Canine Mesenchymal Stromal Cell Exosomes: State-of-the-Art Characterization, Functional Analysis and Applications in Various Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Physiological Biogenesis of Mesenchymal Stromal Cell Exosomes: A Cellular Process
2.1. Formation of Exosomes
2.2. Cargo Organization into Intraluminal Vessels
2.3. Multivesicular Bodies to Plasma Membrane Fusion
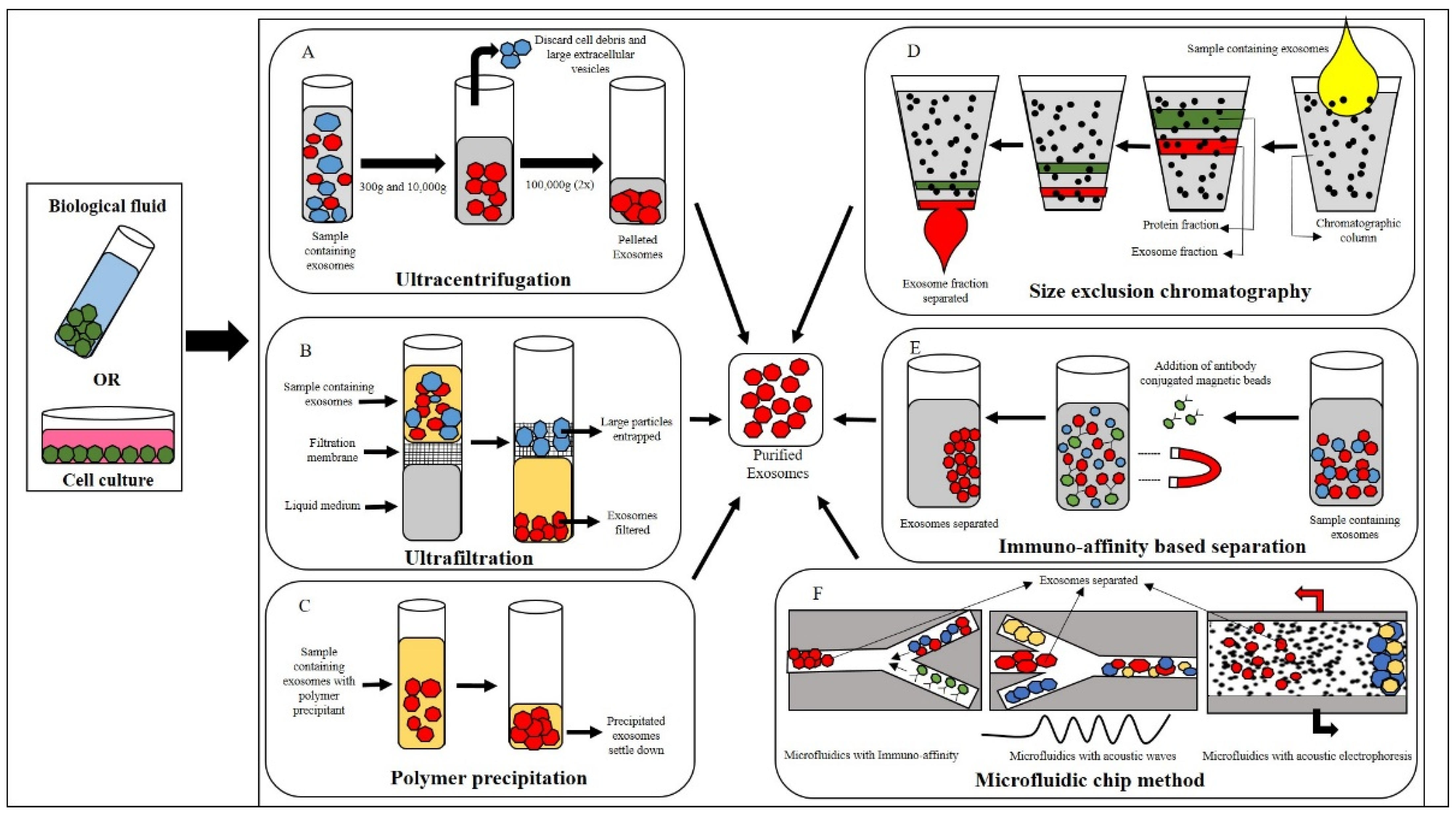
3. Isolation of Canine Exosomes
3.1. Ultracentrifugation
3.2. Ultrafiltration
3.3. Polymer Precipitation
3.4. Size-Exclusion Chromatography (SEC)
3.5. Immunomagnetic Bead-Based Method
3.6. Microfluidic-Based Method
3.6.1. Immunoaffinity-Based Isolation
3.6.2. Microfluidics with Acoustic Fields and Electrophoresis
3.6.3. Electrophoresis
4. Characterization of Exosomes
4.1. Transmission Electron Microscopy (TEM)
4.2. Atomic Force Microscopy (AFM)
4.3. Nanoparticle Tracking Analysis (NTA)
4.4. Dynamic Light Scattering (DLS)
4.5. Tunable Resistive Pulse Sensing (TRPS)
4.6. Flow Cytometry
4.6.1. Western Blot
4.6.2. ELISA
5. Characterization of Canine MSC-Derived EVs and Possible Factors Influencing Their Biological Properties
| Source of Canine MSCs | Characteristics and Specific Properties of Exosome/EVs Isolated | Reference |
|---|---|---|
| Bone marrow |
| [39] |
| [43] | |
| [43] | |
| Adipose tissue |
| [45] |
| Umbilical cord |
| [97] |
| [97] | |
| Amniotic tissue |
| [92] |
| [92] | |
| Gingival tissue |
| [98] |
| [98] |
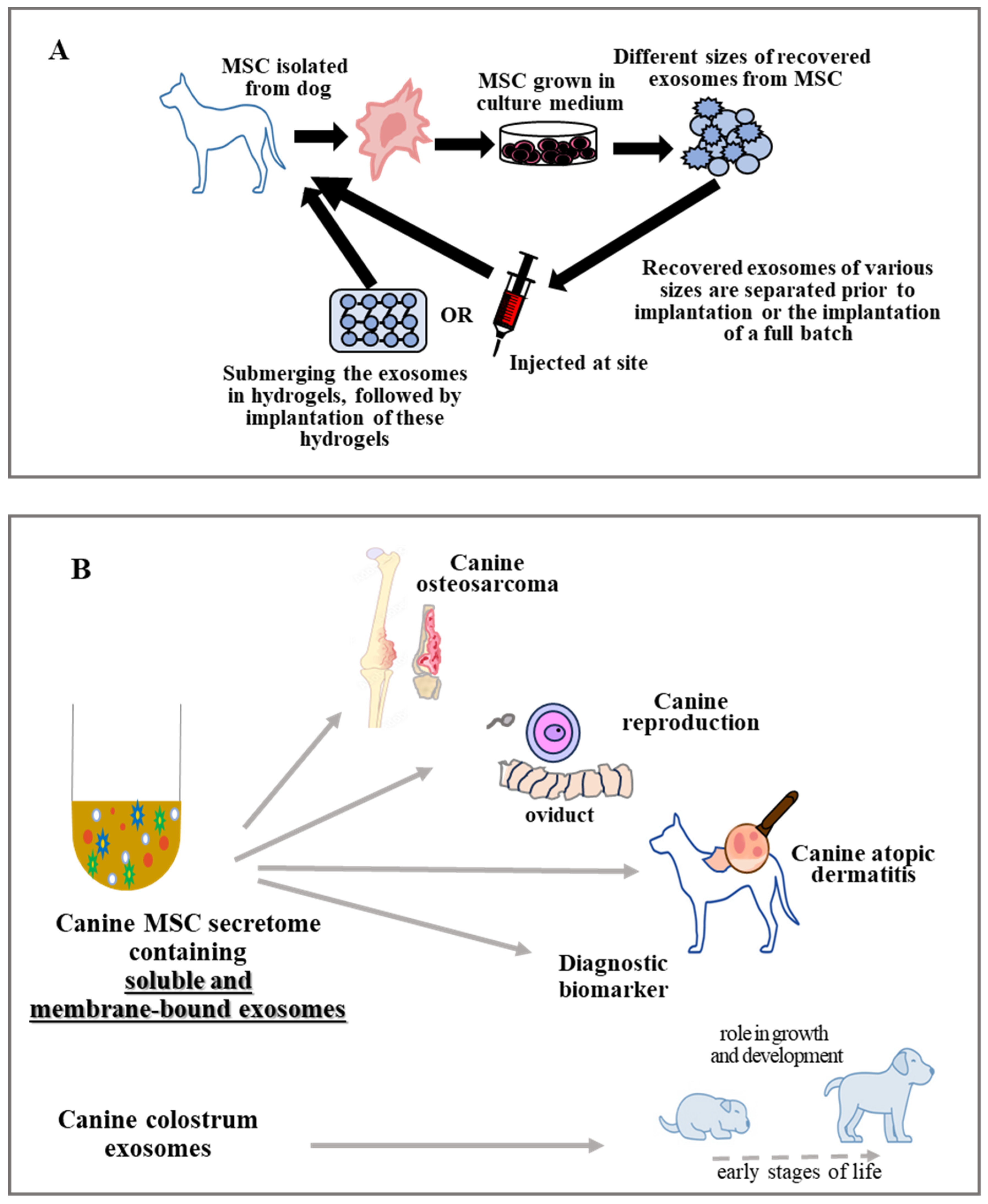
6. Therapeutic Possibilities/Potential of EVs in Multiple Diseases
6.1. Application of Exosome in Bone Disorders
6.2. Exosomes Use in Various Pathological Conditions of Canine Skin
6.3. Exosomes Treatment in Canine Reproduction
6.4. Exosomes Use in Canine Digestive Problems
6.5. Exosomes Use in Canine Cardiovascular System Problems
6.6. Exosomes as Emerging Diagnostic Biomarkers
7. What Are the Advantages and Disadvantages of Using Mesenchymal Stromal Cells or their Exosomes?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgkiss-Geerea, H.M.; Argyle, D.J.; Corcoran, B.M.; Whitelaw, B.; Milne, E.; Bennett, D.; Argyle, S.A. Characterization and differentiation potential of bone marrow derived canine mesenchymal stem cells. Vet. J. 2012, 194, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lopez, M.J. Canine adult adipose tissue-derived multipotent stromal cell isolation and characterization. Methods Mol. Biol. 2018, 1773, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-Ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Park, S.B.; Sun Kang, K. Isolation and characterization of canine Wharton’s jelly-derived mesenchymal stem cells. Cell Transpl. 2012, 21, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell. Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Rojas, M.; Badewien-Rentzsch, B.; Plendl, J.; Kohn, B.; Einspanier, R. Exploration of serum- and cell culture-derived exosomes from dogs. BMC Vet. Res. 2018, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Yoshizaki, K.; Nishida, H.; Kamishina, H.; Maeda, S.; Takano, K.; Fujita, N.; Nishimura, R.; Jo, J.I.; Tabata, Y.; et al. Extracellular vesicles derived from canine mesenchymal stromal cells in serum free culture medium have anti-inflammatory effect on microglial cells. Front. Vet. Sci. 2021, 8, 633426. