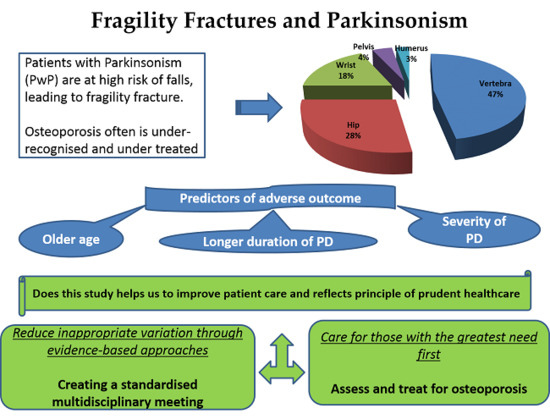
Fragility Fractures and Parkinsonism: Relationship of Fractures with Demography, Severity and Predictors of Adverse Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
2.3. Data and Statistical Analysis
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclosure
References
- Contreras, A.; Grandas, F. Risk of falls in Parkinson’s disease: A cross-sectional study of 160 patients. Park. Dis. 2012, 2012, 362572. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.; Walker, R. Emergency hospital admissions in idiopathic Parkinson’s disease. Mov. Disord. 2005, 20, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent falls in Parkinson’s disease: A systematic review. Park. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.J.W.; Wenn, R.T.; Sahota, O.; Moran, CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: Prospective observational cohort study. BMJ 2005, 331, 1374. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.; Beck, S.; Hood, K.; Johansen, A. Using dietetic assistants to improve the outcome of hip fracture: A randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing 2006, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lyell, V.; Henderson, E.; Devine, M.; Gregson, C. Assessment and management of fracture risk in patients with Parkinson’s disease. Age Ageing 2015, 44, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Kuskowski, M.A.; Taylor, B.C.; Schousboe, J.T.; Orwoll, E.S.; Ensrud, K.E.; Osteoporotic Fractures