Influence of Freeze-Dried Yeast Starter Cultures on Volatile Compounds of Tchapalo, a Traditional Sorghum Beer from Côte d’Ivoire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Production of Freeze-Dried Starter Cultures
2.3. Fermentation Conditions
2.4. Analytical Assays
2.5. Volatile Compound Analysis
2.6. Statistical Analysis
3. Results and Discussion
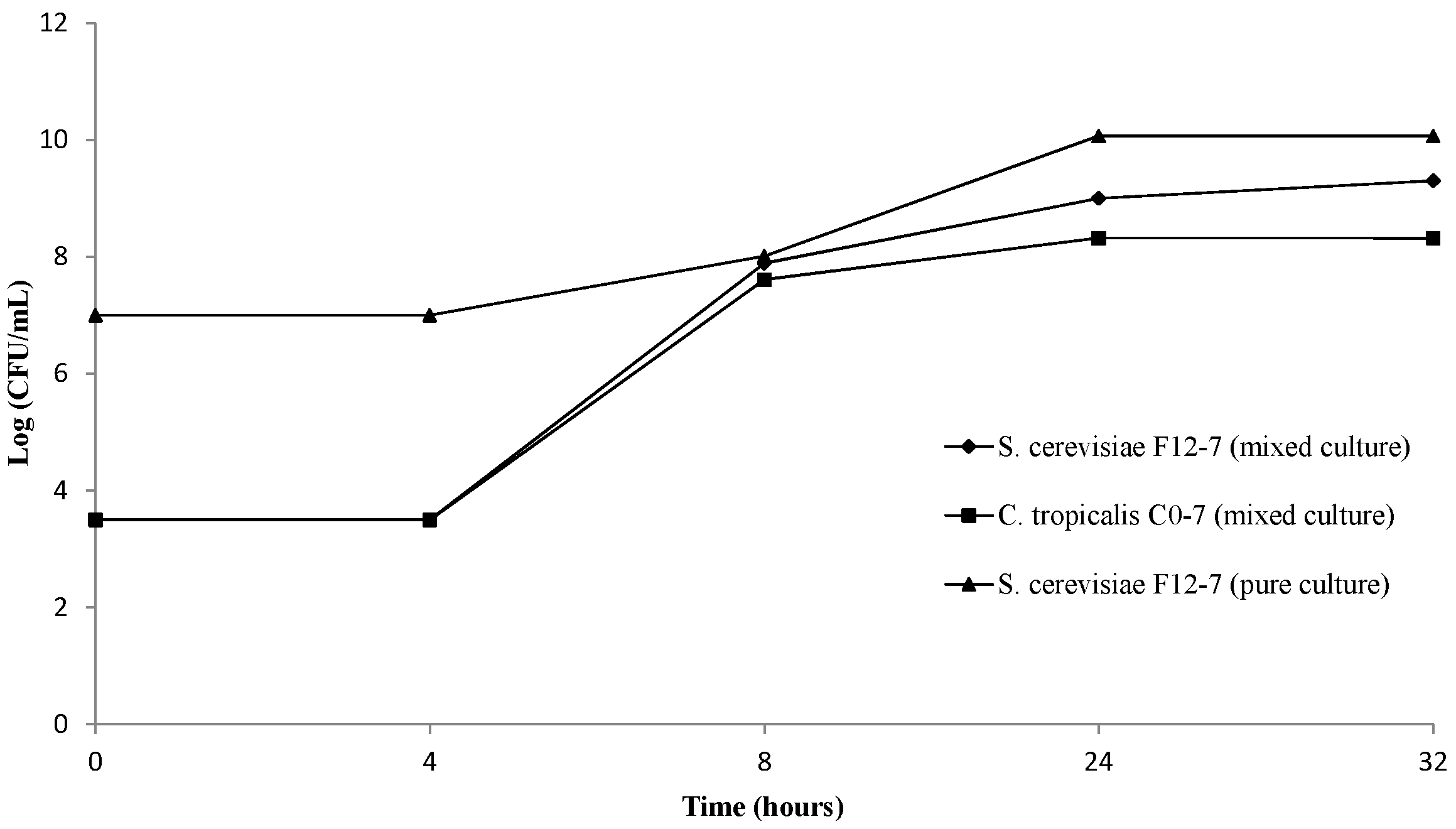
3.1. Evolution of Yeast Biomass during Beer Production
3.2. Biochemical Characteristics of the Produced Beers
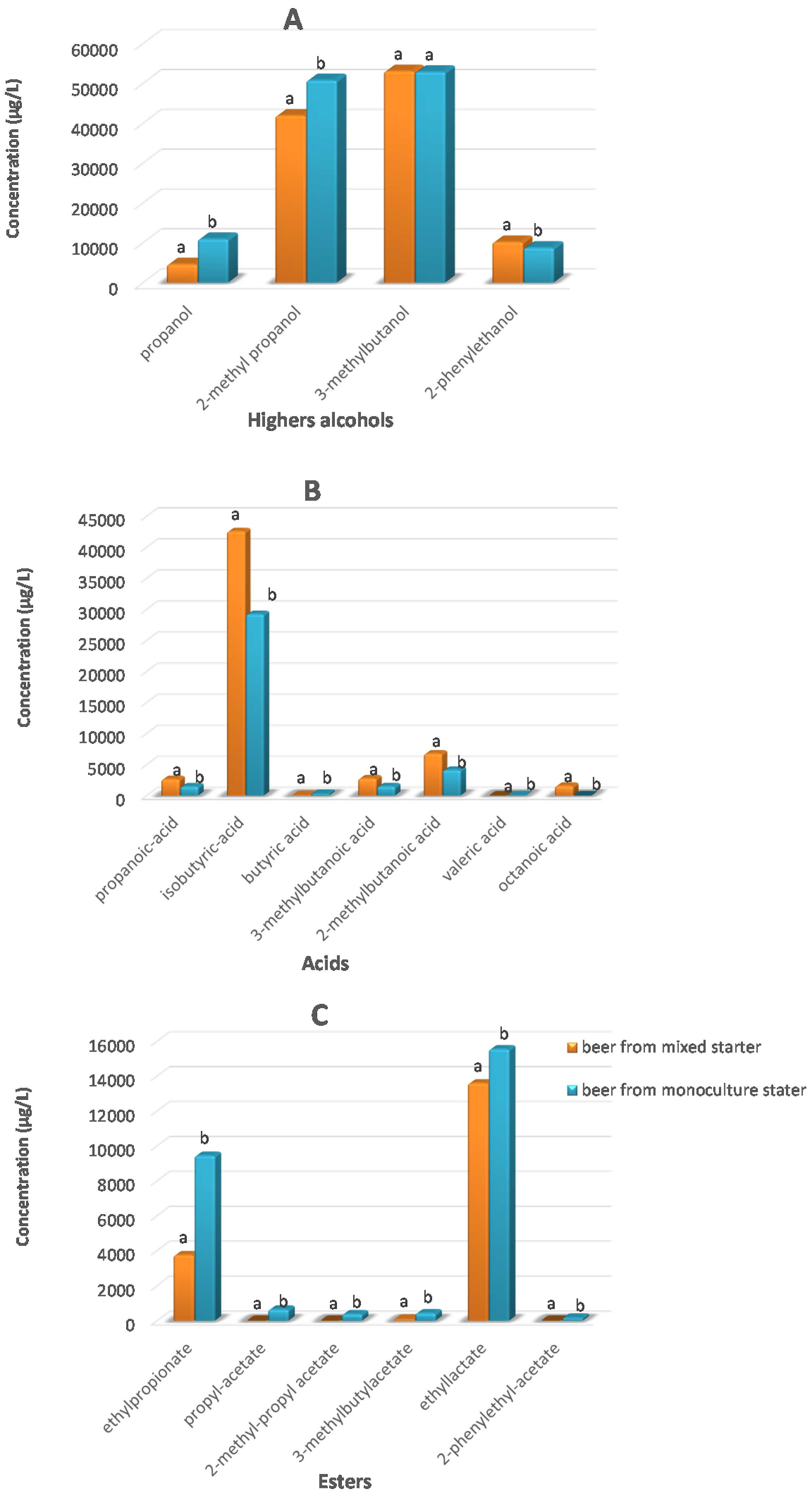
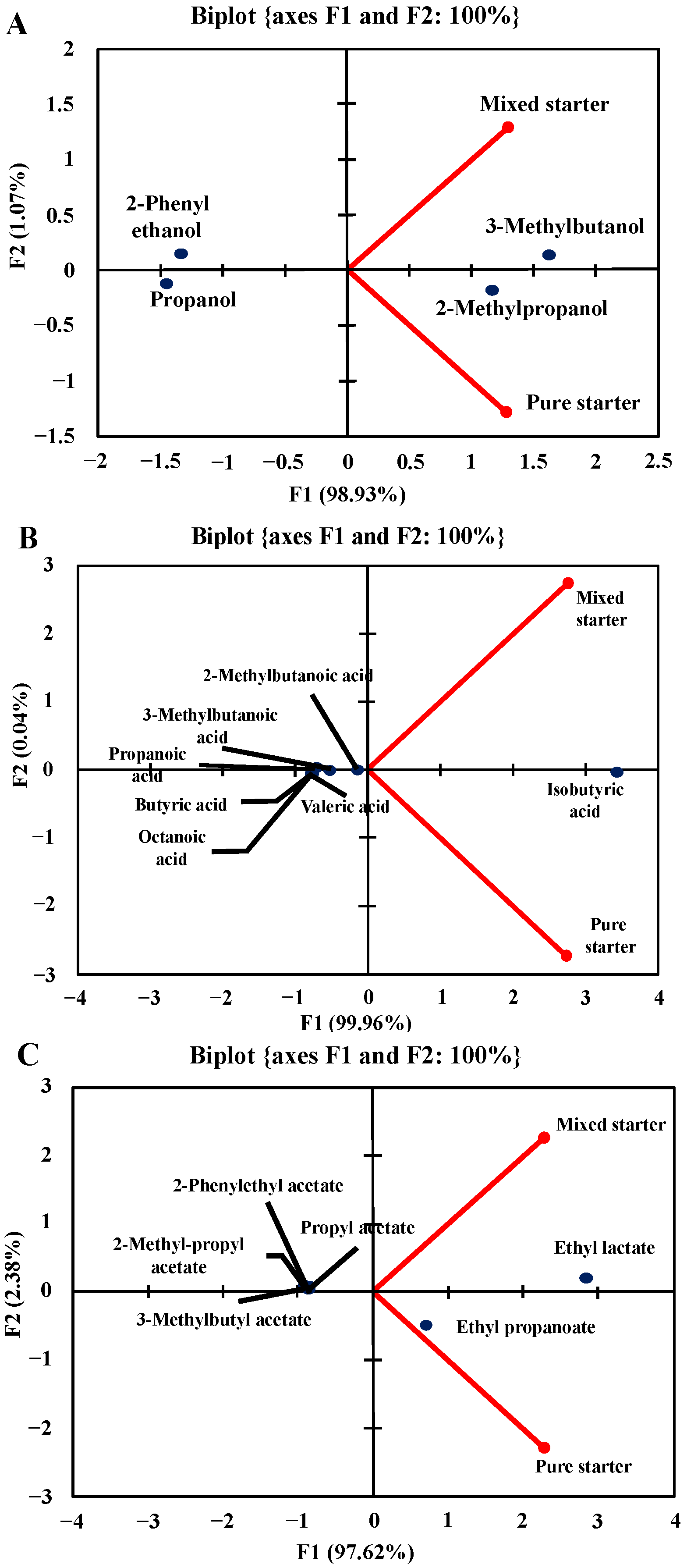
3.3. Volatile Compounds of the Beers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanni, A.I. The need for process optimization of African fermented foods and beverages. Int. J. Food Microbiol. 1993, 18, 85–95. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Van der Aa, K.A.; Jespersen, L.; Glover, R.L.K.; Diawara, B.; Jakobsen, M. Identification and characterization of Saccharomyces cerevisiae strains isolated from West African sorghum beer. Yeast 2001, 18, 1069–1079. [Google Scholar] [CrossRef]
- Maoura, N.; Mbaiguinam, M.; Nguyen, H.V.; Gaillardin, C.; Pourquie, J. Identification and typing of the yeast strains isolated from bilibili, a traditional sorghum beer of Tchad. Afr. J. Biotechnol. 2005, 4, 646–656. [Google Scholar]
- Kayode, A.P.P.; Vieira-Dalodé, G.; Kotchoni, S.O.; Linnemann, A.R.; Nout, M.J.R.; van Boekel, M.A.J.S.; Hounhouigan, D.J. Diversity of yeasts involved in the fermentation of tchoukoutou, anopaque sorghum beer from Benin. Afr. J. Microbiol. Res. 2011, 5, 2737–2742. [Google Scholar]
