Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges
Abstract
:1. Introduction
2. Intricacies and Clinical Impact of MDR Sepsis in Geriatric ICU Patients
3. Epidemiology and Healthcare Costs of Sepsis in Geriatric Patients
4. Factors Contributing to the Rise of MDR in Sepsis in Geriatric ICU Patients
5. Common Pathogens Involved in MDR Sepsis
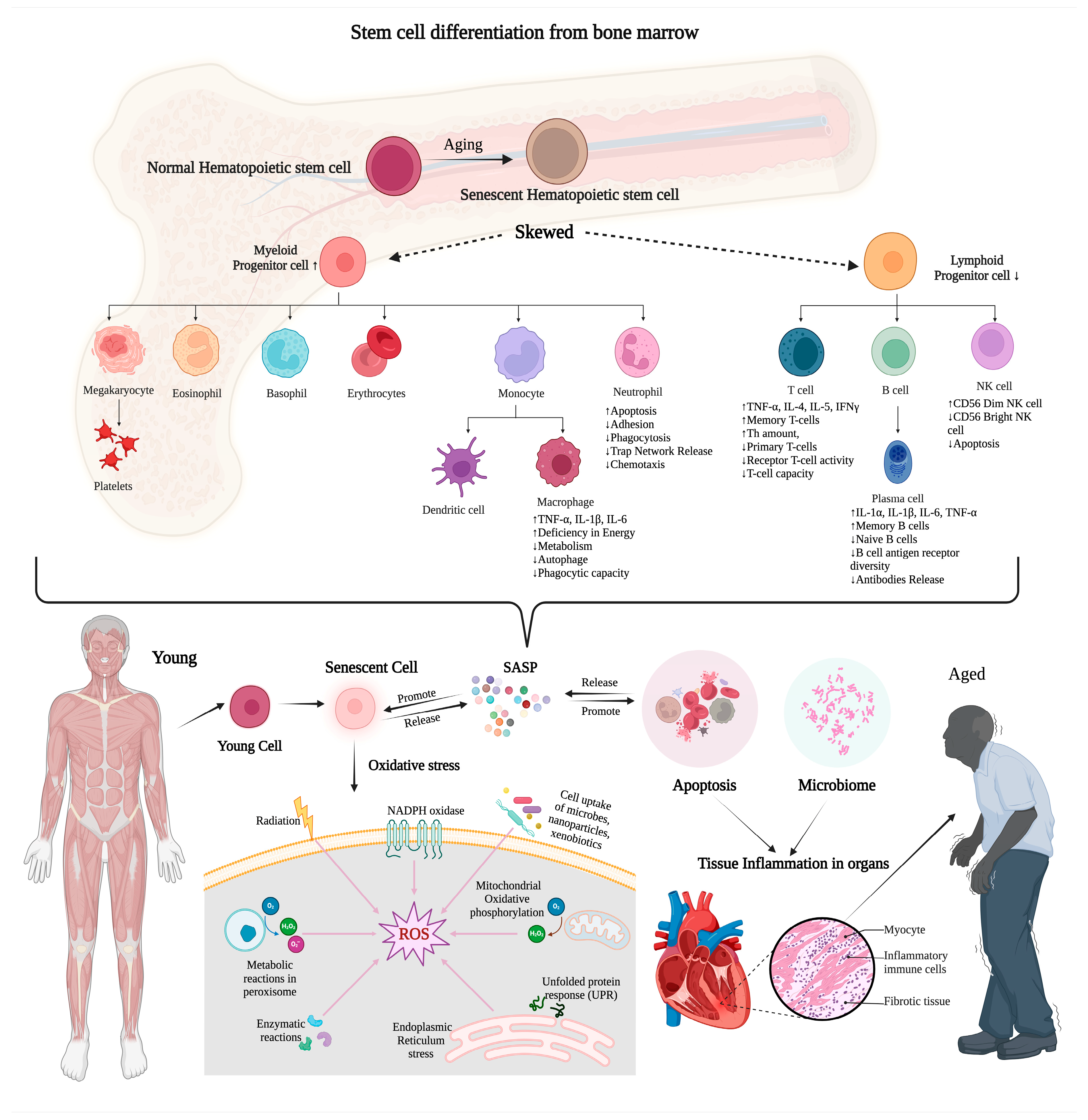
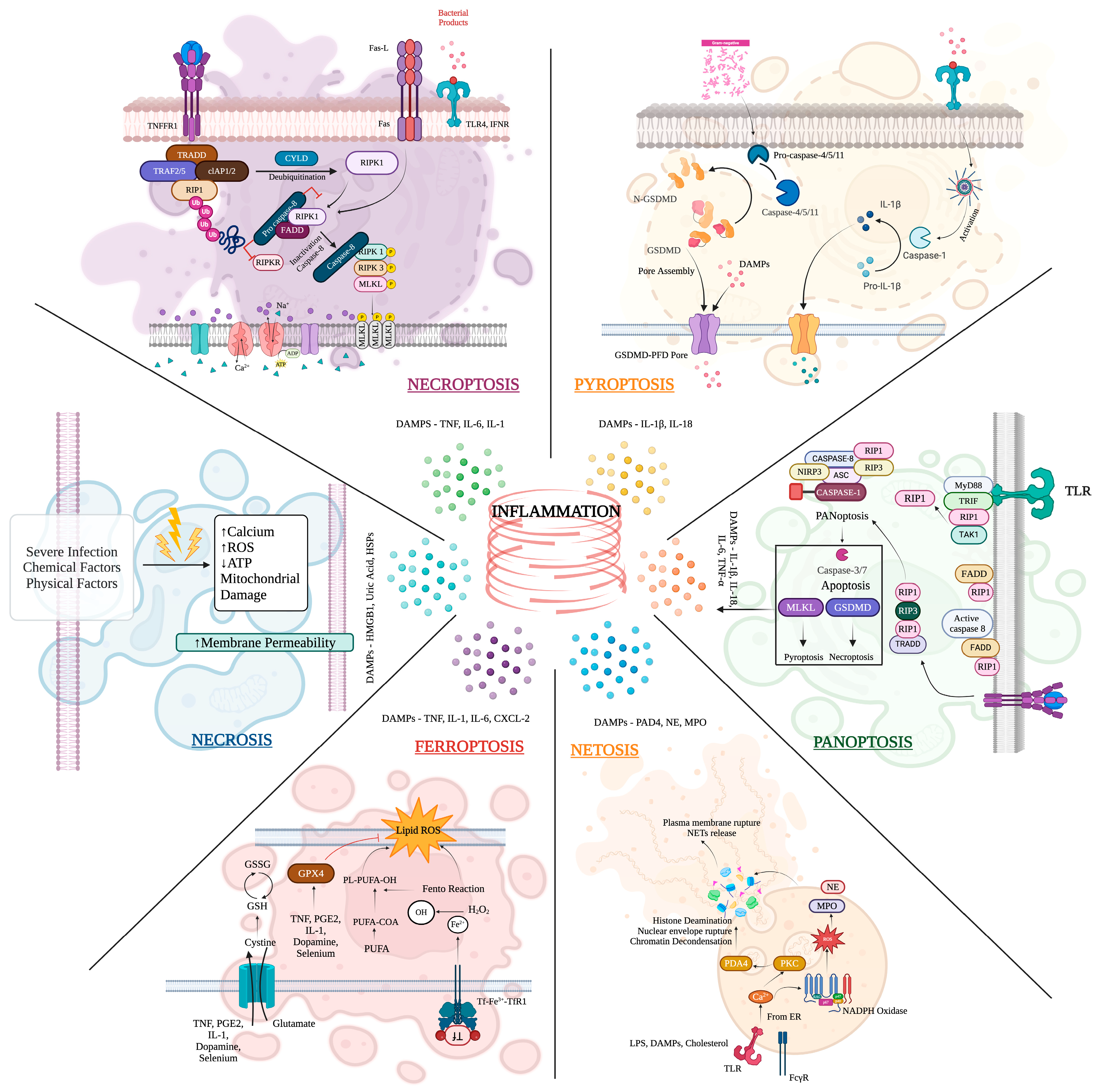
6. Mechanism of Inflammation on MDR Sepsis and Aging in Critical Care
7. Diagnostic Challenges and Innovations of MDR Sepsis in Geriatric Patients
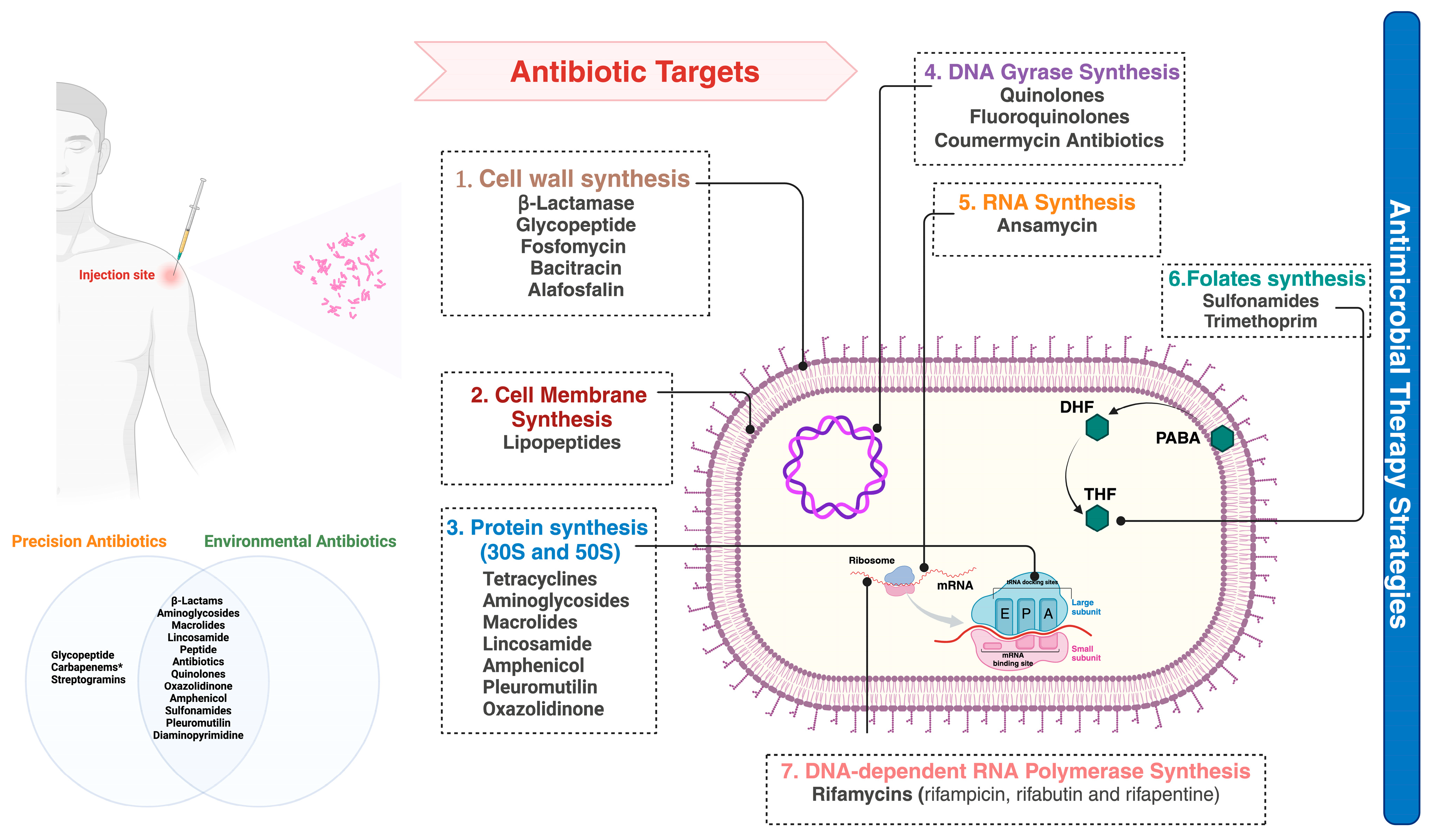
8. Clinical Management of MDR Sepsis in Geriatric ICU Patients
9. Preventive Measures and Infection Control Strategies Impacting MDR Sepsis in Aging
10. Future Directions in Research and Therapeutics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant |
| HAIs | Hospital-acquired infections |
| EPIC II | Extended Prevalence of Infection in Intensive Care II |
| SASP | Senescence-associated secretory phenotype |
| PABA | Paraaminobenzoic acid |
| DHF | Two precursors of folic acid, dihydrofolic acid |
| THF | Tetrahydrofolic acid |
| pK | Pharmacokinetics |
| pD | Pharmacodynamics |
| ABC | ATP-binding cassette |
| MATE | Multidrug and toxic compound extrusion |
