Evaluation of Probiotic L. rhamnosus GG as a Protective Culture in Sea Buckthorn-Based Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cultures
2.2. Preparation of Juice Matrix
2.3. Development of Unfermented Sea Buckthorn Beverage Fortified with L. rhamnosus GG
2.4. Evaluation of L. rhamnosus as Protective Culture
2.5. Statistical Analysis
3. Results and Discussion
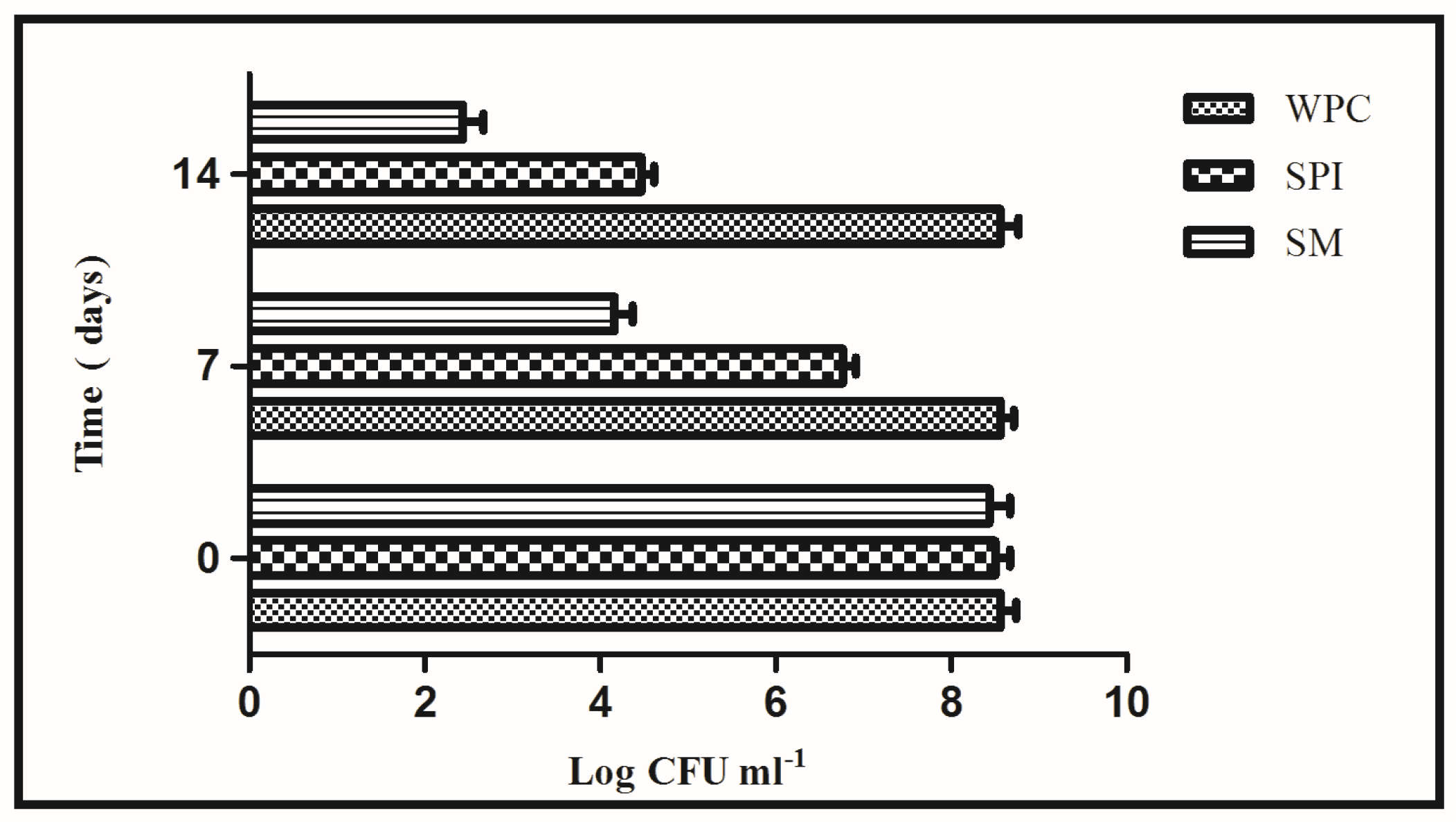
3.1. Development of Unfermented Sea Buckthorn Beverage Fortified with L. rhamnosus GG
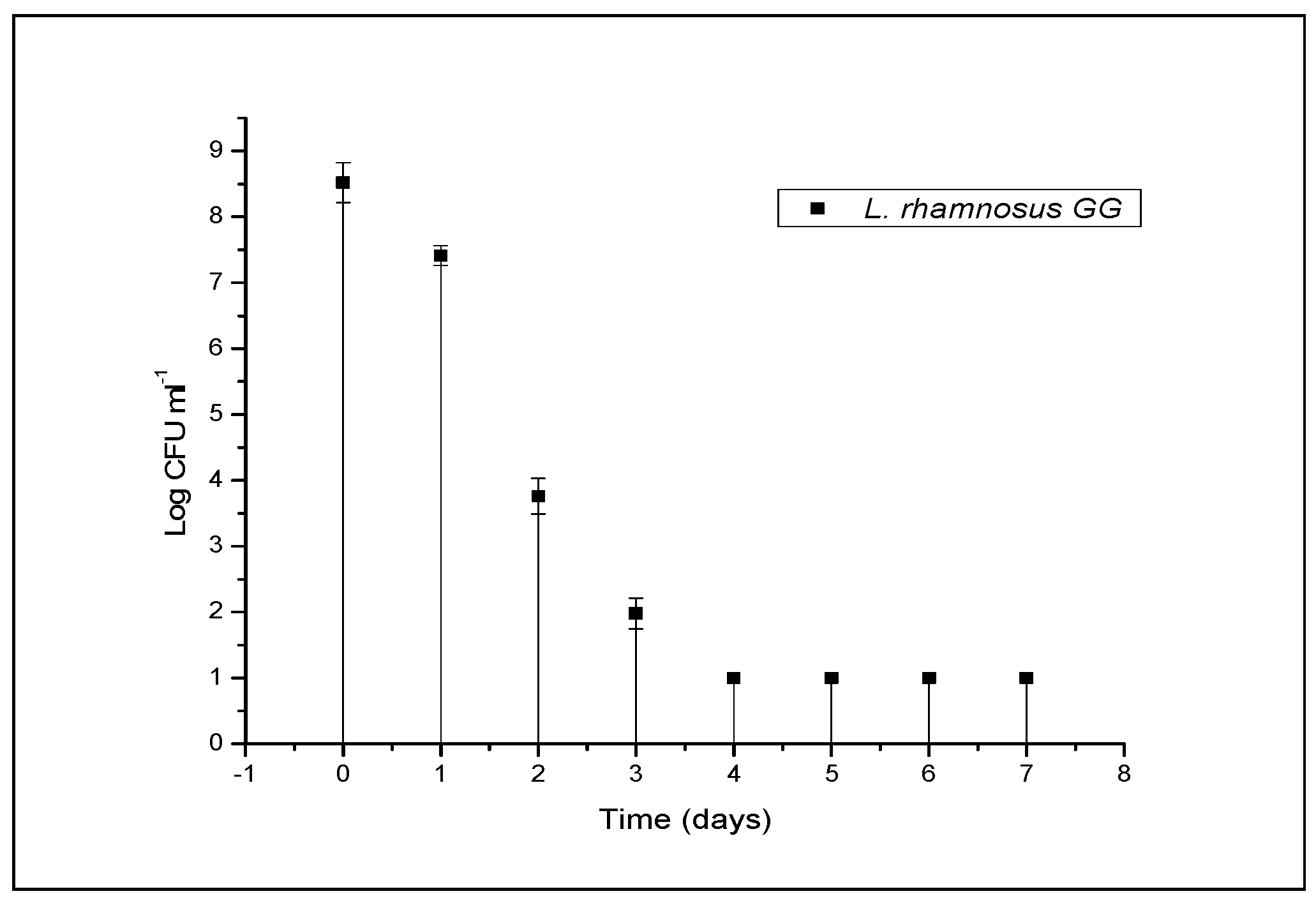
3.2. Viability of L. rhamnosus GG in Beverage
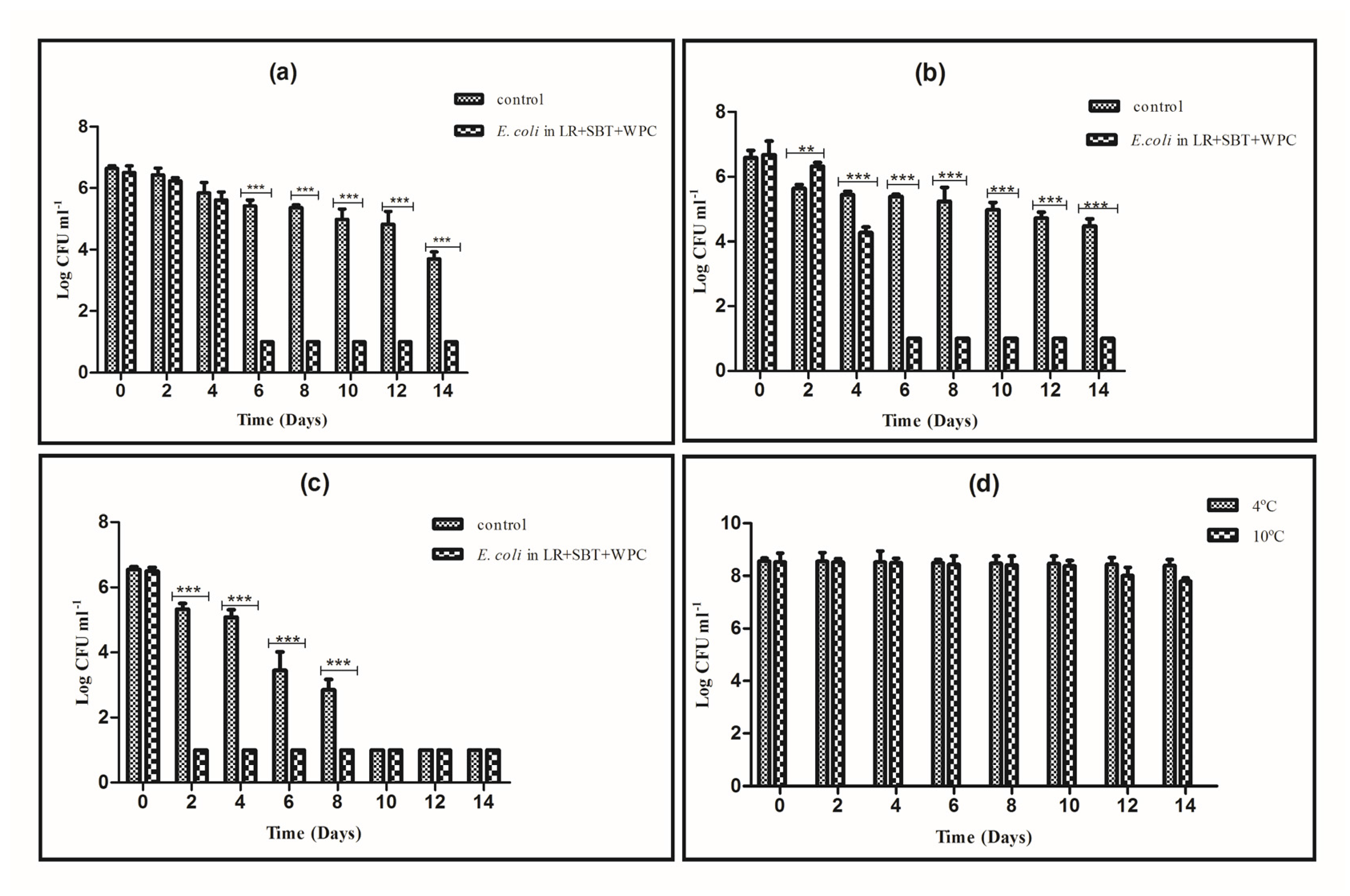
3.3. Evaluation of L. rhamnosus as Protective Culture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raffo, A.; Paoletti, F.; Antonelli, M. Changes in sugar, organic acid, flavonol and carotenoid composition during ripening of berries of three sea buckthorn (Hippophae rhamnoides L.) cultivars. Eur. Food Res. Technol. 2004, 219, 360–368. [Google Scholar] [CrossRef]
- Gutzeit, D.; Wray, V.; Winterhalter, P.; Jerz, G. Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophae rhamnoides L. ssp. rhamnoides) by high-speed counter-current chromatography. Chromatographia 2007, 65, 1–7. [Google Scholar]
- Choi, S.Y.; Beuchat, L.R. Growth inhibition of Listeria monocytogenes by a bacteriocin of Pediococcus acidilactici M during fermentation of kimchi. Food Microbiol. 1994, 11, 301–307. [Google Scholar] [CrossRef]
- Rodgers, S.; Kailasapathy, K.; Cox, J.; Peiris, P. Bacteriocin production by protective cultures. Food Serv. Technol. 2002, 2, 59–68. [Google Scholar] [CrossRef]
- Rodgers, S. Novel applications of live bacteria in food services: Probiotics and protective cultures. Trends Food Sci. Technol. 2008, 191, 88–97. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Cathcart, D.P.; Warner, P.J.; Jiménez-Díaz, R. Use of Lactobacillus plantarum LPCO10, a bacteriocin producer, as a starter culture in Spanish-style green olive fermentations. Appl. Environ. Microb. 1994, 60, 2059–2064. [Google Scholar]
