Sequence Instability in the Proviral Long Terminal Repeat and gag Regions from Peripheral Blood and Tissue-Derived Leukocytes of FIV-Infected Cats during the Late Asymptomatic Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Procurement
2.2. Viral Transcription Status in Peripheral Blood over Time
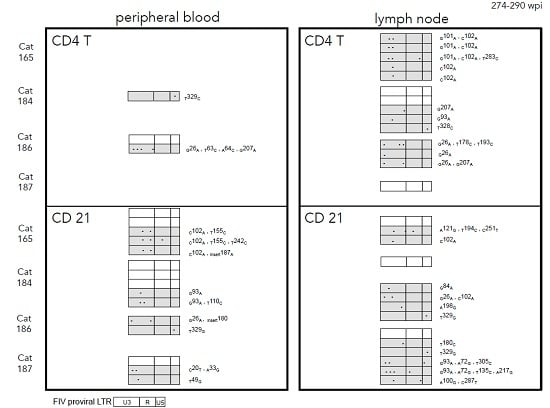
2.3. Sequence Instability of the Proviral LTR and gag Isolated from PBMC and PLN-Derived Leukocytes
2.4. Promoter Functionality Assays
3. Results
3.1. Viral Transcription Status in Peripheral Blood over Time
3.2. Sequence Stability of the Proviral LTR and gag Isolated from PBMC and PLN-Derived Leukocytes
3.3. Promoter Functionality Assays
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Sparger, E.; Ho, E.W.; Andersen, P.R.; O’Connor, T.P.; Mandell, C.P.; Lowenstine, L.; Munn, R.; Pedersen, N.C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 1988, 49, 1246–1258. [Google Scholar] [PubMed]
- McDonnel, S.J.; Sparger, E.E.; Murphy, B.G. Feline immunodeficiency virus latency. Retrovirology 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Vapniarsky, N.; Hillman, C.; Castillo, D. FIV establishes a latent infection in feline peripheral blood CD4+ T lymphocytes in vivo during the asymptomatic phase of infection. Retrovirology 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- McDonnel, S.J.; Sparger, E.E.; Luciw, P.A.; Murphy, B.G. Transcriptional regulation of latent feline immunodeficiency virus in peripheral CD4+ T-lymphocytes. Viruses 2012, 4, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Hillman, C.; McDonnel, S. Peripheral immunophenotype and viral promoter variants during the asymptomatic phase of feline immunodeficiency virus infection. Virus Res. 2014, 179, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Mutation rates and intrinsic fidelity of retroviral reverse transcriptases. Viruses 2009, 1, 1137–1165. [Google Scholar] [CrossRef] [PubMed]
- Svarovskaia, E.S.; Cheslock, S.R.; Zhang, W.-H.; Hu, W.-S.; Pathak, V.K. Retroviral mutation rates and reverse transcriptase fidelity. Front. Biosci. 2003, 8, d117–d134. [Google Scholar] [PubMed]
- Meyerhans, A.; Jung, A.; Maier, R.; Vartanian, J.-P.; Bocharov, G.; Wain-Hobson, S. The non-clonal and transitory nature of HIV in vivo. Swiss Med. Wkly. 2003, 133, 451–454. [Google Scholar] [PubMed]
- Diehl, L.J.; Mathiason-Dubard, C.K.; O’Niel, L.L.; Obert, L.A.; Hoover, E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase viral passage. J. Virol. 1995, 69, 6149–6157. [Google Scholar] [PubMed]
- Eckstrand, C.; Hillman, C.; Smith, A.; Sparger, E.; Murphy, B. Viral reservoirs in lymph nodes of FIV-infected progressor and long-term non-progressor cats during the asymptomatic phase. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Sharmeen, L.; Kimpton, J.; Romeo, J.M.; Garcia, J.V.; Peterlin, B.M.; Groudine, M.; Emerman, M. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 1994, 91, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Hillman, C.; Castillo, D.; Vapniarsky, N.; Rowe, J. The presence or absence of the gamma-activated site determines IFN gamma-mediated transcriptional activation in CAEV promoters cloned from the mammary gland and joint synovium of a single CAEV-infected goat. Virus Res. 2012, 163, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.T.; Mustafa, F.; Schmidt, R.D.; Lew, K.A.; Rizvi, T.A. Sequences within the gag gene of feline immunodeficiency virus (FIV) are important for efficient RNA encapsidation. Virus Res. 2003, 93, 199–209. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.S.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.; Reubel, G. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 1996, 70, 5165–5169. [Google Scholar] [PubMed]
- Report, B.; Park, R.; Health, P.; Service, I.; Animal, F.; Diagnostic, D. Nucleotide sequences of Australian isolates of the feline immunodeficiency virus: Comparison with other feline lentiviruses. Arch. Virol. 1993, 132, 369–379. [Google Scholar]
- Queen, S.E.; Mears, B.M.; Kelly, K.M.; Dorsey, J.L.; Liao, Z.; Dinoso, J.B.; Gama, L.; Adams, R.J.; Zink, M.C.; Clements, J.E.; et al. Replication-competent simian immunodeficiency virus (SIV) gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J. Virol. 2011, 85, 9167–9175. [Google Scholar] [CrossRef] [PubMed]
- Nikolaitchik, O.; Rhodes, T.D.; Ott, D.; Hu, W. Effects of mutations in the human immunodeficiency virus type 1 gag gene on RNA packaging and recombination. Society 2006, 80, 4691–4697. [Google Scholar] [CrossRef] [PubMed]







| Cat # | PBMC gag cDNA | Short R Copies |
|---|---|---|
| 165 | BLD | 8.4 × 105 ± 3.6 × 105 |
| 184 | BLD | 1.31 × 106 ± 3.9 × 105 |
| 186 | BLD | 1.69 × 107 ± 4.8 × 106 |
| 187 | BLD | 2.44 × 107 ± 4.3 × 106 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstrand, C.D.; Hillman, C.; Murphy, B.G. Sequence Instability in the Proviral Long Terminal Repeat and gag Regions from Peripheral Blood and Tissue-Derived Leukocytes of FIV-Infected Cats during the Late Asymptomatic Phase. Vet. Sci. 2016, 3, 10. https://doi.org/10.3390/vetsci3020010
Eckstrand CD, Hillman C, Murphy BG. Sequence Instability in the Proviral Long Terminal Repeat and gag Regions from Peripheral Blood and Tissue-Derived Leukocytes of FIV-Infected Cats during the Late Asymptomatic Phase. Veterinary Sciences. 2016; 3(2):10. https://doi.org/10.3390/vetsci3020010
Chicago/Turabian StyleEckstrand, Christina D., Chadwick Hillman, and Brian G. Murphy. 2016. "Sequence Instability in the Proviral Long Terminal Repeat and gag Regions from Peripheral Blood and Tissue-Derived Leukocytes of FIV-Infected Cats during the Late Asymptomatic Phase" Veterinary Sciences 3, no. 2: 10. https://doi.org/10.3390/vetsci3020010
APA StyleEckstrand, C. D., Hillman, C., & Murphy, B. G. (2016). Sequence Instability in the Proviral Long Terminal Repeat and gag Regions from Peripheral Blood and Tissue-Derived Leukocytes of FIV-Infected Cats during the Late Asymptomatic Phase. Veterinary Sciences, 3(2), 10. https://doi.org/10.3390/vetsci3020010