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-derived extracellular vesicles in inter-organism, cross-species communication and drug delivery. Proteomes 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, E.; Van Ryssen, B.; De Schauwer, C.; Meyer, E. Canine mesenchymal stem cells: State of the art, perspectives as therapy for dogs and as a model for man. Vet. Q. 2013, 33, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.E.; Seo, M.S.; Kang, K.K.; Choi, J.H.; Lee, S.; Sung, M.; Kim, K.; Lee, G.W.; Lim, J.H.; Yang, S.Y.; et al. Mesenchymal stem cell exosomes derived from feline adipose tissue enhance the effects of anti-inflammation compared to fibroblasts-derived exosomes. Vet. Sci. 2021, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, X.; Li, Y.; He, Z.; Qin, K.; Buhl, E.M.; Mert, Ü.; Horst, K.; Hildebrand, F.; Balmayor, E.R.; et al. Porcine mandibular bone marrow-derived mesenchymal stem cell (BMSC)-derived extracellular vesicles can promote the osteogenic differentiation capacity of porcine tibial-derived BMSCs. Pharmaceutics 2024, 16, 279. [Google Scholar] [CrossRef] [PubMed]
- Voga, M.; Kovač, V.; Majdic, G. Comparison of canine and feline adipose-derived mesenchymal stem cells/medicinal signaling cells with regard to cell surface marker expression, viability, proliferation, and differentiation potential. Front. Vet. Sci. 2021, 7, 610240. [Google Scholar] [CrossRef]
- Schwarz, C.; Leicht, U.; Rothe, C.; Drosse, I.; Luibl, V.; Röcken, M.; Schieker, M. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res. Vet. Sci. 2012, 93, 457–462. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Wang, Y.; Zhou, K.; Li, H.; Bi, S.; Wang, Y.; Wu, W.; Huang, Y.; Peng, B.; et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 2023, 11, 1029671. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Balmus, I.M.; Ciobica, A.; Nicoara, M.N.; Luca, A.C.; Palade, D.O. The Role of microglial exosomes and mir-124-3p in neuroinflammation and neuronal repair after traumatic brain injury. Life 2023, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
- Csobonyeiova, M.; Smolinska, V.; Harsanyi, S.; Ivantysyn, M.; Klein, M. The Immunomodulatory role of cell-free approaches in sars-cov-2-induced cytokine storm-a powerful therapeutic tool for COVID-19 patients. Biomedicines 2023, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, K.; Hazrati, A.; Zahar, M.; Markov, A.; Zekiy, A.O.; Navashenaq, J.G.; Roshangar, L.; Ahmadi, M. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev. Rep. 2022, 18, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, L.; Andriessen, A.; Wauben, M.H.M.; Nolte-t’ Hoen, E.N.M.; de Bruin, A. Extracellular vesicles: Novel opportunities to understand and detect neoplastic diseases. Vet. Pathol. 2021, 58, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Diomaiuto, E.; Principe, V.; De Luca, A.; Laperuta, F.; Alterisio, C.; Di Loria, A. Exosomes in dogs and cats: An innovative approach to neoplastic and non-neoplastic diseases. Pharmaceuticals 2021, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.A.; Jurek, S.; Trappe, S.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Influence of bovine serum lipids and fetal bovine serum on the expression of cell surface markers in cultured bovine preadipocytes. Cells Tissues Organs 2017, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.T.; Michurina, V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. International Society for Cellular. