Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drying of Rosehip
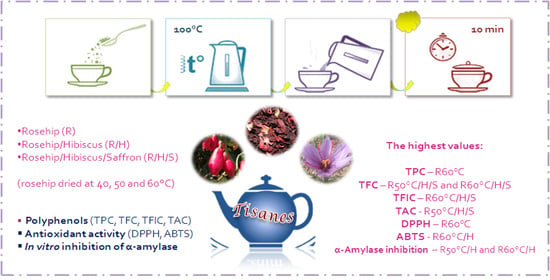
2.3. Experimental Design and Preparation of Tisanes
2.4. Determination of Total Phenolic Content in Tisanes Preparations
2.5. Determination of Total Flavonoid Content in Tisanes Preparations
2.6. Determination of Total Flavonol Content in Tisanes Preparations
2.7. Determination of Total Anthocyanin Content in Tisanes Preparations
2.8. Evaluation of Antioxidant Activity
2.9. Inhibition of α-Amylase
2.10. Statistical Analysis
3. Results and Discussion
3.1. Drying of Rosehip
3.2. Effect of Rosehip Drying Temperature on the Phenolic Composition and Antioxidant Properties of Tisanes
3.3. Effect of Hibiscus and Saffron Addition on the Phenolic Composition and Antioxidant Properties of Tisanes
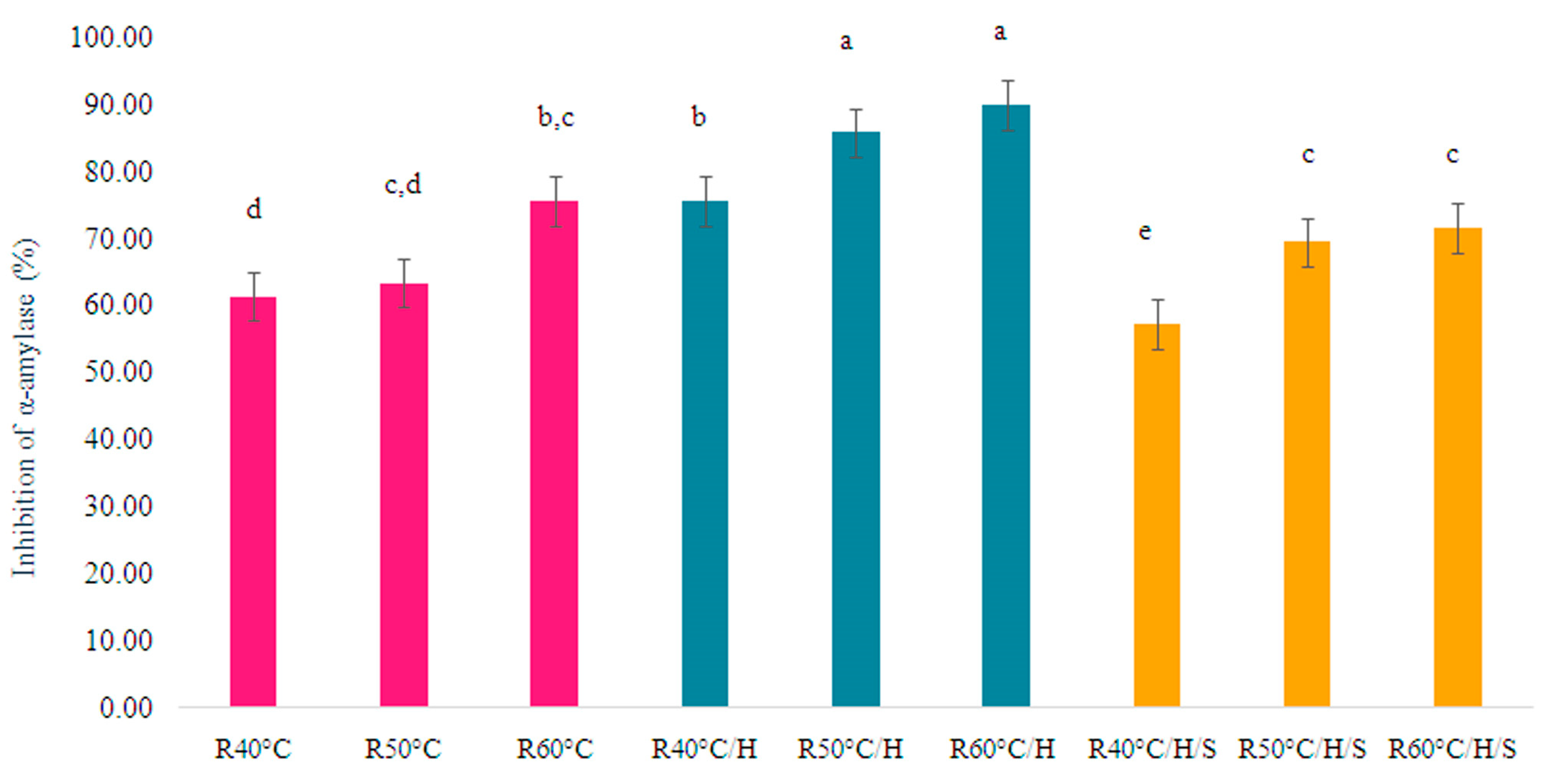
3.4. Inhibition of α-Amylase
3.5. Relationship between the Antioxidant Capacity, Total Phytochemical Composition and Inhibition of α-Amylase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-AmyI | α-amylase inhibition |
| ABTS∙+ | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium (ABTS) radical-cation scavenging activity (mg TE/g dw) |
| Cy 3-glc | cyanidin-3-glycoside equivalents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (mg TE/g dw) |
| dw | dry weight |
| GAE | gallic acid equivalents |
| R | rosehip |
| R/H | rosehip/hibiscus mixture |
| R/H/S | rosehip/hibiscus/saffron mixture |
| QUE | quercetin equivalents |
| TAC | total anthocyanin content (mg Cy 3-glc/g dw) |
| TE | Trolox equivalents |
| TFC | total flavonoid content (mg QUE/g dw) |
| TFlC | total flavonol content (mg QUE/g dw) |
| TPC | total phenolic content (mg GAE/g dw) |
References
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Shazly, M.; Eldahshan, O.A. The role of plant-derived compounds in managing diabetes mellitus: A review of literature from 2014 to 2019. Curr. Med. Chem. 2020, 28, 4694–4730. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E.A. Review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Kostić, Ž.A.; Kamiloglu, S.; Mićanović, N.; Tomas, M.; Capanoglu, E. Chemical composition, nutritional and health related properties of medlar (Mespilus germanica L.): From medieval glory to underutilized fruit. Phytochem. Rev. 2023. [Google Scholar] [CrossRef]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic bioactives from plant-based foods for glycemic control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Naumovski, N.; Foscolou, A.; D’Cunha, N.M.; Tyrovolas, S.; Chrysohoou, C.; Sidossis, L.S.; Rallidis, L.; Matalas, A.L.; Polychronopoulos, E.; Pitsavos, C. The association between green and black tea consumption on successful aging: A combined analysis of the ATTICA and MEDiterranean ISlands (MEDIS) epidemiological studies. Molecules 2019, 24, 1862. [Google Scholar] [CrossRef]