in Men (MrOS) Study Group. Association of Parkinson’s disease with accelerated bone loss, fractures and mortality in older men: The Osteoporotic Fractures in Men (MrOS) study. Osteoporos. Int. 2008, 19, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Genever, R.W.; Downes, T.W.; Medcalf, P. Fracture rates in Parkinson’s disease compared with age and gender-matched controls: A retrospective cohort study. Age Ageing 2005, 34, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Torsney, K.M.; Torsney, K.M.; Noyce, A.J.; Doherty, K.M.; Bestwick, J.P.; Dobson, R.; Lees, A.J. Bone health in Parkinson’s disease: A systemic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kaji, M.; Tsuru, T.; Oizumi, K. Risk factors for hip fracture among elderly patients with Parkinson’s disease. J. Neurol. Sci. 2001, 182, 89–93. [Google Scholar] [CrossRef]
- Dennison, E.M.; Compston, J.E.; Flahive, J.; Siris, E.S.; Gehlbach, S.H.; Adachi, J.D.; Boonen, S.; Chapurlat, R.; Díez-Pérez, A.; Anderson, F.A.; et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 2012, 50, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Honda, Y.; Iwamoto, J. Risedronate and ergocalciferol prevent hip fracture in elderly men with Parkinson disease. Neurology 2007, 68, 911–915. [Google Scholar] [PubMed]