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Bonetto, V. Extracellular vesicles and a novel form of communication in the brain. Front. Neurosci. 2016, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, J.; Lee, H. Emerging role of extracellular vesicles in the respiratory system. Exp. Mol. Med. 2020, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Chevallier, J.; Mayran, N.; Le Blanc, I.; Ferguson, C.; Faure, J.; Blanc, N.S.; Matile, S.; Dubochet, J.; Sadoul, R.; et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Holz, R.W.; Zimmerberg, J. Dynamic relationship of the snare complex with a membrane. Biophys. J. 2019, 117, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Alcoholado, C.; Martin-Astorga, M.C.; Fernandez, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Kim, S.B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine mesenchymal-stem-cell-derived extracellular vesicles attenuate atopic dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshizaki, K.; Nishida, H.; Tabata, Y.; Jo, J.; Nakased, I.; Akiyoshi, H. Controlled release of canine MSC-derived extracellular vesicles by cationized gelatin hydrogels. Regen. Therap. 2013, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Li, Q.; Bhang, D.H.; Song, W.J.; Youn, H.Y. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Yuchi, Y.; Suzuki, R.; Matsumoto, H.; Koyama, H. Immunomodulatory effects of canine adipose tissue mesenchymal stem cell-derived extracellular vesicles on stimulated CD4+ T cells isolated from peripheral blood mononuclear cells. J. Immunol. Res. 2021, 2021, 2993043. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.P.; Caro-Vegas, C.P.; Costantini, L.M.; Landis, J.T.; Griffith, J.D.; Damania, B.A.; Dittmer, D.P. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1541396. [Google Scholar] [CrossRef] [PubMed]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Renal. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in exosome isolation techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Palaniyandi, K.; Ramalingam, S.; Sahabudeen, S.; Raja, N.S. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183490. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Chen, X.; Niu, X.; Cai, Y.; Zhang, Q.; Chen, T.; Yang, H. Integrated immunomagnetic bead-based microfluidic chip for exosomes isolation. Micromachines 2020, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Court, J.; Navabi, H.; Adams, M.; Mason, M.D.; Hobot, J.A.; Newman, G.R.; Jasani, B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Meth. 2001, 247, 163–174. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab. Chip 2014, 14, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab. Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab. Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Lim, H.C.; Evander, M.; Lilja, H.; Laurell, T.; Scheding, S.; Ceder, Y. Acoustic enrichment of extracellular vesicles from biological fluids. Anal. Chem. 2018, 90, 8011–8019. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Lu, Y.; Luo, X.; Huang, Y.; Xie, T.; Pilarsky, C.; Dang, Y.; Zhang, J. Microfluidic technology for the isolation and analysis of exosomes. Micromachines 2022, 13, 1571. [Google Scholar] [CrossRef] [PubMed]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Mao, Z.; Chen, K.; Bachman, H.; Chen, Y.; Rufo, J.; Ren, L.; Li, P.; Wang, L.; Huang, T.J. Acoustic separation of nanoparticles in continuous flow. Adv. Funct. Mater. 2017, 27, 1606039. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Jo, W.; Heo, Y.; Kang, J.Y.; Kwak, R.; Park, J.J.S. Chemical AB. Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens. Actuators B Chem. 2016, 233, 289–297. [Google Scholar] [CrossRef]