- Giraffa, G. Enterococcal bacteriocins: Their potential as anti-Listeria factors in dairy technology. Food Microbiol. 1995, 12, 291–299. [Google Scholar] [CrossRef]
- Stecchini, M.L.; Aquili, V.; Sarais, I. Behavior of Listeria monocytogenes in Mozzarella cheese in presence of Lactococcus lactis. Int. J. Food Microbiol. 1995, 25, 301–310. [Google Scholar] [CrossRef]
- Schillinger, U.; Kaya, M.; Lücke, F.K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sakei. J. Appl. Bacteriol. 1991, 70, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Anon, *!!! REPLACE !!!*; Rodgers, S. 10. Anon; Rodgers, S. Novel applications of live bacteria in food services: probiotics and protective cultures. Trends Food Sci Technol 2008, 19, 188–197. [Google Scholar]
- Zocco, M.A.; Dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharm. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Lorea-Baroja, M.; Kirjavainen, P.V.; Hekmat, S.; Reid, G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. J. Clin. Exp. Immunol. 2007, 149, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Salleh-Mack, S.Z.; Roberts, J.S. Ultrasound pasteurization: The effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrason. Sonochem. 2007, 14, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Moraru, C.I. Inactivation of Escherichia coli ATCC 25922 and Escherichia coli O157: H7 in apple juice and apple cider, using pulsed light treatment. J. Food Prot. 2009, 72, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Tippayatum, P.; Chonhenchob, V. Antibacterial activities of thymol, eugenol and nisin against some food spoilage bacteria. Nat. Sci. 2007, 41, 319–323. [Google Scholar]
