1105. [Google Scholar]
- Lampe, J.W.; Padmanaban, S.; Becker, L.B.; Zanos, T.P. Towards Personalized Closed-Loop Mechanical CPR: A Model Relating Carotid Blood Flow to Chest Compression Rate and Duration. IEEE Trans. Biomed. Eng. 2020, 67, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D. Fundamentals of Aerodynamics, 6th ed.; McGraw-Hill Series in Aeronautical and Aerospace Engineering; McGraw Hill Education: New York, NY, USA, 2017; ISBN 978-1-259-12991-9. [Google Scholar]
- Gruben, K.G.; Halperin, H.R.; Popel, A.S.; Tsitlik, J.E. Canine sternal force-displacement relationship during cardiopulmonary resuscitation. IEEE Trans. Biomed. Eng. 1999, 46, 788–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalali, A.; Berg, R.A.; Nadkarni, V.M.; Nataraj, C. Mathematical Modeling of Cardiopulmonary Resuscitation. In Proceedings of the Volume 2: Diagnostics and Detection; Drilling; Dynamics and Control of Wind Energy Systems; Energy Harvesting; Estimation and Identification; Flexible and Smart Structure Control; Fuels Cells/Energy Storage; Human Robot Interaction; HVAC Building Energy Management; Industrial Applications; Intelligent Transportation Systems; Manufacturing; Mechatronics; Modelling and Validation; Motion and Vibration Control Applications; American Society of Mechanical Engineers, Columbus, OH, USA, 28–30 October 2015; p. V002T34A011. [Google Scholar]
- Babbs, C.F. Effects of an impedance threshold valve upon hemodynamics in Standard CPR: Studies in a refined computational model. Resuscitation 2005, 66, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, M.T.; Olsen, J.-A.; Eriksen, M.; Neset, A.; Norseng, P.A.; Kramer-Johansen, J.; Hardig, B.M.; Wik, L. Haemodynamic outcomes during piston-based mechanical CPR with or without active decompression in a porcine model of cardiac arrest. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 31. [Google Scholar] [CrossRef] [Green Version]
- Ewy, G.A. The mechanism of blood flow during chest compressions for cardiac arrest is probably influenced by the patient’s chest configuration. Acute Med. Surg. 2018, 5, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Iordanova, B.; Li, L.; Clark, R.S.B.; Manole, M.D. Alterations in Cerebral Blood Flow after Resuscitation from Cardiac Arrest. Front. Pediatr. 2017, 5, 174. [Google Scholar]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- John, A.R.; Manivannan, M.; Ramakrishnan, T.V. Computer-Based CPR Simulation Towards Validation of AHA/ERC Guidelines. Cardiovasc. Eng. Technol. 2017, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Buléon, C.; Delaunay, J.; Parienti, J.-J.; Halbout, L.; Arrot, X.; Gérard, J.-L.; Hanouz, J.-L. Impact of a feedback device on chest compression quality during extended manikin CPR: A randomized crossover study. Am. J. Emerg. Med. 2016, 34, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Gyory, R.A.; Buchle, S.E.; Rodgers, D.; Lubin, J.S. The Efficacy of LUCAS in Prehospital Cardiac Arrest Scenarios: A Crossover Mannequin Study. West. J. Emerg. Med. 2017, 18, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichhorn, S.; Mendoza Garcia, A.; Polski, M.; Spindler, J.; Stroh, A.; Heller, M.; Lange, R.; Krane, M. Corpuls cpr resuscitation device generates superior emulated flows and pressures than LUCAS II in a mechanical thorax model. Australas. Phys. Eng. Sci. Med. 2017, 40, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Daya, M.R.; Morrison, L.J.; Aufderheide, T.P.; Vaillancourt, C.; Vilke, G.M.; Idris, A.; Brown, S. Compression depth measured by accelerometer vs. outcome in patients with out-of-hospital cardiac arrest. Resuscitation 2021, 167, 95–104. [Google Scholar] [CrossRef]
- Scott, G.; Barron, T.; Gardett, I.; Broadbent, M.; Downs, H.; Devey, L.; Hinterman, E.; Clawson, J.; Olola, C. Can a Software-Based Metronome Tool Enhance Compression Rate in a Realistic 911 Call Scenario Without Adversely Impacting Compression Depth for Dispatcher-Assisted CPR? Prehospital Disaster Med. 2018, 33, 399–405. [Google Scholar] [CrossRef]
- Tanaka, S.; Rodrigues, W.; Sotir, S.; Sagisaka, R.; Tanaka, H. CPR performance in the presence of audiovisual feedback or football shoulder pads. BMJ Open Sport Exerc. Med. 2017, 3, e000208. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Oh, J.; Lee, J.; Kang, H.; Lim, T.H.; Ko, B.S.; Cho, Y.; Song, S.Y. Retrospective Study Using Computed Tomography to Compare Sufficient Chest Compression Depth for Cardiopulmonary Resuscitation in Obese Patients. J. Am. Heart Assoc. 2019, 8, e013948. [Google Scholar] [CrossRef]