| MFS | Major facilitator superfamily |
| RND | Resistance nodulation and cell division |
| SMR | Small multidrug resistance |
| POCT | Point-of-care testing |
| SARS | Severe acute respiratory syndrome-related coronavirus |
| MERS | Middle East respiratory syndrome |
| VAP | Ventilator-associated pneumonia |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| ICU | Intensive care unit |
| WHO | World Health Organization |
| ESBLs | Extended spectrum-β-lactamases |
| COVID-19 | Coronavirus disease 2019 |
| AMR | Antimicrobial resistance |
| ESKAPE | Enterococcus faecium, S. aureus, Acinetobacter baumannii, Klebsiella pneumonia, Enterobacter species, and Pseudomonas. Aeruginosa |
| HAP | Hospital-acquired pneumonia |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPs | Danger-associated molecular patterns |
| DNA | Deoxyribonucleic |
| RNA | Ribonucleic acid |
| HMGB1 | High-mobility group box-1 protein |
| HSPs | Heat shock proteins |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| APCs | Antigen-presenting cells |
| PPR | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| IFNs | Interferons |
| NF-κB | Nuclear factor-κB |
| IRF | Interferon regulatory factor |
| TNF | Tumor necrosis factor |
| IL | Interleukins |
| IFNγ | interferon-gamma |
