Addressing Combative Behaviour in Spanish Bulls by Measuring Hormonal Indicators
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bullfighting
- Third of sticks: the animal receives one or two pricks in the front area of the back.
- Third of skewers: three pairs of skewers are placed in the front area of the animal’s back.
- Third of crutch: The bullfighter passes the crutch to the bull and the fight ends with the death of the animal.
2.3. Blood Sample Collection, Processing and Hormonal Analysis
2.4. Direct Observational Method
- Very slightly aggressive;
- Not very aggressive;
- Combative;
- Aggressive;
- Very aggressive.
2.5. Statistical Analysis
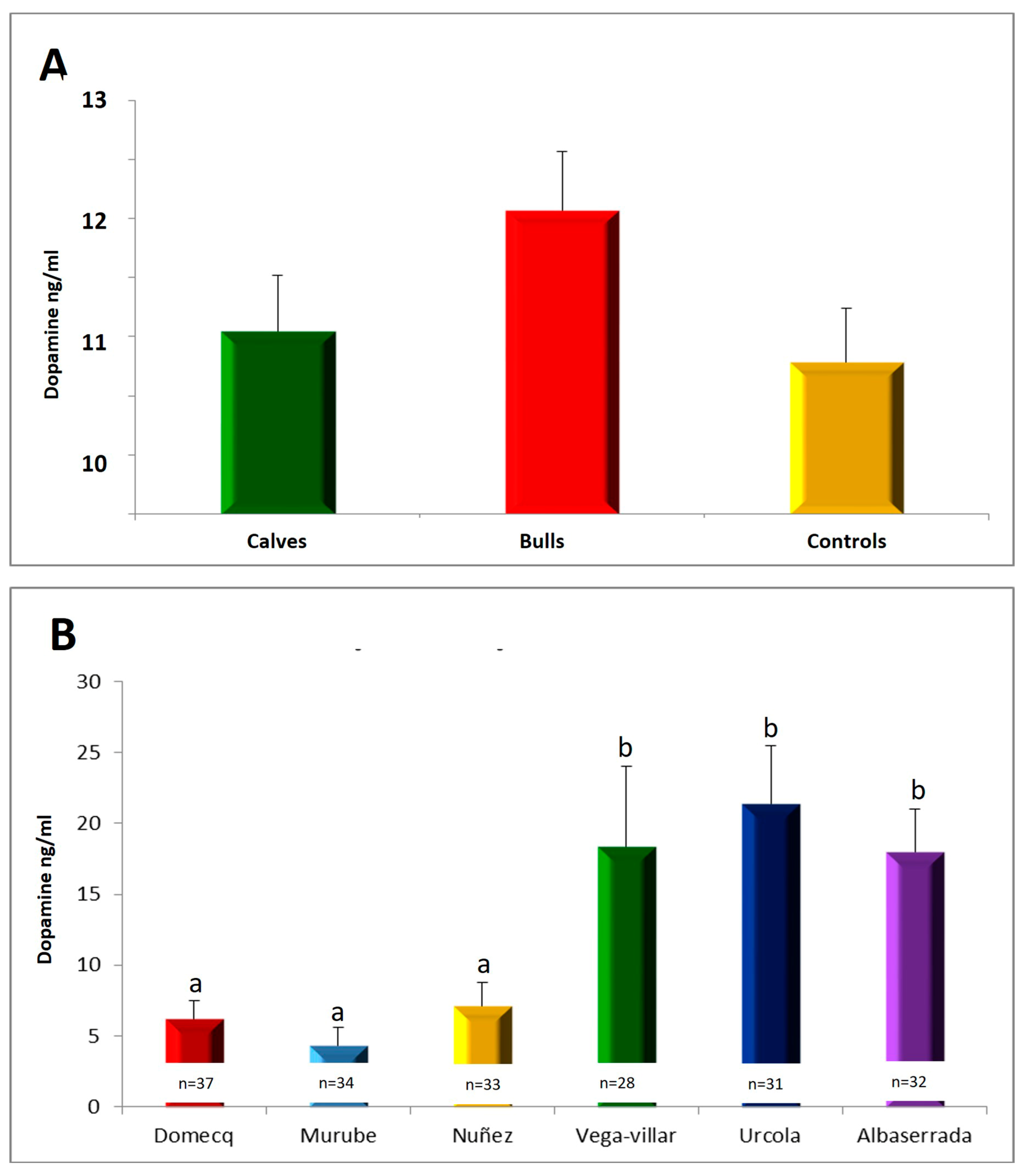
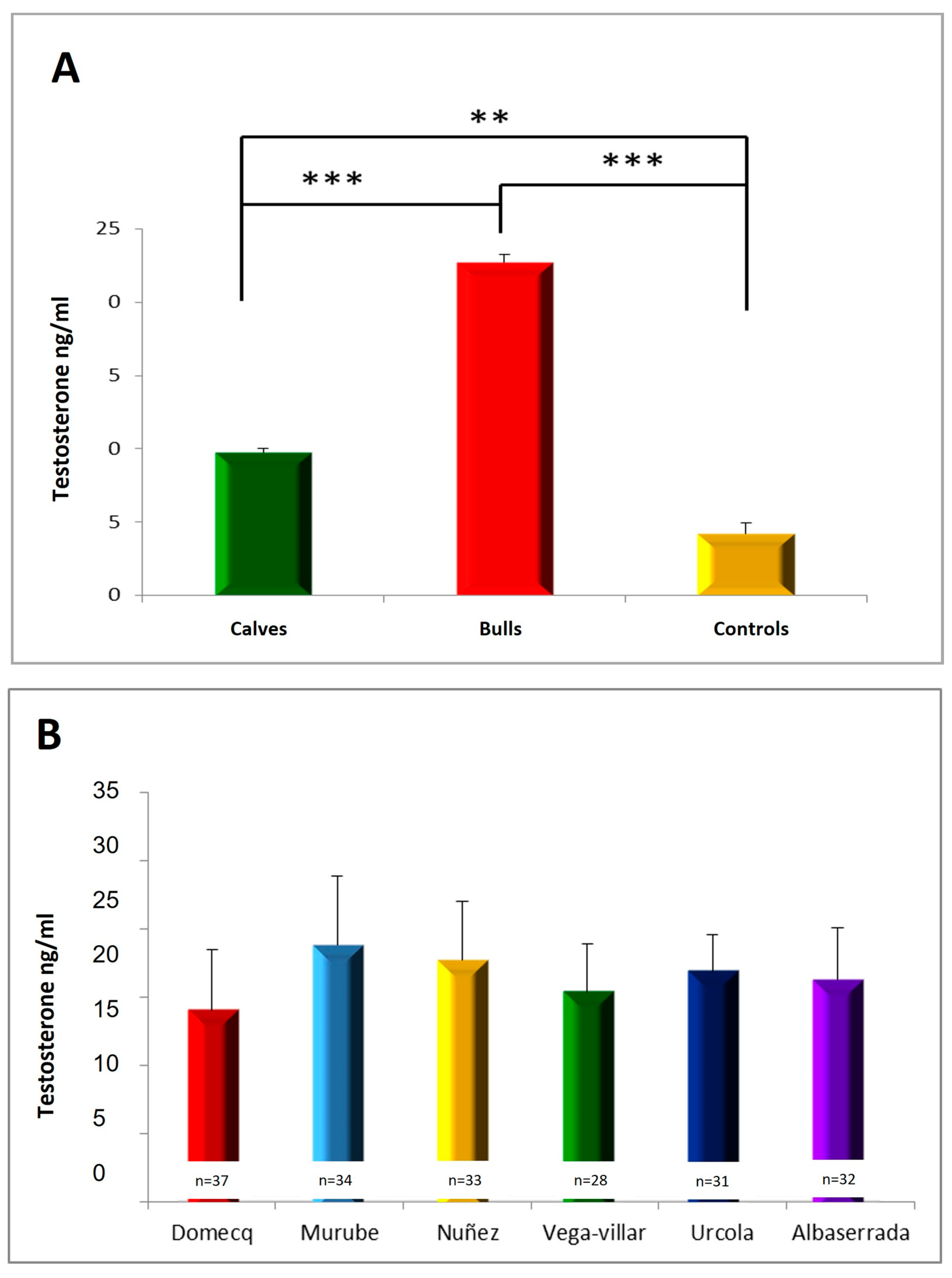
3. Results