- N’guessan, K.F.; Brou, K.; Noemie, J.; Casaregola, S.; Djè, K.M. Identification of yeasts during alcoholic fermentation of tchapalo, a traditional sorghum beer from Côte d’Ivoire. Antonie Van Leeuwenhoek 2011, 99, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Sefa-deheh, S.; Sanni, A.I.; Tetteh, G.; Sakyi-Dawson, E. Yeasts in the traditional brewing of pito in Ghana. World J. Microbiol. Biotechnol. 1999, 15, 593–597. [Google Scholar] [CrossRef]
- Lyumugabe, F.; Kamaliza, G.; Bajyana, E.; Thonart, P.H. Microbiological and physico-chemical characteristic of Rwandese traditional beer “Ikigage”. Afr. J. Biotechnol. 2010, 9, 4241–4246. [Google Scholar]
- Greppi, A.; Rantisou, K.; Padonou, W.; Hounhouigan, J.; Jespersen, L.; Jakobsen, M.; Cocolin, L. Yeast dynamics during spontaneous fermentation of mawè and tchoukoutou, two traditional products from Benin. Int. J. Food Microbiol. 2013, 165, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Glover, R.L.K.; Abaidoo, R.C.; Jakobsen, M.; Jespersen, L. Biodiversity of Saccharomyces cerevisiae isolated from a survey of pito production sites in various parts of Ghana. Syst. Appl. Microbiol. 2005, 28, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulasporadelbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non-Saccharomyces sector with Torulasporadelbrueckii as model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.M.; Van Landschoot, A.; de Vuyst, L.; Vandamme, P. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estela-Escalante, W.D.; Rosales-Mendoza, S.; Moscosa-Santillán, M.; González-Ramírez, J.E. Evaluation of the fermentative potential of Candida zemplinina yeasts for craft beer fermentation. J. Inst. Brew. 2016, 122, 530–535. [Google Scholar] [CrossRef]