- Wang, J.; Ma, P.; Kim, D.H.; Liu, B.F.; Demirci, U. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today 2021, 37, 101066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab. Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Iacono, E. Beyond canine adipose tissue-derived mesenchymal stem/stromal cells transplantation: An update on their secretome characterisation and application. Animals 2023, 13, 3571. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell. Biol. 2006, 3, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-based high-throughput nanomechanical screening of single extracellular vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Martin-Astorga, M.D.C.; Alcoholado, C.; Becerra, J. Canine colostrum exosomes: Characterization and influence on the canine mesenchymal stem cell secretory profile and fibroblast anti-oxidative capacity. BMC Vet. Res. 2020, 16, 417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Oosterkamp, T.H.; Bahatyrova, S.; Ashcroft, B.; Garcia Rodriguez, P.; Bertina, R.M.; Osanto, S. Atomic force microscopy: A novel approach to the detection of nanosized blood microparticles. J. Thromb. Haemost. 2010, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Šuštar, V.; Frank, M.; Janša, V.; Zavec, A.B.; Rozman, B.; Mozetic, M.; Hagerstrand, H.; Kralj-Iglic, V. Blood and synovial microparticles as revealed by atomic force and scanning electron microscope. Open Autoimmun. J. 2009, 1, 50–58. [Google Scholar] [CrossRef]
- de Necochea-Campion, R.; Gonda, A.; Kabagwira, J.; Mirshahidi, S.; Cao, H.; Reeves, M.E.; Wall, N.R. A practical approach to extracellular vesicle characterization among similar biological samples. Biomed. Phys. Eng. Express 2018, 4, 065013. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The methods of choice for extracellular vesicles (evs) characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yoon, T.H.; Na, J.; Yi, S.J.; Jin, Y.; Kim, M.; Oh, T.H.; Chung, T.W. Mesenchymal stem cells and extracellular vesicles derived from canine adipose tissue ameliorates inflammation, skin barrier function and pruritus by reducing jak/stat signaling in atopic dermatitis. Int. J. Mol. Sci. 2022, 23, 4868. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, A.S.; Albanyan, A.; Cardigan, R.A.; Mackie, I.J.; Harrison, P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009, 96, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.; Broekman, M.L.; de Vrij, J. Tunable resistive pulse sensing for the characterization of extracellular vesicles. Methods Mol. Biol. 2017, 1545, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.; Lane, R.; Korbie, D.; Trau, M. Observations of tunable resistive pulse sensing for exosome analysis: Improving system sensitivity and stability. Langmuir 2015, 31, 6577–6587. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Pal, A.K.; Jambhrunkar, S.; Patel, P.; Thakur, S.S.; Reategui, E.; Parekh, H.S.; Saa, P.; Stassinopoulos, A.; Broom, M.F. High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Sci. Rep. 2017, 7, 17479. [Google Scholar] [CrossRef] [PubMed]