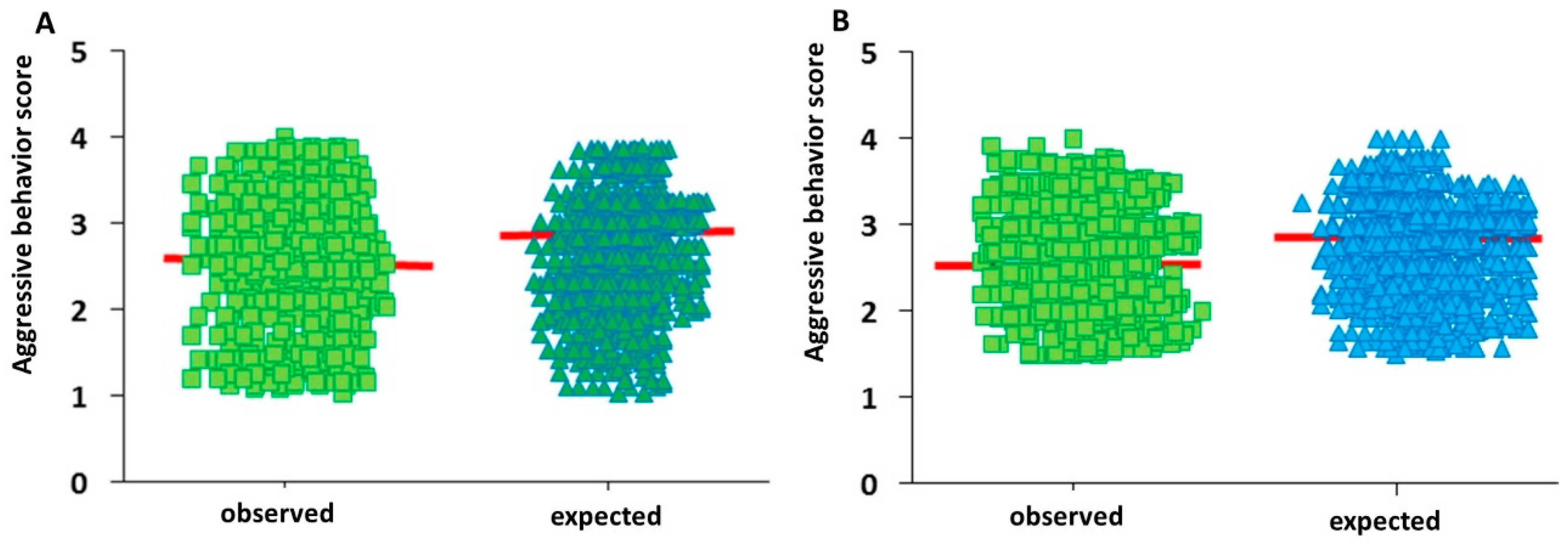
3.1. Distribution of Aggressive Behaviour Scores in Bull Populations
Serum Serotonin Concentrations
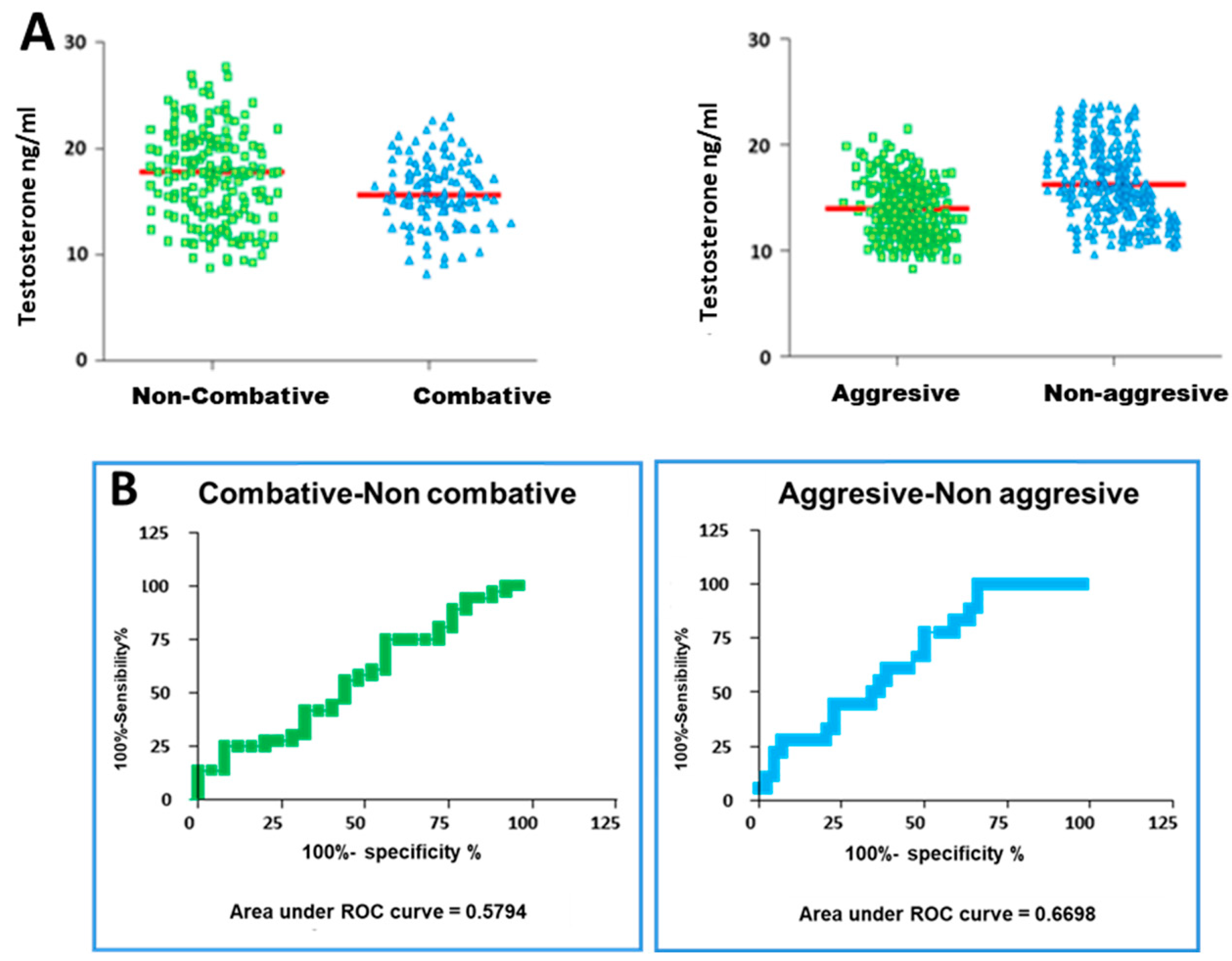
3.2. Serum Testosterone Concentrations
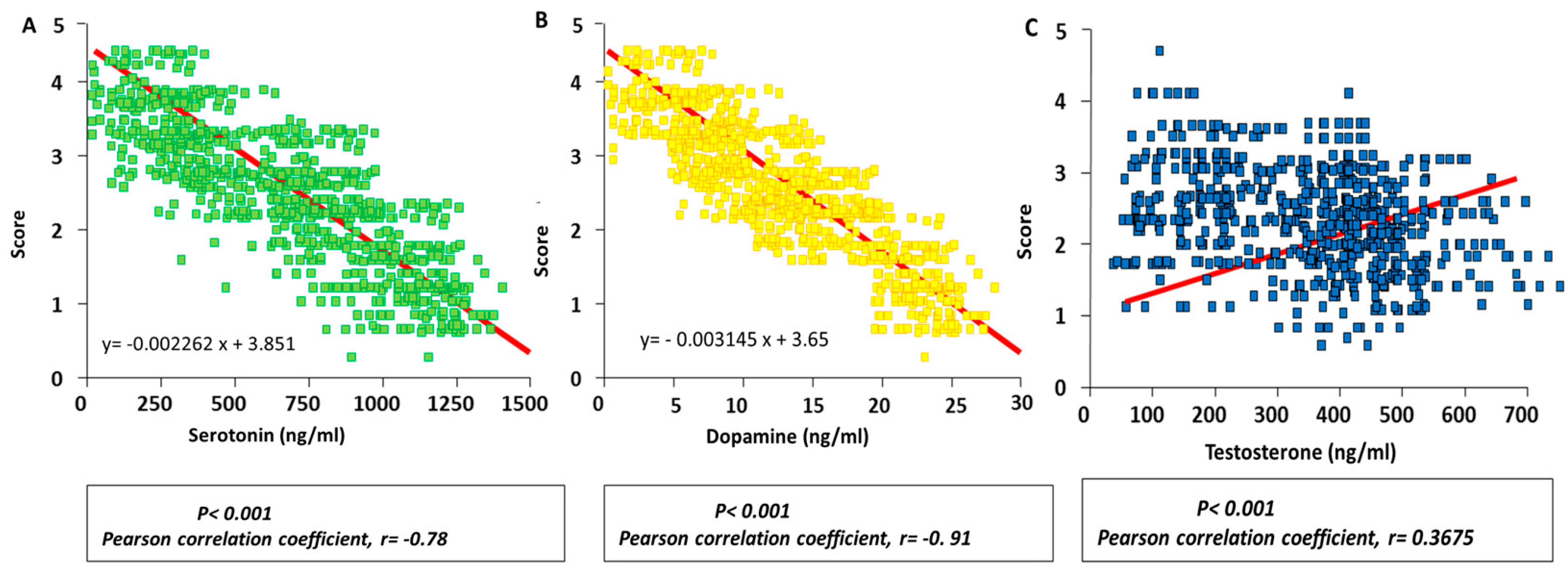
3.3. Correlation between Hormonal Concentrations Measured in Fighting Bulls and Aggressiveness Scores during Their Fights