- Fink, H.A.; Kuskowski, M.A.; Orwoll, E.S.; Cauley, J.A.; Ensurd, K.E. Association between Parkinson’s disease and low bone density and falls in older men: The osteoporotic fractures in men study. J. Am. Geriatr. Soc. 2005, 53, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.; Walker, R. Osteoporosis in Parkinson’s disease. Mov. Dis. 2005, 20, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Vaserman, N. Parkinson’s disease and osteoporosis. Jt. Bone Spine 2005, 72, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Bazelier, M.T.; Boer, A.; Weber, W.E.; Neef, C.; Cooper, C.; de Vries, F. Risk of fracture in patients with Parkinson’s disease. Osteoporos. Int. 2013, 24, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Derivation and validation of updated QFracture algorithm to predict the risk of osteoporotic fracture in primary care in the United Kingdom: A prospective open cohort study. BMJ 2012, 344, e3427. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.; Yahr, M. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1966, 14, 317–335. [Google Scholar] [CrossRef]
- Masud, T.; Morris, R. Epidemiology of falls. Age Aging 2001, 30, 3–7. [Google Scholar] [CrossRef]
- Singh, I. Approach to falls in the young, middle aged, and the elderly. In Differential Diagnosis in Neurology; Prabhakar, S., Singh, G., Eds.; Jaypee Brothers Medical Publishers: New Delhi, India, 2015; pp. 281–286. [Google Scholar]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.; Glatt, S.; Vetere-Overfield, B.; Hassanein, R. Falls and Parkinson’s disease. Clin. Neuropharmacol. 1989, 2, 98–105. [Google Scholar]