- Liu, Y.; Guoa, C.; Zang, E.; Shi, R.; Liu, Q.; Zhang, M.; Zhang, K.; Li, M. Review on herbal tea as a functional food: Classification, active compounds, biological activity, and industrial status. J. Future Foods 2023, 3, 206–219. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Kyriakoudi, A.; Tsimidou, M.Z. Enhanced bioaccessibility of crocetin sugar esters from saffron in infusions rich in natural phenolic antioxidants. Molecules 2015, 20, 17760–17774. [Google Scholar] [CrossRef]
- Ramphinwa, M.L.; Mchau, G.R.A.; Mashau, M.E.; Madala, N.E.; Chimonyo, V.G.P.; Modi, T.A.; Mabhaudhi, T.; Thibane, V.S.; Mudau, F.N. Eco-physiological response of secondary metabolites of teas: Review of quality attributes of herbal tea. Front. Sustain. Food Syst. 2023, 7, 990334. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From herbal teabag to infusion-impact of brewing on polyphenols and antioxidant capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- İlyasoğlu, H.; Arpa, T.E. Effect of brewing conditions on antioxidant properties of rosehip tea beverage: Study by response surface methodology. J. Food Sci. Technol. 2017, 54, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ognyanov, M.; Kirchev, M.; Stancheva, M. Bioactive compounds in water extracts prepared from rosehip-containing herbal blends. J. Food Process Preserv. 2021, 45, e14645. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidib, F. Herbal beverages: Bioactive compounds and their role in disease riskreduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Obón, C.; Rivera, D.; Fonollá, E.; Alcaraz, F.; Attieh, L. A comparison study on traditional mixtures of herbal teas used in eastern Mediterranean area. Front. Pharmacol. 2021, 12, 632692. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Scariot, V. Hot and cold drying of edible flowers affect metabolite patterns of extracts and decoctions. Folia Hortic. 2023, 35, 193–207. [Google Scholar] [CrossRef]
- Etheridge, C.; Derbyshire, E. Hibiscus tea and health: A scoping review of scientific evidence. Nutr. Food Sci. 2020, 50, 969–985. [Google Scholar] [CrossRef]
- Joshi, D.D.; Deb, L.; Somkuwar, B.G.; Rana, V.S. Relevance of Indian traditional tisanes in the management of type 2 diabetes mellitus: A review. Saudi Pharm. J. 2023, 31, 626–638. [Google Scholar] [CrossRef]
- Abdullah, R.; Zaheer, S.; Kaleem, A.; Iqtedar, M.; Aftab, M.; Saleem, F. Formulation of herbal tea using Cymbopogon citratus, Foeniculum vulgare and Murraya koenigii and its anti-obesity potential. J. King Saud. Univ-Sci. 2023, 35, 102734. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial effects of natural bioactive compounds from Hibiscus sabdariffa L. on obesity. Molecules 2019, 24, 21. [Google Scholar] [CrossRef]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal teas and their health benefits: A scoping review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Pećinar, I.; Krstić, D.J.; Caruso, G.; Popović-Djordjević, B.J. Rapid characterisation of hypanthium and seed in wild and cultivated rosehip: Application of Raman microscopy combined with multivariate analysis. R. Soc. Open Sci. 2021, 8, 202064. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Fotirić-Akšić, M.; Katanić-Stanković, J.S.; Pantelić, N.Đ.; Mihailović, V. Wild-growing species in the service of medicine: Environmental challenges and sustainable production. In Environmental Challenges and Medicinal Plants: Sustainable Production Solutions under Adverse Conditions; Aftab, T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 49–104. Available online: https://link.springer.com/chapter/10.1007/978-3-030-92050-0_3 (accessed on 12 June 2023).
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Gorgulu, T.Y.; Ozdemir, O.D.; Kipcak, A.S.; Piskin, M.B.; Derun, E.M. The effect of lemon on the essential element concentrations of herbal and fruit teas. Appl. Biol. Chem. 2016, 59, 425–431. [Google Scholar] [CrossRef]
- Aptin, R.; Ghavamaldin, A.; Ahmad, T.; Mariamalsadat, T. Evaluation of biochemical compounds Rosa canina L. in north of Iran (Ramsar and Tonekabon Heights). J. Med. Plants Res. 2013, 7, 3319–3324. [Google Scholar]
- Dabić-Zagorac, D.Č.; Fotirić-Akšić, M.M.; Glavnik, V.; Gašić, U.M.; Vovk, I.; Tešić, Ž.L.; Natić, M.M. Establishing the chromatographic fingerprints of flavan-3-ols and proanthocyanidins from rose hip (Rosa sp.) species. J. Sep. Sci. 2020, 43, 1431–1439. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A.; Zapater, J.M.M.; Fernandez, S.D.P.T. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 2020, 13, 1105–1119. [Google Scholar] [CrossRef]
- Daels-Rakotoarison, D.A.; Gressier, B.; Trotin, F.; Brunet, C.; Luyckx, M.; Dine, T.; Bailleul, F.; Cazin, M.; Cazin, J.C. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother. Res. 2002, 16, 157–161. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Paunović, D.; Milić, A.; Krstić, Đ.; Moghaddam, S.; Roje, V. Multi-elemental analysis, pattern recognition techniques of wild and cultivated rosehips from Serbia, and nutritional aspect. Biol. Trace Elem. Res. 2021, 199, 1110–1122. [Google Scholar] [CrossRef]
- Briliantama, A.; Oktaviani, N.M.D.; Rahmawati, S.; Setyaningsih, W.; Palma, M. Optimization of ultrasound-assisted extraction (UAE) for simultaneous determination of individual phenolic compounds in 15 dried edible flowers. Horticulturae 2022, 8, 1216. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.M.; Başyiğit, B.; Alaşalvar, H.; Salum, P.; Berktaş, S.; Erbay, Z.; Çam, M. Instant soluble roselle (Hibiscus sabdariffa L.) powder rich in bioactive compounds: Effect of the production process on volatile compounds. J. Food Meas. Charact. 2023, 17, 108–120. [Google Scholar] [CrossRef]