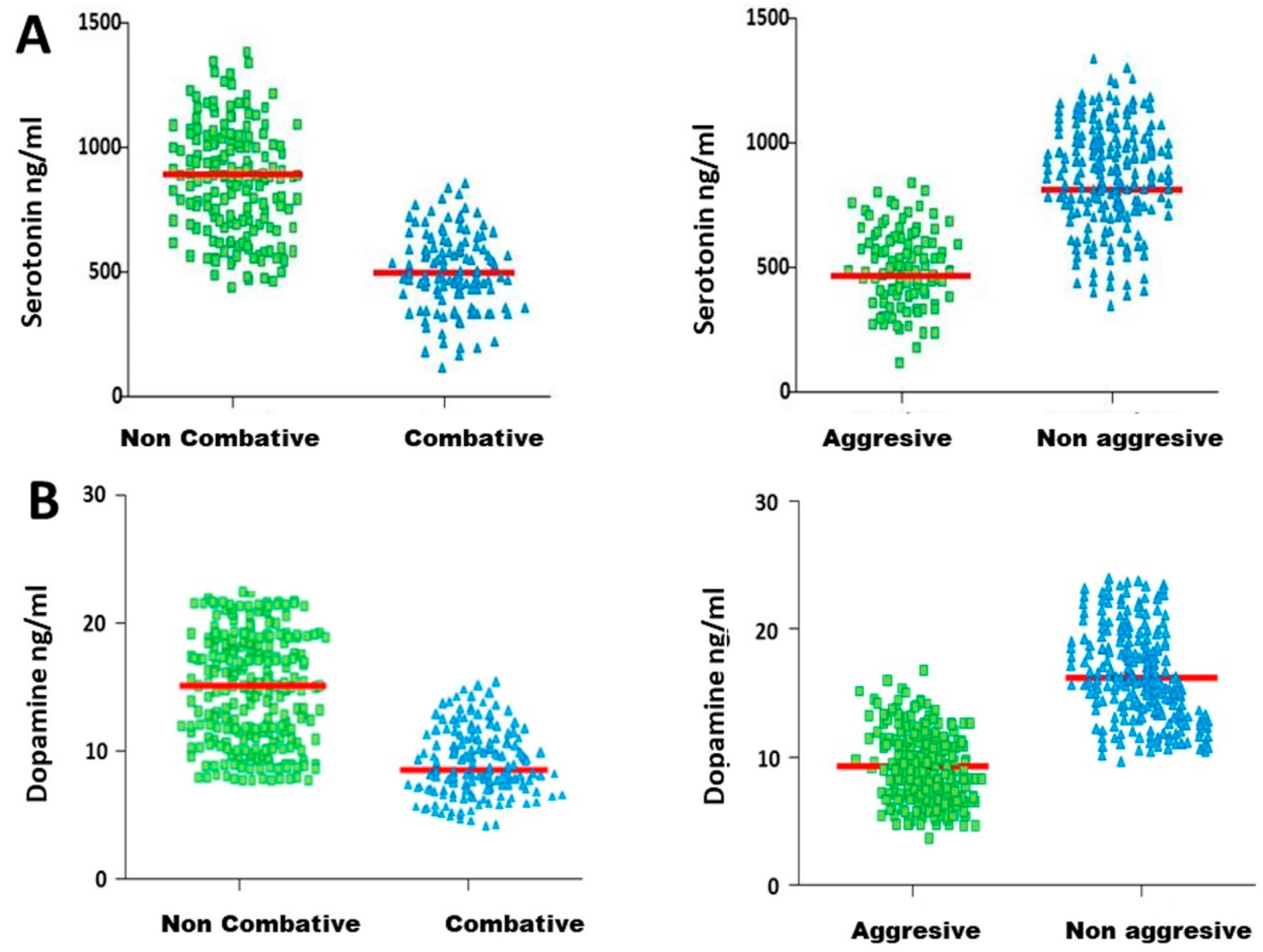
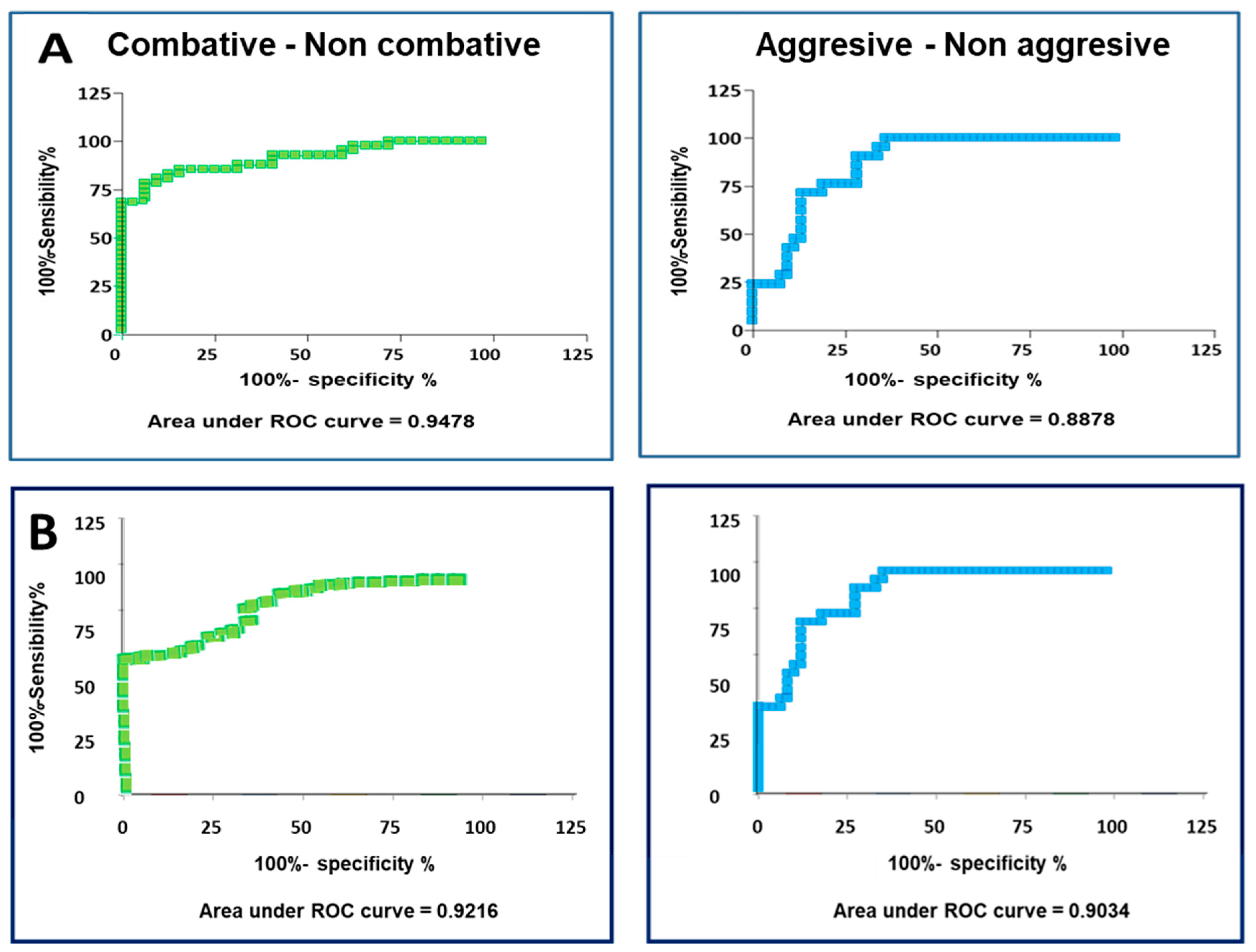
3.4. Determination of the Hormonal Threshold Value in Fighting Bulls to Differentiate Subgroups of Animals with Different Behaviours
- -
- Aggressive: Animals that scored between 3 (inclusive) and 5. These are animals that clearly manifested aggressive actions during all parts of the fight.
- -
- Non-aggressive: Animals that scored between 1 and 3. These are the animals excluded from the previous group.
- -
- Combative: Animals with scores between 2.5 (inclusive) and 5. These animals showed behaviour of combativity and zeal, clearly showing aggressive behaviour or not.
- -
- Non-combative: Animals with scores between 1 and 2.5. These are the animals excluded from the previous group.