- Daudre-Vignier, C.; Laviola, M.; Das, A.; Bates, D.G.; Hardman, J.G. Identification of an optimal CPR chest compression protocol. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 5459–5462. [Google Scholar]
- Duval, S.; Pepe, P.E.; Aufderheide, T.P.; Goodloe, J.M.; Debaty, G.; Labarère, J.; Sugiyama, A.; Yannopoulos, D. Optimal Combination of Compression Rate and Depth During Cardiopulmonary Resuscitation for Functionally Favorable Survival. JAMA Cardiol. 2019, 4, 900. [Google Scholar] [CrossRef] [Green Version]
- Nas, J.; Thannhauser, J.; van Geuns, R.M.; van Royen, N.; Bonnes, J.L.; Brouwer, M.A. Optimal Combination of Chest Compression Depth and Rate in Virtual Reality Resuscitation Training: A Post Hoc Analysis of the Randomized Lowlands Saves Lives Trial. J. Am. Heart Assoc. 2021, 10, e017367. [Google Scholar] [CrossRef]
- Cipani, S.; Bartolozzi, C.; Ballo, P.; Sarti, A. Blood flow maintenance by cardiac massage during cardiopulmonary resuscitation: Classical theories, newer hypotheses, and clinical utility of mechanical devices. J. Intensive Care Soc. 2019, 20, 2–10. [Google Scholar] [CrossRef] [PubMed]















| Start to Compress (s) | Fully Compressed (s) | Start to Decompress (s) | Fully Decompressed (s) | |
|---|---|---|---|---|
| Real Wave | 0.028 | 0.165 | 0.310 | 0.465 |
| Simulation | 0.007 | 0.198 | 0.304 | 0.474 |
| Depths | Start to Compress (s) | Fully Compressed (s) | Start to Decompress (s) | Fully Decompressed (s) |
|---|---|---|---|---|
| 20 mm | 0.043 | 0.236 | 0.317 | 0.554 |
| 25 mm | 0.039 | 0.240 | 0.319 | 0.563 |
| 30 mm | 0.039 | 0.227 | 0.330 | 0.431 |
| 35 mm | 0.039 | 0.253 | 0.328 | 0.471 |
| 40 mm | 0.034 | 0.249 | 0.328 | 0.492 |
| 45 mm | 0.030 | 0.249 | 0.322 | 0.523 |
| 50 mm | 0.028 | 0.165 | 0.310 | 0.465 |
| Frequencies | Start to Compress (s) | Fully Compressed (s) | Start to Decompress (s) | Fully Decompressed (s) |
| 90 press/min | 0.031 | 0.171 | 0.354 | 0.505 |
| 100 press/min | 0.028 | 0.165 | 0.310 | 0.465 |
| 110 press/min | 0.028 | 0.175 | 0.293 | 0.444 |
| 120 press/min | 0.031 | 0.168 | 0.270 | 0.420 |
| 130 press/min | 0.030 | 0.172 | 0.250 | 0.399 |
| 140 press/min | 0.028 | 0.165 | 0.229 | 0.376 |
| 150 press/min | 0.031 | 0.171 | 0.220 | 0.363 |
| Duty Cycles | Start to Compress (s) | Fully Compressed (s) | Start to Decompress (s) | Fully Decompressed (s) |
| 0.2 | 0.032 | 0.133 (34.4 mm) | 0.133 | 0.223 |
| 0.3 | 0.032 | 0.177 | 0.207 | 0.344 |
| 0.4 | 0.028 | 0.187 | 0.252 | 0.397 |
| 0.5 | 0.028 | 0.165 | 0.310 | 0.465 |
| 0.6 | 0.031 | 0.181 | 0.362 | 0.505 |
| 0.7 | 0.031 | 0.181 | 0.429 | 0.562 |
| 0.8 | 0.032 | 0.185 | 0.494 | - |
| Aortic | Right Atrial | |||
|---|---|---|---|---|
| Maximum Value | Reached Time | Maximum Value | Reached Time | |
| Manual | 45.88 mmHg | 0.135 s | 24.44 mmHg | 0.074 s |
| Mechanical | 43.47 mmHg | 0.167 s | 21.50 mmHg | 0.167 s |
| Cardiac Output (L/min) | Coronary Perfusion Pressure (mmHg) | Cerebral Flow (L/min) | ||||
|---|---|---|---|---|---|---|
| Real Wave | Simulation | Real Wave | Simulation | Real Wave | Simulation | |
| Frequency (press/min) | 1.2241 (110) | 1.2181 (107) | 30.431 (100) | 30.318 (100) | 0.314 (110) | 0.312 (110) |
| Duty Cycle | 1.2237 (0.6) | 1.2227 (0.55) | 30.715 (0.4) | 30.787 (0.43) | 0.318 (0.6) | 0.315 (0.59) |
| Depth (mm) | 1.2218 (50) | 1.2158 (50) | 30.431 (50) | 30.318 (50) | 0.313 (50) | 0.311 (50) |
| Study | Depth (mm) | Frequency (Press/min) | Duty Cycle | Methods |
|---|---|---|---|---|
| Daudre-Vignier [46] | 50 | 137 | 0.28 | Sine waves in lumped model |
| John [38] | 57 | 110 | - | Sine waves in lumped model |
| Suval [47] | 47 | 107 | - | Sine waves in clinical experiments |
| Nas [48] | 47 | 107 | - | Sine waves in clinical experiments |
| Lampe [9] | 51 | 125 | 0.73 | Trapezoidal waves in clinical experiments |
| Ours | 50 | 110 | 0.6 | Mechanical waves in lumped model |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, S.; Wang, S.; Liu, G. Mathematical Model of Blood Circulation with Compression of the Prototype’s Mechanical CPR Waveform. Bioengineering 2022, 9, 802. https://doi.org/10.3390/bioengineering9120802
Xu X, Wang S, Wang S, Liu G. Mathematical Model of Blood Circulation with Compression of the Prototype’s Mechanical CPR Waveform. Bioengineering. 2022; 9(12):802. https://doi.org/10.3390/bioengineering9120802
Chicago/Turabian StyleXu, Xingyuan, Shaoping Wang, Shangyu Wang, and Guiling Liu. 2022. "Mathematical Model of Blood Circulation with Compression of the Prototype’s Mechanical CPR Waveform" Bioengineering 9, no. 12: 802. https://doi.org/10.3390/bioengineering9120802