| PCT | Procalcitonin |
| CRP | C-reactive protein |
| WBC | White blood cells |
| SIRS | Systemic inflammatory response syndrome |
| ASP | Antimicrobial Stewardship Program |
| SER | Surface-enhanced Raman spectroscopy |
| MALDI-TOFMS | Matrix-assisted laser desorption ionization time-of-flight mass spectrometry |
| PCR | Polymerase chain reaction |
| NGS | Next-generation sequencing |
| Tɑ1 | Thymosin ɑ1 |
| UTI | Urinary tract infection |
References
- Detweiler, K.; Mayers, D.; Fletcher, S.G. Bacteruria and Urinary Tract Infections in the Elderly. Urol. Clin. N. Am. 2015, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E. High antimicrobial resistant rates among gram-negative pathogens in intensive care units a retrospective study at a tertiary care hospital in southwest saudi arabia. Saudi Med. J. 2018, 39, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Nguyen, N.T.Q.; Hughes, C.M.; O’neill, C. Trends and impact of antimicrobial resistance on older inpatients with urinary tract infections (UTIs): A national retrospective observational study. PLoS ONE 2019, 14, e0223409. [Google Scholar] [CrossRef]
- McConville, T.H.; Sullivan, S.B.; Gomez-Simmonds, A.; Whittier, S.; Uhlemann, A.-C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS ONE 2017, 12, e0186195. [Google Scholar] [CrossRef] [PubMed]