- Wielinski, C.L.; Erickson-Davis, C.; Wichmann, R.; Walde-Douglas, M.; Parashos, S.A. Falls and injuries resulting from falls among patients with Parkinson’s disease and other Parkinsonian syndromes. Mov. Disord. 2005, 20, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Bezza, A.; Ouzzif, Z.; Naji, H.; Achemlal, L.; Mounach, A.; Nouijai, M.; Bourazza, A.; Mossadeq, R.; El Maghraoui, A. Prevalence and risk factors of osteoporosis in patients with Parkinson’s disease. Rheumatol. Int. 2008, 28, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Carda, S.; Viscontini, G.S.; Cisari, C. Osteoporosis in Parkinson’s disease. Park. Relat. Disord. 2009, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Cheng, P.Y.; Wu, S.L.; Lai, C.H. Parkinson’s disease and risk of hip fracture: An 8-year follow-up study in Taiwan. Park. Relat. Disord. 2012, 18, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Gallacher, J.; Davis, K.; Johansen, A.; Eeles, E.; Hubbard, R.E. Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing 2012, 41, 242–246. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Centre for Metabolic Bone Diseases, FRAX®. Available online: https://www.shef.ac.uk/FRAX/tool.jsp/ (accessed on 15 February2017).
- ClinRisk. QFracture®—2012 risk calculator. ClinRisk Ltd., 2012. Available online: http://www.qfracture.org/ (accessed on 15 February 2017).
- Singh, I.; Fletcher, R.; Scanlon, L.; Tyler, M.; Aithal, S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual. Improv. Rep. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
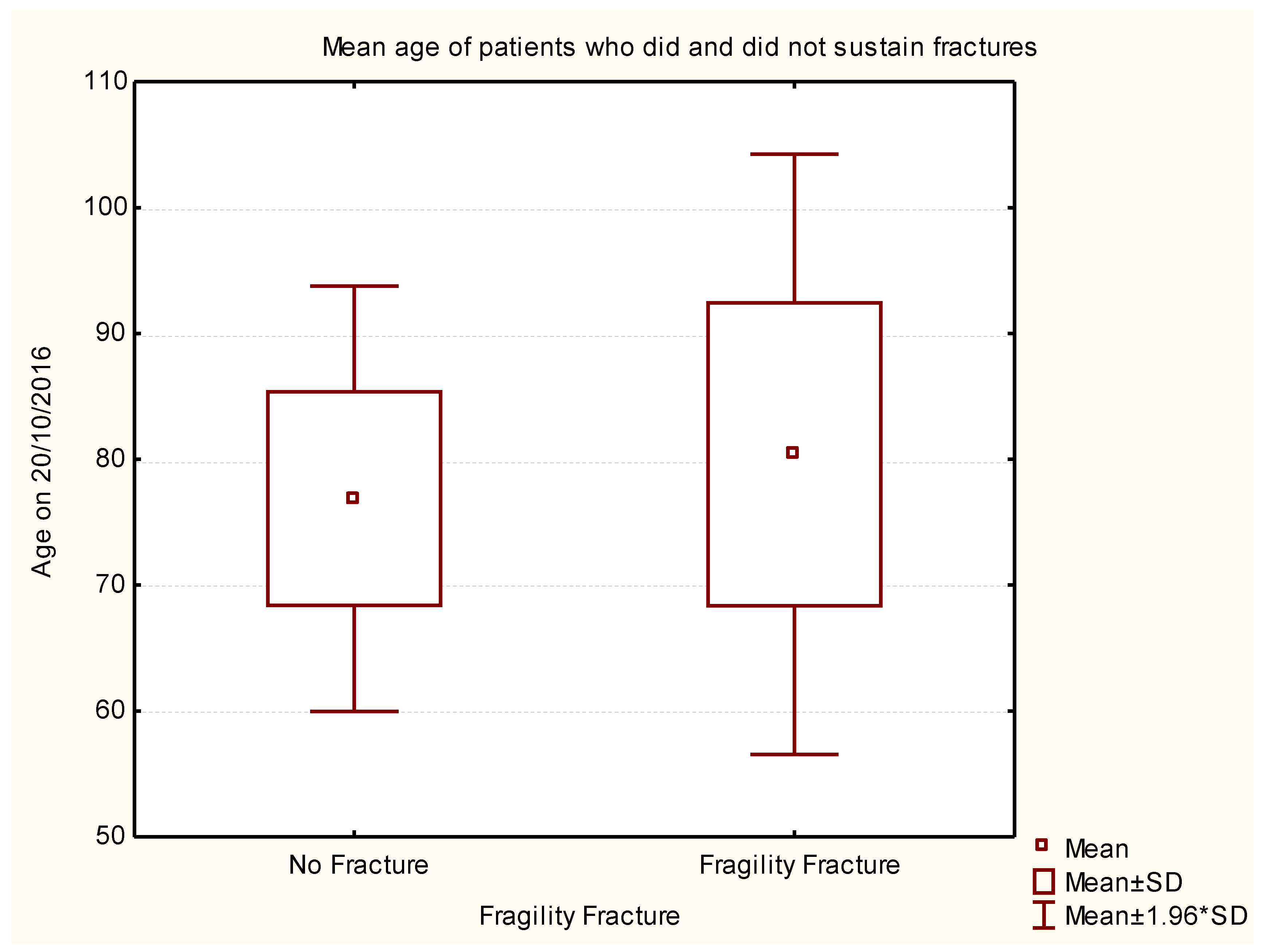