- Patko, D.; Gyorgy, B.; Nemeth, A.; Szabó-Taylor, K.; Kittel, A.; Buzás, E.I.; Horvath, R.J.S. Chemical AB. Label-free optical monitoring of surface adhesion of extracellular vesicles by grating coupled interferometry. Sens. Actuators B Chem. 2013, 188, 697–701. [Google Scholar] [CrossRef]
- Nolan, J.P.; Duggan, E. Analysis of individual extracellular vesicles by flow cytometry. Methods Mol. Biol. 2018, 1678, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. Methods Enzymol. 2020, 645, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Madan, A.; Bhunia, B.; Arora, D. Isolation, Characterization, and Detailed History of exosomes derived from stem cells and their epigenetic biology. In Applications of Stem Cells and Derived Exosomes in Neurodegenerative Disorders; Springer: Singapore, 2023; pp. 139–168. [Google Scholar]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital detection of exosomes by interferometric imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.A.; Chow, N.H.; Dukoff, D.; Gibson, T.W.; LaMarre, J.; Betts, D.H.; Koch, T.G. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS ONE 2016, 11, e0167442. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Coy, J.; Regan, D.; Dow, S. Mechanisms of immune suppression utilized by canine adipose and bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2017, 26, 374–389. [Google Scholar] [CrossRef]
- Karam, R.G.; Branco Motta, L.C.; Ferreira de Almeida, M.; Bridi, A.; Coelho da Silveira, J.; Ambrósio, C.E. Secretion pattern of canine amniotic stem cells derived extracellular vesicles. Anim. Reprod. 2022, 19, e20220063. [Google Scholar] [CrossRef]
- Ferreira Scassiotti, R.; de Paula Coutinho, M.; Pinto Santos, S.I.; Ferreira Pinto, P.A.; Ferreira de Almeida, M.; Karam, R.G.; da Silva Rosa, P.M.; dos Santos Martins, D.; Coelho da Silveira, J.; Ambrosio, C.E. Adipose and amnion-derived mesenchymal stem cells: Extracellular vesicles characterization and implication for reproductive biotechnology. Theriogenology 2023, 198, 264e272. [Google Scholar]
- Wright, A.; Snyder, O.; He, H.; Christenson, L.K.; Fleming, S.; Weiss, L. Procoagulant activity of umbilical cord-derived mesenchymal stromal cells’ extracellular vesicles (MSC-EVs). Int. J. Mol. Sci. 2023, 24, 9216. [Google Scholar] [CrossRef]
- Park, S.M.; An, J.H.; Lee, J.H.; Kim, K.B.; Chae, H.K.; Oh, Y.I.; Song, W.J.; Youn, H.Y. Extracellular vesicles derived from DFO preconditioned canine AT-MSCs reprogrammacrophages into M2 phase. PLoS ONE 2021, 16, e0254657. [Google Scholar]
- Mocchi, M.; Bari, E.; Dotti, S.; Villa, R.; Berni, P.; Conti, V.; Del Bue, M.; Squassino, G.P.; Segale, L.; Ramoni, R.; et al. Canine mesenchymal cell lyosecretome production and safety evaluation after allogenic intraarticular injection in osteoarthritic dogs. Animals 2021, 11, 3271. [Google Scholar] [CrossRef]
- Wright, A.B. The Isolation, Culture-Expansion, Cryopreservation, Characterization, and Properties of Umbilical Cord-Derived Mesenchymal Stromal Cells and Their Extracellular Vesicles. Ph.D. Thesis, College of Veterinary Medicine Kansas State University, Manhattan, KS, USA, 2021. [Google Scholar]