- Dat, V.Q.; Vu, H.N.; The, H.N.; Nguyen, H.T.; Hoang, L.B.; Viet, D.V.T.; Bui, C.L.; Van Nguyen, K.; Nguyen, T.V.; Trinh, D.T.; et al. Bacterial bloodstream infections in a tertiary infectious diseases hospital in Northern Vietnam: Aetiology, drug resistance, and treatment outcome. BMC Infect. Dis. 2017, 17, 493. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.-E.; Nicolau, D.P.; Postma, M.J.; et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, B.; Cherubini, A.; Lucarelli, M.; Espinosa, E.; Prospero, E. Multidrug-resistant bacterial infections in geriatric hospitalized patients before and after the COVID-19 outbreak: Results from a retrospective observational study in two geriatric wards. Antibiotics 2021, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Athari, F.; Hillman, K.M.; Frost, S.A. The changing nature of the population of intensive-care patients. J. Hosp. Adm. 2018, 7, 1. [Google Scholar] [CrossRef]
- Zykova, S.N.; Jenssen, T.G.; Berdal, M.; Olsen, R.; Myklebust, R.; Seljelid, R. Altered Cytokine and Nitric Oxide Secretion In Vitro by Macrophages from Diabetic Type II-Like db/db Mice. 2000. Available online: http://diabetesjournals.org/diabetes/article-pdf/49/9/1451/365255/10969828.pdf (accessed on 4 October 2023).