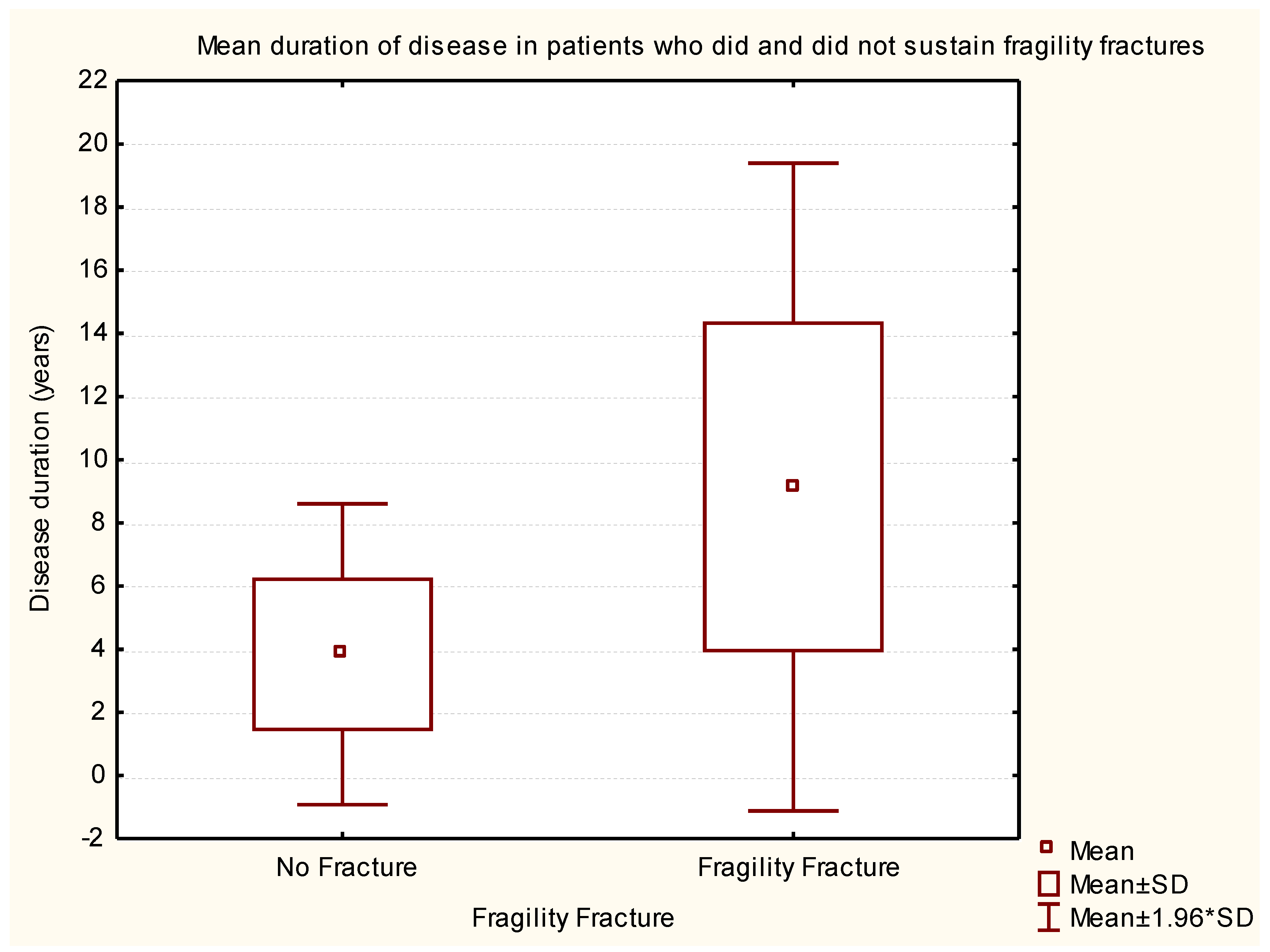
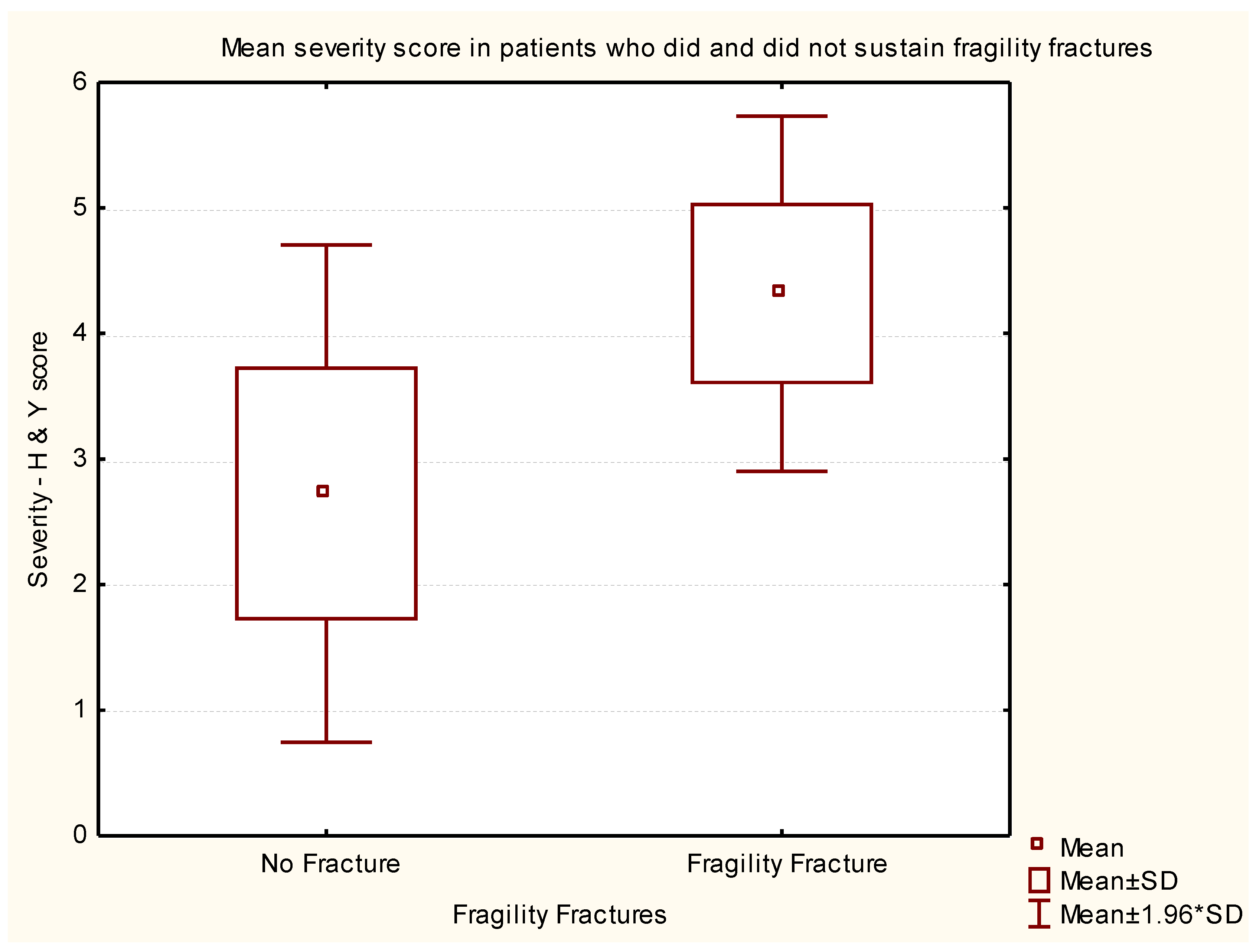
| Rank | Variable 1 | Variable 2 | Variable 3 | Degrees of Freedom | Likelihood - Score | p |
|---|---|---|---|---|---|---|
| 1 | Age on 20/10/2016 | Disease duration (years) | H & Y Score @ Study | 3 | 131.8525 | 0.000 |
| 2 | Disease duration (years) | H & Y Score @ Study | 2 | 131.3147 | 0.000 | |
| 3 | Age on 20/10/2016 | Disease duration (years) | 2 | 98.9471 | 0.000 | |
| 4 | Disease duration (years) | 1 | 98.8608 | 0.000 | ||
| 5 | Age on 20/10/2016 | H & Y Score @ Study | 2 | 94.5216 | 0.000 | |
| 6 | H & Y Score @ Study | 1 | 94.5209 | 0.000 | ||
| 7 | Age on 20/10/2016 | 1 | 6.5888 | 0.010262 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aithal, S.; Sequeira, R.; Edwards, C.; Singh, I. Fragility Fractures and Parkinsonism: Relationship of Fractures with Demography, Severity and Predictors of Adverse Outcomes. Geriatrics 2017, 2, 17. https://doi.org/10.3390/geriatrics2020017
Aithal S, Sequeira R, Edwards C, Singh I. Fragility Fractures and Parkinsonism: Relationship of Fractures with Demography, Severity and Predictors of Adverse Outcomes. Geriatrics. 2017; 2(2):17. https://doi.org/10.3390/geriatrics2020017
Chicago/Turabian StyleAithal, Shridhar, Ruford Sequeira, Chris Edwards, and Inderpal Singh. 2017. "Fragility Fractures and Parkinsonism: Relationship of Fractures with Demography, Severity and Predictors of Adverse Outcomes" Geriatrics 2, no. 2: 17. https://doi.org/10.3390/geriatrics2020017