3.5. Relationship between Serotonin, Dopamine and Testosterone Concentrations in Calves during Shoeing and Aggressive Behaviour during Their Subsequent Fights
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eusebi, P.G.; Sevane, N.; Cortés, O.; Contreras, E.; Cañon, J.; Dunner, S. Aggressive behavior in cattle is associated with a polymorphism in the MAOA gene promoter. Anim. Genet. 2020, 51, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, R.; Valera, M.; Molina, A.; Royo, L.J. Contribution of Lidia cattle breed historical castes to the paternal genetic stock of Spain. Anim. Genet. 2015, 46, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Cañón, J.; Tupac-Yupanqui, I.; García-Atance, M.A.; Cortés, O.; García, D.; Fernández, J.; Dunner, S. Genetic variation within the Lidia bovine breed. Anim. Genet. 2008, 39, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Gonzalo, A.; Cañón, J. Genetic parameters of aggressiveness, ferocity and mobility in the fighting bull breed. Anim. Res. 2006, 55, 65–70. [Google Scholar] [CrossRef]
- Menéndez-Buxadera, A.; Cortés, O.; Cañon, J. Genetic (co)variance and plasticity of behavioural traits in Lidia bovine breed. Ital. J. Anim. Sci. 2017, 16, 208–216. [Google Scholar] [CrossRef]
- Lockwood, S.A.; Kattesh, H.G.; Krawczel, P.D.; Kirkpatrick, F.D.; Saxton, A.M.; Rhinehart, J.D.; Wilkerson, J.B. Relationships among temperament, behavior, and growth during performance testing of bulls1. J. Anim. Sci. 2015, 93, 5856–5862. [Google Scholar] [CrossRef]
- Miczek, K.A.; de Almeida, R.M.M.; Kravitz, E.A.; Rissman, E.F.; de Boer, S.F.; Raine, A. Neurobiology of escalated aggression and violence. J. Neurosci. 2007, 27, 11803–11806. [Google Scholar] [CrossRef]
- Terry, N.; Margolis, K.G. Serotonergic mechanisms regulating the GI tract: Experimental evidence and therapeutic relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.M.; Andreu, J.M. Aggression, and some related psychological constructs (anger, hostility, and impulsivity); some comments from a research project. Neurosci. Biobehav. Rev. 2006, 30, 276–291. [Google Scholar] [CrossRef]
- Çakiroǧlu, D.; Meral, Y.; Sancak, A.A.; Çifti, G. Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet. Rec. 2007, 161, 59–61. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.E.; McCloskey, M.S.; Fanning, J.R.; Schumacher, J.A.; Coccaro, E.F. serotonin augmentation reduces response to attack in aggressive individuals. Psychol. Sci. 2009, 20, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Duke, A.A.; Bègue, L.; Bell, R.; Eisenlohr-Moul, T. Revisiting the serotonin–aggression relation in humans: A meta-analysis. Psychol. Bull. 2013, 139, 1148–1172. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, N.J.; Siegel, A. Bimodal classification of aggression: Affective defense and predatory attack. Aggress. Violent Behav. 2002, 7, 237–250. [Google Scholar] [CrossRef]
- Waltes, R.; Chiocchetti, A.G.; Freitag, C.M. The neurobiological basis of human aggression: A review on genetic and epigenetic mechanisms. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 557–760. [Google Scholar] [CrossRef] [PubMed]
- Wrangham, R.W. Two types of aggression in human evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Daw, N.D.; Kakade, S.; Dayan, P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002, 15, 603–616. [Google Scholar] [CrossRef]
- Davidson, R.J.; Putnam, K.M.; Larson, C.L. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science 2000, 289, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Rosado, B.; García-Belenguer, S.; Palacio, J.; Chacón, G.; Villegasa, A.; Alcalde, A.I. Serotonin transporter activity in platelets and canine ag-gression. Vet. J. 2010, 186, 104–105. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Juárez, H.; Calderón, D.; Hernández-García, E.; Barragán, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2016, 9730467. [Google Scholar] [CrossRef]
- Mahadevia, D.; Saha, R.; Manganaro, A.; Chuhma, N.; Ziolkowski-Blake, A.; Morgan, A.A.; Dumitriu, D.; Rayport, S.; Ansorge, M.S. Dopamine promotes aggression in mice via ventral tegmental area to lateral septum projections. Nat. Commun. 2021, 12, 6796. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.M.; Ferrari, P.F.; Parmigiani, S.; Miczek, K.A. Escalated aggressive behavior: Dopamine, serotonin and GABA. Eur. J. Pharmacol. 2005, 526, 51–64. [Google Scholar] [CrossRef]
- Huertas, D.; Aliño, J.L.I.; Hervás, M.D.C. Neurobiología de la agresividad humana; ARS Médica: Doylestown, PA, USA, 2005; ISBN 8497060946. [Google Scholar]
- van Erp, A.M.; Miczek, K.A. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, K. Behavioural effects of androgen in men and women. J. Endocrinol. 2001, 170, 39–48. [Google Scholar] [CrossRef]
- Lischinsky, J.E.; Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 2020, 23, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Huijgens, P.T.; Snoeren, E.M.S.; Meisel, R.L.; Mermelstein, P.G. Effects of gonadectomy and dihydrotestosterone on neuronal plasticity in motivation and reward related brain regions in the male rat. J. Neuroendocr. 2020, 33, e12918. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Galbany, J.; Mcfarlin, S.C.; Ndayishimiye, E. Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim. Behav. 2019, 151, 87–102. [Google Scholar] [CrossRef]
- Oliveira, V.E.d.M.; Bakker, J. Neuroendocrine regulation of female aggression. Front. Endocrinol. 2022, 13, 957114. [Google Scholar] [CrossRef]
- Peterson, C.K.; Harmon-Jones, E. Anger and testosterone: Evidence that situationally-induced anger relates to situationally-induced tes-tosterone. Emotion 2012, 12, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 145/1996, de 2 de Febrero, Por el Que se Modifica y da Nueva Redacción al Reglamento de Espectáculos Taurinos. Available online: https://www.boe.es/eli/es/rd/1996/02/02/145 (accessed on 21 November 2023).