- Orji, M.U.; Mbata, T.I.; Aniche, G.N.; Ahonkhai, I. The use of starter cultures to produce ‘Pito’, a Nigerian fermented alcoholic beverage. World J. Microbiol. Biotechnol. 2003, 19, 733–736. [Google Scholar] [CrossRef]
- Sawadogo-Lingani, H.; Lei, V.; Diawara, B.; Nielsen, D.S.; Møller, P.L.; Traoré, A.S.; Jakobsen, M. The biodiversity of predominant lactic acid bacteria in dolo and pito wort, for production of sorghum beer. J. Appl. Microbiol. 2007, 103, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Glover, R.L.K.; Sawadogo-Lingani, H.; Diawara, B.; Jespersen, L.; Jakobsen, M. Utilization of Lactobacillus fermentum and Saccharomyces cerevisiae as starter cultures in the production of ‘dolo’. J. Appl. Biosci. 2009, 22, 1312–1319. [Google Scholar]
- N’guessan, F.K.; N’Dri, D.Y.; Camara, F.; Djè, M.K. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J. Microbiol. Biotechnol. 2010, 26, 693–699. [Google Scholar] [CrossRef]
- Hubalek, Z. Protectants used in the cryopreservation of microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- N’Guessan, F.K.; Coulibaly, H.W.; Alloue-Boraud, M.W.; Cot, M.; Djè, K.M. Production of freeze-dried yeast culture for the brewing of traditional sorghum beer, tchapalo. Food Sci. Nutr. 2016, 4, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbol. Biotechnol. 