- Ji, Y.; Jiang, W.; Zeng, F.; Zou, D.; Li, S.; Zhang, X.; Zhu, Q.; Liang, Q.; Li, M.; Li, D. Characterization of canine gingival-derived mesenchymal stem cells and their exosomes. J. Vet. Dent. 2023, 16, 8987564231206459. [Google Scholar] [CrossRef]
- Liang, B.; Liang, J.M.; Ding, J.N.; Xu, J.; Xu, J.G.; Chai, Y.M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mai, Z.; Cui, L.; Zhao, X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res. Ther. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xue, X.; Yin, Z.; Gao, F.; Wang, X.; Geng, Z. Research progress of exosomes in bone diseases: Mechanism, diagnosis and therapy. Front. Bioeng. Biotechnol. 2022, 10, 866627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Wang, S.; Cui, J.; Ren, X.; Su, J. Bone-targeted exosomes: Strategies and applications. Adv. Healthc. Mater. 2023, 12, e2203361. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.; Dash, P.; Dash, M. Drug delivery to the bone microenvironment mediated by exosomes: An axiom or enigma. Int. J. Nanomed. 2021, 16, 3509–3540. [Google Scholar] [CrossRef] [PubMed]
- Vig, S.; Fernandes, M.H. Bone cell exosomes and emerging strategies in bone engineering. Biomedicines 2022, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, Y.; Li, S.; Li, A.; Li, Y.; Pei, D. Engineering exosomes for bone defect repair. Front. Bioeng. Biotechnol. 2022, 10, 1091360. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cao, H.; Hua, W.; Gao, L.; Yuan, Y.; Zhou, X.; Zeng, Z. Mesenchymal stem cell-derived extracellular vesicles for bone defect repair. Membranes 2022, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Y.; Dong, W.; Jiang, B.; Yu, Y.; Chen, Y. Role of nano-hydrogels coated exosomes in bone tissue repair. Fron. Bioeng. Biotechnol. 2023, 11, 1167012. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1alpha-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; He, T.; Jiang, J.; Li, Y.; Chen, P. Osteogenic effect of tsrna-10277-loaded exosome derived from bone mesenchymal stem cells on steroid-induced osteonecrosis of the femoral head. Drug Des. Devel. Ther. 2020, 14, 4579–4591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. MicroRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021, 272, 119204. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, C.; Li, T.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Xie, P.; Li, Y.S.; Wen, T.; Yang, X.C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020, 70, 109504. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental pulp stem cell-derived exosomes alleviate mice knee osteoarthritis by inhibiting trpv4-mediated osteoclast activation. Int. J. Mol. Sci. 2023, 24, 4926. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ramos, F.; Malard, P.F.; Brunel, H.; Paludo, G.R.; de Castro, M.B.; da Silva, P.H.S.; da Cunha Barreto-Vianna, A.R. Canine atopic dermatitis attenuated by mesenchymal stem cells. J. Adv. Vet. Anim. Res. 2020, 7, 554–565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, M.; Sun, Y.; Yang, D.; Xu, W.; Qian, H. Exosomes: Emerging cell-free based therapeutics in dermatologic diseases. Front. Cell Dev. Biol. 2021, 9, 736022. [Google Scholar] [CrossRef] [PubMed]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological evaluation of experimentally induced critical size defect skin wounds using exosomal