- Woods, P.; Almvik, R. The Brøset violence checklist (BVC). Ac. Psych. Scand. 2002, 412, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.N. Biology of Aggression; Nelson, R.J., Ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Natarajan; Natarajan, D.; Caramaschi, D. Animal violence demystified. Front. Behav. Neurosci. 2010, 4, 9. [Google Scholar] [CrossRef]
- Hermann, H.R. Dominance and Aggression in Humans and Other Animals: The Great Game of Life; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Narvaes, R.; de Almeida, R.M.M. Aggressive behavior and three neurotransmitters: Dopamine, GABA, and serotonin—A review of the last 10 years. Psychol. Neurosci. 2014, 7, 601–607. [Google Scholar] [CrossRef]
- Caceres, S.; Moreno, J.; Crespo, B.; Silvan, G.; Illera, J.C. Physiological stress responses in cattle used in the spanish rodeo. Animals 2023, 13, 2654. [Google Scholar] [CrossRef] [PubMed]
- Gaudioso, V.R.; Sánchez, J.M.; Riol, J.A. Metodología de valoración de la aptitud productiva de lidia. Mem. I Simp. Nac. Toro Lidia. Zafra 1993, 139–149. [Google Scholar]
- Calvo Sáez, L.A. Determinación Evolutiva Hasta el Siglo XXI de los Caracteres Morfo-Etológicos del Bos Taurus Braquíceros, Subespecie Lidia. Doctoral Thesis, Facultad de Veterinaria, Universidad Complutense de Madrid, Madrid, Spain, 2010. [Google Scholar]
- Domecq, A. El Toro Bravo, 2nd ed.; Espasa Calpe: Madrid, Spain, 1985. [Google Scholar]
- Mathews, T.A.; Fedele, D.E.; Coppelli, F.M.; Avila, A.M.; Murphy, D.L.; Andrews, A.M. Gene dose-dependent alterations in extraneuronal seroto-nin but not dopamine in mice with reduced serotonin transporter expresión. J. Neurosci. Meth. 2004, 140, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Quadros, I.M.; de Almeida, R.M.M.; Miczek, K.A. Behavioral and Pharmacogenetics of Aggressive Behavior. Curr. Top. Behav. Neurosci. 2012, 12, 73–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Teixeira, C.M.; Mahadevia, D.; Huang, Y.; Balsam, D.; Mann, J.J.; A Gingrich, J.; Ansorge, M.S. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol. Psychiatry 2014, 19, 688–698. [Google Scholar] [CrossRef]
- Niederkofler, V.; Asher, T.E.; Okaty, B.W.; Rood, B.D.; Narayan, A.; Hwa, L.S.; Beck, S.G.; Miczek, K.A.; Dymecki, S.M. Identification of serotonergic neuronal modules that affect aggressive behavior. Cell Rep. 2016, 17, 1934–1949. [Google Scholar] [CrossRef]
- Ikemoto, S.; Panksepp, J. The role of nucleus accumebs dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999, 31, 6–41. [Google Scholar] [CrossRef] [PubMed]
- Everit, B.J.; Robbins, T.W. Second order schedules of drug reinforcement in rats and monkeys: Measurement of reinforcing efficacy and drug-seeking behavior. Psychopharmacology 2000, 153, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, T.; Winz, O.; Henkel, K.; Prinz, S.; Rademacher, L.; Schmaljohann, J.; Dautzenberg, K.; Cumming, P.; Kumakura, Y.; Rex, S.; et al. The impact of dopamine on aggression: An [18F]-FDOPA Pet Study in healthy males. J. Neurosci. 2013, 23, 16889–16896. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Zanni, G.; Mahadevia, D.; Chuhma, N.; Saha, R.; Spivack, S.; Pini, N.; Stevens, G.S.; Ziolkowski-Blake, A.; Simpson, E.H.; et al. Dopamine transporter blockade during adolescence increases adult dopamine function, impulsivity, and aggression. Mol. Psychiatry 2023, 28, 3512–3523. [Google Scholar] [CrossRef] [PubMed]
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R.J. Dopamine, Time, and Impulsivity in Humans. J. Neurosci. 2010, 30, 8888–8896. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Roiser, J.P. Dopamine, serotonin and impulsivity. Neuroscience 2012, 215, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Miczek, K.A. Neurogenetics of aggressive behavior: Studies in rodents. Curr. Top. Behav. Neurosci. 2014, 17, 3–44. [Google Scholar] [CrossRef]
- Fritz, M.; Soravia, S.-M.; Dudeck, M.; Malli, L.; Fakhoury, M. Neurobiology of aggression—Review of recent findings and relationship with alcohol and trauma. Biology 2023, 12, 469. [Google Scholar] [CrossRef]
- Niederkofler, V.; Asher, T.E.; Dymecki, S.M. Functional interplay between dopaminergic and serotonergic neuronal systems during development and adulthood. ACS Chem. Neurosci. 2015, 6, 1055–1070. [Google Scholar] [CrossRef]