2014, 99, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Verachtert, H.; Shanta Kumara, H.M.C.; Dawoud, E. Yeasts in mixed cultures with emphasis on lambic beer brewing. In Yeasts in Biotechnology and Biocatalysis; Verachtert, H., de Mot, R., Eds.; Marcel Dekker: New York, NY, USA, 1990; pp. 429–449. [Google Scholar]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The role and use of non-Saccharomyces yeasts in wine production. S. Afr. J. Enol. Vitic. 2006, 27, 15–39. [Google Scholar]
- Dufour, J.-P.; Malcorps, P.; Silcock, P. Control of ester synthesis during brewery fermentation. In Brewing Yeast Fermentation Performance; Blackwell Publishing: Hoboken, NJ, USA, 2003; pp. 213–233. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeasts and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Rojas, V.; Gil, J.V.; Pinaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; de Pinho, P.G.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hansenisporauvarum and Hanseniasporaguilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Andorra, I.; Berradre, M.; Rozes, N.; Mas, A.; Guillamon, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed cultures of the main wine yeast species on grap. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.R.; Saputra, A.; Yu, B.; Curran, P.; Liu, S.Q. Effects of pure and mixed-cultures of Saccharomyces cerevisiae and Williopsissaturnus on the volatile profiles of grape wine. Food Biotechnol. 2012, 26, 307–325. [Google Scholar] [CrossRef]
- Lyumugabe, F.; Uyisenga, J.P.; Bajyana, E.S.; Thonart, P. Production of traditional sorghum beer “Ikigage” using Saccharomyces cerevisiae, Lactobacillus fermentum and Issatckenkiaorientalis as starter cultures. Food Nutr. Sci. 2014, 5, 507–515. [Google Scholar] [CrossRef]