solution of mesenchymal stem cells derived microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.M.; Amer, M.S.; Abo-EL-Sooud, K.; Abdallah, A.N.; Shehab, G.G.; El-Tookhy, O.S. Proficiency of carboxymethylcellulose as a cryoprotectant. clinical and histological evaluation of cryopreserved heterogenous mesenchymal stem cell-exosomal hydrogel on critical size skin wounds in dogs. Int J Hematol Oncol Stem Cell Resactions. IJHOSCR 2021, 15, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Saadeldin, I.M. Exosomes as a potential tool for supporting canine oocyte development. Animals 2020, 10, 1971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, H.; Li, D.; Yuan, M.; Li, Q.; Li, N.; Wang, G. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathwaydagger. Biol. Reprod. 2019, 100, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Asante, A.; Taylor, R.N. Endometriosis: The role of neuroangiogenesis. Annu. Rev. Physiol. 2011, 73, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-derived exosomes and metabolic regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.Y.; Sun, J.J.; Ye, R.S.; Cheng, X.; Sun, R.P.; Wie, L.M.; Li, M.; Lin, D.L.; Jiang, Q.Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, W.J.; Li, Q.; Ryu, M.O.; Nam, A.; An, J.H.; Jung, Y.C.; Ahn, J.O.; Youn, H.Y. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res. Vet. Sci. 2019, 125, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Liang, X.L.; Zheng, K.; Ge, G.R.; Chen, X.; Xu, Y.Z.; Bai, J.; Pan, G.; Geng, D. Horizon of exosome-mediated bone tissue regeneration: The all-rounder role in biomaterial engineering. Mater. Today Bio. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xie, L.; Xiao, P.; Chen, X.; Kong, W.; Lou, Q.; Chen, F.; Lu, X. Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury. Mol. Cell. Biochem. 2022, 477, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.V.; Troyer, R.M.; Ramsey, S.A.; Leeper, H.; Yang, L.; Maier, C.S.; Goodall, C.P.; Ruby, C.E.; Albarqi, H.A.; Taratula, O. A preliminary proteomic investigation of circulating exosomes and discovery of biomarkers associated with the progression of osteosarcoma in a clinical model of spontaneous disease. Transl. Oncol. 2018, 11, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Di Loria, A.; Dattilo, V.; Santoro, D.; Guccione, J.; De Luca, A.; Ciaramella, P.; Pirozzi, M.; Iaccino, E. Expression of serum exosomal mirna122 and lipoprotein levels in dogs naturally infected by Leishmania infantum: A preliminary study. Animals 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Chokeshaiusaha, K.; Sananmuang, T.; Puthier, D.; Nguyen, C. A novel cross-species differential tumor classification method based on exosome-derived microRNA biomarkers established by human-dog lymphoid and mammary tumor cell lines’ transcription profiles. Vet. World 2022, 15, 1163. [Google Scholar] [CrossRef] [PubMed]