- Sepsis Alliance. Sepsis and Aging. Available online: https://www.sepsis.org/sepsisand/aging/ (accessed on 4 October 2023).
- Chonchol, M. Hematology: Issues in the Dialysis Patient Neutrophil Dysfunction and Infection Risk in End-Stage Renal Disease; Blackwell Publishing Inc.: Malden, MA, USA, 2006. [Google Scholar]
- Kim, B.H.; Lee, S.; Yoo, B.; Lee, W.Y.; Lim, Y.; Kim, M.-C.; Yon, J.H.; Kim, K.-M. Risk factors associated with outcomes of hip fracture surgery in elderly patients. Korean J. Anesthesiol. 2015, 68, 561–567. [Google Scholar] [CrossRef]
- Ranjit, S.; Kissoon, N. Challenges and Solutions in translating sepsis guidelines into practice in resource-limited settings. Transl. Pediatr. 2021, 10, 2646–2665. [Google Scholar] [CrossRef]
- Chou, C.-H.; Lee, J.-T.; Lin, C.-C.; Sung, Y.-F.; Lin, C.-C.; Muo, C.-H.; Yang, F.-C.; Wen, C.-P.; Wang, I.-K.; Kao, C.-H.; et al. Septicemia Is Associated with Increased Risk for Dementia: A Population-Based Longitudinal Study. Available online: www.impactjournals.com/oncotarget (accessed on 4 October 2023).
- Rowe, T.; Araujo, K.L.B.; Van Ness, P.H.; Pisani, M.A.; Juthani-Mehta, M. Outcomes of older adults with sepsis at admission to an intensive care unit. Open Forum Infect. Dis. 2016, 3, ofw010. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Flevari, A.; Theodorakopoulou, M.; Velegraki, A.; Armaganidis, A. Treatment of invasive candidiasis in the elderly: A review. Clin. Interv. Aging 2013, 8, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Yaluri, N.; Yaluri, A.S.; Žeňuch, P.; Žeňuchová, Z.; Tóth, Š.; Kalanin, P. Cardiac Biomarkers and Their Role in Identifying Increased Risk of Cardiovascular Complications in COVID-19 Patients. Diagnostics 2023, 13, 2508. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Evangelou, K.; Vasileiou, P.V.S.; Vasileiou, P.V.S.; Papaspyropoulos, A.; Papaspyropoulos, A.; Hazapis, O.; Hazapis, O.; Petty, R.; Petty, R.; et al. Cellular senescence and cardiovascular diseases: Moving to the “heart” of the problem. Physiol. Rev. 2023, 103, 609–647. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Ding, H.; Wang, Y.; Xie, Y.; Zhang, X. Cellular Senescence in Cardiovascular Diseases: From Pathogenesis to Therapeutic Challenges. J. Cardiovasc. Dev. Dis. 2023, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, S.M.; Shen, Y.; Wyse, R.J.; Dunne, J.R.; Berg, G.M.; Garland, J.M.; Ludwig, A.D.; Shillinglaw, W.C.D.; Hightower, T.D.B.; Hunt, D.L.; et al. Incidence, outcomes and costs of severe sepsis and septic shock in geriatric trauma patients: Analysis of 2,563,463 hospitalizations at 3284 hospitals. J. Trauma Inj. Infect. Crit. Care 2023, 95, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Koulenti, D.; Blot, S.; Sakr, Y.; Anzueto, A.; Spies, C.; Solé-Violán, J.; Kett, D.; Armaganidis, A.; Martin, C.; et al. Critically Ill elderly adults with infection: Analysis of the extended prevalence of infection in intensive care study. J. Am. Geriatr. Soc. 2013, 61, 2065–2071. [Google Scholar] [CrossRef]
- Chiu, C.; Legrand, M. Epidemiology of Sepsis and Septic Shock. Curr. Opin. Anaesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L.; et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- WHO. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Weng, L.; Xu, Y.; Yin, P.; Wang, Y.; Chen, Y.; Liu, W.; Li, S.; Peng, J.-M.; Dong, R.; Hu, X.-Y.; et al. National incidence and mortality of hospitalized sepsis in China. Crit. Care 2023, 27, 1–12. [Google Scholar] [CrossRef]