- Sasaki, K.; Harada, M. Dopamine production in the brain is associated with caste-specific morphology and behavior in an artificial intermediate honey bee caste. PLoS ONE 2020, 15, e0244140. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.F.; Van Erp, A.M.M.; Tornatzky, W.; Miczek, K.A. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur. J. Neurosci. 2003, 17, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Valente, F.; Alonso, M.E.; Lomillos, J.M.; Gaudioso, V.R.; Alonso, J.; González-Montaña, J.R. Effect of intense exercise on plasma macrominerals and trace elements in lidia bulls. Veter- Sci. 2021, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Almenara-Barrios, J.; García González, G.R. Assessment scale for behaviour in bulfighting cattle (ebl 10). reliability and validity studies. Arch. Zootec. 2011, 60, 215–224. [Google Scholar] [CrossRef]
- Veenema, A.H. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models? Front. Neuroendocrinol. 2009, 30, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; Ruiz, J.L.; Manteca, X. Differences in serotonin serum concentration between aggressive English cocker spaniels and aggressive dogs of other breeds. J. Vet. Behav. 2013, 8, 19–25. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Tanaka, K.F.; Barr, M.M.; Tritschler, L.; Le Dantec, Y.; David, D.J.; Gardier, A.M.; Blanco, C.; Hen, R.; Ahmari, S.E. Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 2015, 86, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, H.; Blennow, K.; Manhem, A.; Forsman, A. CSF studies in violent offenders¶I. 5-HIAA as a negative and HVA as a positive predictor of psychopathy. J. Neural Transm. 2001, 108, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Tsutsui, K. Review: Neuroestrogen regulation of socio-sexual behavior of males. Front. Neurosci. 2014, 8, 323. [Google Scholar] [CrossRef]
- Goldey, K.L.; Van Anders, S.M. Sexual modulation of testosterone: Insights for humans from across species. Adapt. Hum. Behav. Physiol. 2014, 1, 93–123. [Google Scholar] [CrossRef]
- Salvador, A.; Suay, F.; Martinezsanchis, S.; Simon, V.; Brain, P. Correlating testosterone and fighting in male participants in judo contests. Physiol. Behav. 1999, 68, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R. Influencia de la Lidia Sobre los Perfiles Hormonales Plasmáticos en el Ganado Bravo. Doctoral Thesis, Facultad de Veteri-naria de Madrid, Universidad Complutense de Madrid, Madrid, Spain, 2003. [Google Scholar]
- Praschak-Rieder, N.; Willeit, M.; Wilson, A.A.; Houle, S.; Meyer, J.H. Seasonal variation in human brain serotonin transporter binding. Arch. Gen. Psychiatry 2008, 65, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Karrer, T.M.; McLaughlin, C.L.; Guaglianone, C.P.; Samanez-Larkin, G.R. Reduced serotonin receptors and transporters in normal aging adults: A meta-analysis of PET and SPECT imaging studies. Neurobiol. Aging 2019, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, E.; Jaroenporn, S.; Ueda, Y.; Arai, K.; Katsumata, H.; Watanabe, G.; Taya, K. Circulating gonadotropins and testicular hormones during sexual maturation and annual changes in male bottlenose dolphins (Tursiops truncatus). J. Veter-Med. Sci. 2017, 79, 1899–1905. [Google Scholar] [CrossRef] [PubMed]




| Score | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 |
| % | 5.96 | 5.96 | 17.89 | 3.15 | 25.61 | 11.22 | 26.31 | 3.15 | 0.35 |
| Cut-Off Point | % Specificity | % Sensitivity | |
|---|---|---|---|
| Serotonin | 708.5 ng/mL | 90.63% | 80.49% |
| Dopamine | 12.24 ng/mL | 91.23% | 82.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illera, J.C.; Jimenez-Blanco, F.; Centenera, L.; Gil-Cabrera, F.; Crespo, B.; Lopez, P.R.; Silvan, G.; Caceres, S. Addressing Combative Behaviour in Spanish Bulls by Measuring Hormonal Indicators. Vet. Sci. 2024, 11, 182. https://doi.org/10.3390/vetsci11040182
Illera JC, Jimenez-Blanco F, Centenera L, Gil-Cabrera F, Crespo B, Lopez PR, Silvan G, Caceres S. Addressing Combative Behaviour in Spanish Bulls by Measuring Hormonal Indicators. Veterinary Sciences. 2024; 11(4):182. https://doi.org/10.3390/vetsci11040182
Chicago/Turabian StyleIllera, Juan Carlos, Francisco Jimenez-Blanco, Luis Centenera, Fernando Gil-Cabrera, Belen Crespo, Paula Rocio Lopez, Gema Silvan, and Sara Caceres. 2024. "Addressing Combative Behaviour in Spanish Bulls by Measuring Hormonal Indicators" Veterinary Sciences 11, no. 4: 182. https://doi.org/10.3390/vetsci11040182