- Bump, V.L. Apple pressing and juice extraction. In Processed Apple Products; Springer: Boston, MA, USA, 1989; pp. 53–82. [Google Scholar]
- Sireswar, S.; Dey, G.; Sreesoundarya, T.K.; Sarkar, D. Design of probiotic-fortified food matrices influence their antipathogenic potential. Food Biosci. 2017. [Google Scholar] [CrossRef]
- Millette, M.; Luquet, F.M.; Lacroix, M. In vitro growth control of selected pathogens by Lactobacillus acidophilus-and Lactobacillus casei-fermented milk. Lett. Appl. Microbiol. 2007, 44, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.A.; Mountzouris, K.C.; Psyrras, D.; Cremonese, S.; Fischer, J.; Cantor, M.D.; Tsakalidou, E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int. J. Food Microbiol. 2009, 130, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fall, P.A.; Leroi, F.; Chevalier, F.; Guérin, C.; Pilet, M.F. Protective effect of a non-bacteriocinogenic Lactococcus piscium CNCM I-4031 strain against Listeria monocytogenes in sterilized tropical cooked peeled shrimp. J. Aquat. Food Prod. Technol. 2010, 19, 84–92. [Google Scholar] [CrossRef]
- Saraoui, T.; Fall, P.A.; Leroi, F.; Antignac, J.P.; Chereau, S.; Pilet, M.F. Inhibition mechanism of Listeria monocytogenes by a bioprotective bacteria Lactococcus piscium CNCM I-4031. Food Microbiol. 2016, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the addition of probiotic cultures to foods. CRC Crit. Rev. Food Sci. 2005, 45, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, R.N.; Troncoso, E. Improving Nutrition through the Design of Food Matrices; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Bulatović, M.L.; Rakin, M.B.; Vukašinović-Sekulić, M.S.; Mojović, L.V.; Krunić, T.Ž. Effect of nutrient supplements on growth and viability of Lactobacillus johnsonii NRRL B-2178 in whey. Int. Dairy J. 2014, 34, 109–115. [Google Scholar] [CrossRef]