- Popović-Djordjević, B.J.; Katanić-Stanković, S.J.; Mihailović, V.; Akram, M. Biochemistry and metabolism. In Saffron; Galanakis, C., Ed.; Elsevier S&T: Amsterdam, The Netherlands, 2021; pp. 1–40. [Google Scholar] [CrossRef]
- Popović-Djordjević, B.J.; Katanić-Stanković, S.J.; Mihailović, V.; Akram, M. Antioxidant activities of bioactive compounds and various extracts obtained from saffron. In Saffron; Galanakis, C., Ed.; Elsevier S&T: Amsterdam, The Netherlands, 2021; pp. 41–97. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot. 2015, 99, 80–87. [Google Scholar] [CrossRef]
- Gismondi, A.; Serio, M.; Canuti, L.; Canini, A. Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am. J. Plant Sci. 2012, 03, 1573–1580. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A multitask neuroprotective agent for retinal degenerative diseases. Antioxidants 2019, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Khorasanchi, Z.; Shafiee, M.; Kermanshahi, F.; Khazaei, M.; Ryzhikov, M.; Parizadeh, M.R.; Kermanshahi, B.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine 2018, 43, 21–27. [Google Scholar]
- Razak, S.I.A.; Anwar-Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A review on medicinal properties of saffron toward major diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Xu, X.R. Antiproliferative activities of tea and herbal infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef]
- Akar, Z.; Orhan, E.; Durmuş, S. Effects of cold and hot infusions on antioxidant activities of rosehip tea bag. Turkish J. Agric-Food Sci. Technol. 2021, 9, 1455–1459. [Google Scholar] [CrossRef]
- Paunović, D.; Kalušević, A.; Petrović, T.; Urošević, T.; Djinović, D.; Nedović, V.; Popović-Djordjević, J. Assessment of chemical and antioxidant properties of fresh and dried rosehip (Rosa canina L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 108–113. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Zannou, O.; Koca, I. Modeling and optimization of drying conditions of dog rose for preparation of a functional tea. J. Food Process Eng. 2021, 44, e13632. [Google Scholar] [CrossRef]
- Singelton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Yermakov, A.I.; Arasimov, V.V.; Yarosh, N.P. Methods of Biochemical Analysis of Plants; Agropromizdat: Leningrad, Russia, 1987. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins. Characterization and Measurement with UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley and Sons, Inc.: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vunduk, J.; Kozarski, M.; Djekic, I.; Tomašević, I.; Klaus, A. Effect of modified atmosphere packaging on selected functional characteristics of Agaricus bisporus. Eur. Food Res. Technol. 2021, 247, 829–838. [Google Scholar] [CrossRef]
- Jangam, S.V.; Law, C.L.; Mujumdar, A.S. Drying of Foods, Vegetables and Fruits. Slideshare, 1. 2010. Available online: https://www.slideshare.net/didididi2/drying-of-foods-vegetables-and-fruits-volume-1 (accessed on 13 July 2023).