| Sorghum Wort | Beer with Mixed Starter | Beer with Pure Starter | |
|---|---|---|---|
| Glucose (g/L) | 11.1 ± 0a | 7.0 ± 0b | 6.0 ± 0b |
| Maltose (g/L) | 187.2 ± 0.4a | 30.5 ± 2.7b | 28.6 ± 1.3b |
| Ethanol (%) | n.d | 4.4 ± 0.9a | 4.5 ± 0.8a |
| Oxalic acid (mg/L) | 11.7 ± 0a | 11.0 ± 0a | 10.0 ± 0a |
| Citric acid (g/L) | 0.1 ± 0a | 1.1 ± 0.6b | 0.8 ± 0.4b |
| Tartaric acid (mg/L) | 11.0 ± 0.1a | 0.0b | 8.0 ± 0a |
| Acetic acid (mg/L) | 21.0 ± 0.1a | 0.0b | 0.0b |
| Malic acid (g/L) | 0.7 ± 0.1a | 4.7 ± 0.8b | 2.7 ± 0.1c |
| Lactic acid (g/L) | 11.0 ± 0.1a | 6.6 ± 0.8b | 5.4 ± 0.3b |
| Propionic acid (g/L) | 3.3 ± 0.1a | 3.3 ± 0.6a | 2.1 ± 0.1a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulibaly, W.H.; N’guessan, K.F.; Coulibaly, I.; Cot, M.; Rigou, P.; Djè, K.M. Influence of Freeze-Dried Yeast Starter Cultures on Volatile Compounds of Tchapalo, a Traditional Sorghum Beer from Côte d’Ivoire. Beverages 2016, 2, 35. https://doi.org/10.3390/beverages2040035
Coulibaly WH, N’guessan KF, Coulibaly I, Cot M, Rigou P, Djè KM. Influence of Freeze-Dried Yeast Starter Cultures on Volatile Compounds of Tchapalo, a Traditional Sorghum Beer from Côte d’Ivoire. Beverages. 2016; 2(4):35. https://doi.org/10.3390/beverages2040035
Chicago/Turabian StyleCoulibaly, Wahauwouélé Hermann, Kouadio Florent N’guessan, Ibourahema Coulibaly, Marlène Cot, Peggy Rigou, and Koffi Marcellin Djè. 2016. "Influence of Freeze-Dried Yeast Starter Cultures on Volatile Compounds of Tchapalo, a Traditional Sorghum Beer from Côte d’Ivoire" Beverages 2, no. 4: 35. https://doi.org/10.3390/beverages2040035