- Pathomrungsiyounggul, P.; Lewis, M.J.; Grandison, A.S. Effects of calcium-chelating agents and pasteurisation on certain properties of calcium-fortified soy milk. Food Chem. 2010, 118, 808–814. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Bryant, C.; Mcclements, D.J. Influence of sucrose on the thermal denaturation, gelation, and emulsion stabilization of whey proteins. J. Agric. Food Chem. 2000, 48, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.E. A discussion of the dynamics of Salmonella enrichment. J. Hyg. 1962, 60, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Aljewicz, M.; Cichosz, G. Protective effects of Lactobacillus cultures in Dutch-type cheese-like products. LWT Food Sci. Technol. 2015, 63, 52–56. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.H.; Lievin-Le Moal, V.; Servin, A.L. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013. [Google Scholar] [CrossRef] [PubMed]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Mogna, L.; Del Piano, M.; Deidda, F.; Nicola, S.; Soattini, L.; Debiaggi, R.; Sforza, F.; Strozzi, G.; Mogna, G. Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J. Clin. Gastroenterol. 2012, 46, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Darehabi, H.K.; Nikmaram, P. Assessment of the growth and survival of Escherichia coli O157: H7 during the manufacture and storage of Iranian white cheese and probiotic cheese. Glob. Vet. 2011, 63, 228–232. [Google Scholar]
- Pisano, M.B.; Viale, S.; Conti, S.; Fadda, M.E.; Deplano, M.; Melis, M.P.; Deiana, M.; Cosentino, S. Preliminary evaluation of probiotic properties of Lactobacillus strains isolated from Sardinian dairy products. Biomed. Res. Int. 