- Ju-Hyun, A.N.; Li, Q.; Ok Ryu, M.; Ryung Nam, A.; Ha Bhang, D.; Jung, Y.C.; Song, W.J.; Youn, H.Y. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS ONE 2020, 15, e0220756. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death. Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.K.; Sponder, G.; Sandhu, M.A.; Trappe, S.; Kolisek, M.; Aschenbach, J.R. The combined influence of magnesium and insulin on central metabolic functions and expression of genes involved in magnesium homeostasis of cultured bovine adipocytes. Int. J. Mol. Sci. 2021, 22, 5897. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Saba, E.; Yousaf, A.; Tareen, W.A.; Sarfraz, A.; Rhee, M.H.; Sandhu, M.A. Autologous platelet lysate is an alternative to fetal bovine serum for canine adipose-derived mesenchymal stem cell culture and differentiation. Animals 2023, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sun, R.; Origuchi, M.; Kanehira, M.; Takahata, T.; Itoh, J.; Umezawa, A.; Kijima, H.; Fukuda, S.; Saijo, Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol. Med. 2011, 17, 579–587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassem, M. Mesenchymal stem cells: Biological characteristics and potential clinical applications. Cloning Stem Cells 2004, 6, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Muthu, S.; Mankuzhy, P.D.; Pawde, A.M.; Chandra, V.; Lorenzo, J.M.; Dhama, K.; Taru Sharma, A.G. Cell-free therapy for canine osteoarthritis: Current evidence and prospects. Vet. Q. 2022, 42, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, D.; Bresolin, N.; Comi, G.P.; Corti, S. Extracellular vesicles and amyotrophic lateral sclerosis: From misfolded protein vehicles to promising clinical biomarkers. Cell Mol. Life Sci. 2021, 78, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ananbeh, H.; Novak, J.; Juhas, S.; Juhasova, J.; Klempir, J.; Doleckova, K.; Rysankova, I.; Turnovcova, K.; Hanus, J.; Hansikova, H. Huntingtin co-isolates with small extracellular vesicles from blood plasma of tghd and ki-hd pig models of huntington’s disease and human blood plasma. Int. J. Mol. Sci. 2022, 23, 5598. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhao, Z.; He, C.; Li, J.; Li, X.; Lu, M. Removing the stumbling block of exosome applications in clinical and translational medicine: Expand production and improve accuracy. Stem Cell Res. Ther. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Morsy, M.A.; Alkhodair, K.M.; Alhojaily, S. Mesenchymal stem cell-derived extracellular vesicles: An emerging diagnostic and therapeutic biomolecules for neurodegenerative disabilities. Biomolecules 2023, 13, 1250. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Krockenberger, M.; Bennett, P. Review of exosomes and their potential in veterinary medicine. Res. Vet. Sci. 2024, 168, 105141. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Disadvantages | |
|---|---|---|
| Mesenchymal stromal cells (MSCs) |
| |
| Exosomes |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba, E.; Sandhu, M.A.; Pelagalli, A. Canine Mesenchymal Stromal Cell Exosomes: State-of-the-Art Characterization, Functional Analysis and Applications in Various Diseases. Vet. Sci. 2024, 11, 187. https://doi.org/10.3390/vetsci11050187
Saba E, Sandhu MA, Pelagalli A. Canine Mesenchymal Stromal Cell Exosomes: State-of-the-Art Characterization, Functional Analysis and Applications in Various Diseases. Veterinary Sciences. 2024; 11(5):187. https://doi.org/10.3390/vetsci11050187
Chicago/Turabian StyleSaba, Evelyn, Mansur Abdullah Sandhu, and Alessandra Pelagalli. 2024. "Canine Mesenchymal Stromal Cell Exosomes: State-of-the-Art Characterization, Functional Analysis and Applications in Various Diseases" Veterinary Sciences 11, no. 5: 187. https://doi.org/10.3390/vetsci11050187