- Afifah, R.A.; Niwat, C. Phenolic contents and antioxidant activities of various infused tea liquids made from leaves of green tea (Camellia sinensis), banaba (Lagestroemia speciosa) and moringa (Moringa oleifera L.). J. Teknol. Pengolah. Pertan. 2020, 2, 15–20. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Hussain, M.M.; Dincer, I. Heat and moisture diffusion in slab products due to convective boundary condition. Heat. Mass. Transf. 2003, 39, 471–476. [Google Scholar] [CrossRef]
- Milanović, M.P.; Komatina, M.S.; Zlatanović, I.J.; Manić, N.G.; Antonijević, D.L. Kinetic parameters identification of conductive enhanced hot air drying process of food waste. Therm. Sci. 2021, 25, 1795–1807. [Google Scholar] [CrossRef]
- Faal, S.; Tavakoli, T.; Ghobadian, B. Mathematical modeling of thin layer hot air drying of apricot with combined heat and power dryer. J. Food Sci. Technol. 2015, 52, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Babalis, S.J.; Belessiotis, V.G. Influence of the drying conditions on the drying constants and moisture diffusivity during the thin-layer drying of figs. J. Food Eng. 2004, 65, 449–458. [Google Scholar] [CrossRef]
- Veljković, J.N.; Pavlović, A.N.; Mitić, S.S.; Tosić, S.B.; Stojanović, G.S.; Kaličanin, B.M.; Stanković, D.M.; Stojković, M.B.; Mitić, M.N.; Bracanović, J.M. Evaluation of individual phenolic compounds and antioxidant properties of black, green, herbal and fruit tea infusions consumed in Serbia: Spectrophotometrical and electrochemical approaches. J. Food Nutr. Res. 2013, 52, 12–24. [Google Scholar]
- Sales, P.M.; Souza, P.M.; Alberto-Simeoni, L.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. Available online: http://journal.chemistrycentral.com/content/7/1/73 (accessed on 13 June 2023). [CrossRef]
- Sun, L.; Gidley, M.J.; Warren, F.J. The mechanism of interactions between tea polyphenols and porcine pancreatic alpha-amylase: Analysis by inhibition kinetics, fluorescence quenching, differential scanning calorimetry and isothermal titration calorimetry. Mol. Nutr. Food Res. 2017, 61, 1700324. [Google Scholar] [CrossRef]
- Proenca, C.; Freitas, M.; Ribeiro, D.; Tome, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araujo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Ozkan, G.; Esatbeyoglu, T.; Capanoglu, E. Bioavailability of rosehip (Rosa canina L.) infusion phenolics prepared by thermal, pulsed electric field and high pressure processing. Foods 2022, 11, 1955. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]



| Herbal Constituents | Mass Ratio 1 (w/w) | Tisane Label 2 |
|---|---|---|
| Rosehip 40 °C 3 | 100% | R40 °C |
| Rosehip 50 °C | 100% | R50 °C |
| Rosehip 60 °C | 100% | R60 °C |
| Rosehip 40 °C/Hibiscus | 60:40% | R40 °C/H |
| Rosehip 50 °C/Hibiscus | 60:40% | R40 °C/H |
| Rosehip 60 °C/Hibiscus | 60:40% | R40 °C/H |
| Rosehip 40 °C/Hibiscus/Saffron | 60:35:5% | R40 °C/H/S |
| Rosehip 50 °C/Hibiscus/Saffron | 60:35:5% | R50 °C/H/S |
| Rosehip 60 °C/Hibiscus/Saffron | 60:35:5% | R60 °C/H/S |
| TPC 1 | TFC | TFlC | TAC | DPPH· | ABTS∙+ | α-AmyI | |
|---|---|---|---|---|---|---|---|
| TPC | 1 | ||||||
| TFC | −0.44 | 1 | |||||
| TFlC | −0.40 | 1.00 | 1 | ||||
| TAC | −0.44 | 0.93 | 0.94 | 1 | |||
| DPPH· | 0.83 | −0.43 | −0.37 | −0.32 | 1 | ||
| ABTS∙+ | 0.44 | −0.31 | −0.32 | −0.24 | 0.44 | 1 | |
| α-AmyI | 0.23 | −0.19 | −0.19 | 0.04 | 0.16 | 0.57 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasić, D.; Katanić Stanković, J.S.; Urošević, T.; Kozarski, M.; Naumovski, N.; Khan, H.; Popović-Djordjević, J. Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.). Beverages 2024, 10, 1. https://doi.org/10.3390/beverages10010001
Vasić D, Katanić Stanković JS, Urošević T, Kozarski M, Naumovski N, Khan H, Popović-Djordjević J. Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.). Beverages. 2024; 10(1):1. https://doi.org/10.3390/beverages10010001
Chicago/Turabian StyleVasić, Dušan, Jelena S. Katanić Stanković, Tijana Urošević, Maja Kozarski, Nenad Naumovski, Haroon Khan, and Jelena Popović-Djordjević. 2024. "Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.)" Beverages 10, no. 1: 1. https://doi.org/10.3390/beverages10010001