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rolim, F.R.; dos Santos, KM.; de Barcelos, S.C.; do Egito, A.S.; Ribeiro, T.S.; da Conceição, M.L.; Magnani, M.; de Oliveira, M.E.; do Egypto, R.D. Survival of Lactobacillus rhamnosus EM1107 in simulated gastrointestinal conditions and its inhibitory effect against pathogenic bacteria in semi-hard goat cheese. LWT Food Sci. Technol. 2015, 63, 807–813. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and physical properties of protein films added with cell-free supernatant of Lactobacillus rhamnosus. Food Control 2016, 62, 44–51. [Google Scholar] [CrossRef]
- Cottage, V. North America to Remain the Largest Market for Probiotic Supplements. Available online: http://www.prnewswire.com/news-releases/north-america-to-remain-the-largest-market-for-probiotic-supplements-633606643.html (accessed on 10 July 2017).
- Raja, B.R.; Arunachalam, K.D. Market potential for probiotic nutritional supplements in India. Afr. J. Bus. Manag. 2011, 5, 5418–5423. [Google Scholar]
- Techsci Research. India Probiotic Market Forecast and Opportunities, 2019. Available online: https://www.techsciresearch.com/report/india-probiotic-market-forecast-and-opportunities-2019/375.html (accessed on 21 August 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sireswar, S.; Dey, G.; Dey, K.; Kundu, A. Evaluation of Probiotic L. rhamnosus GG as a Protective Culture in Sea Buckthorn-Based Beverage. Beverages 2017, 3, 48. https://doi.org/10.3390/beverages3040048
Sireswar S, Dey G, Dey K, Kundu A. Evaluation of Probiotic L. rhamnosus GG as a Protective Culture in Sea Buckthorn-Based Beverage. Beverages. 2017; 3(4):48. https://doi.org/10.3390/beverages3040048
Chicago/Turabian StyleSireswar, Srijita, Gargi Dey, Kinjoll Dey, and Arkasish Kundu. 2017. "Evaluation of Probiotic L. rhamnosus GG as a Protective Culture in Sea Buckthorn-Based Beverage" Beverages 3, no. 4: 48. https://doi.org/10.3390/beverages3040048