- Torio, C.M.; Moore, B.J. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016. [Google Scholar]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef]
- Rubens, M.; Saxena, A.; Ramamoorthy, V.; Das, S.; Khera, R.; Hong, J.; Armaignac, D.; Veledar, E.; Nasir, K.; Gidel, L. Increasing Sepsis Rates in the United States: Results From National Inpatient Sample, 2005 to 2014. J. Intensive Care Med. 2020, 35, 858–868. [Google Scholar] [CrossRef]
- Garg, P.; Krishak, R.; Shukla, D.K. NICU in a Community Level Hospital. Indian J. Pediatr. 2005, 72, 27–30. [Google Scholar] [CrossRef]
- M. Publications PvtLtd., ‘IJCCM_April_08.indd’. Available online: https://www.ijccm.org/journalDetails/IJCCM (accessed on 10 October 2023).
- Farrah, K.; McIntyre, L.; Doig, C.J.; Talarico, R.; Taljaard, M.; Krahn, M.; Fergusson, D.; Forster, A.J.; Coyle, D.; Thavorn, K. Sepsis-associated Mortality, Resource Use, and Healthcare Costs: A Propensity-Matched Cohort Study. Crit. Care Med. 2021, 49, 215–227. [Google Scholar] [CrossRef]
- Oami, T.; Imaeda, T.; Nakada, T.; Abe, T.; Takahashi, N.; Yamao, Y.; Nakagawa, S.; Ogura, H.; Shime, N.; Umemura, Y.; et al. Temporal trends of medical cost and cost-effectiveness in sepsis patients: A Japanese nationwide medical claims database. J. Intensive Care 2022, 10, 33. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Alrahmany, D.; Omar, A.F.; Alreesi, A.; Harb, G.; Ghazi, I.M. Acinetobacter baumannii Infection-Related Mortality in Hospitalized Patients: Risk Factors and Potential Targets for Clinical and Antimicrobial Stewardship Interventions. Antibiotics 2022, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Vakkalanka, J.P.; Harland, K.K.; Swanson, M.B.; Mohr, N.M. Clinical and epidemiological variability in severe sepsis: An ecological study. J. Epidemiol. Community Health 2018, 72, 741–745. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, A.; Cortés, J.A. Bacterial and atypical infections in critically ill cancer patients. In Oncologic Critical Care; Nates Joseph, K.J., Price, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1379–1400. [Google Scholar] [CrossRef]
- Di Franco, S.; Alfieri, A.; Pace, M.C.; Sansone, P.; Pota, V.; Fittipaldi, C.; Fiore, M.; Passavanti, M.B. Blood stream infections from mdr bacteria. Life 2021, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Retamar, P.; López-Prieto, M.D.; Rodríguez-López, F.; de Cueto, M.; García, M.V.; González-Galan, V.; del Arco, A.; Pérez-Santos, M.J.; Téllez-Pérez, F.; Becerril-Carral, B.; et al. Predictors of early mortality in very elderly patients with bacteremia: A prospective multicenter cohort. Int. J. Infect. Dis. 2014, 26, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Juneja, D.; Nasa, P.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 2012, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.S.; Varghese, D. Geriatric Evaluation and Treatment of Age-Related Cognitive Decline. [Updated 2023 Sep 28]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580536/ (accessed on 10 October 2023).
- Macias, J.; Kahly, O.; Pattik-Edward, R.; Khan, S.; Qureshi, A.; Shaik, A.; Shala, A.; Shah, D. Sepsis: A Systematic Review of Antibiotic Resistance and Antimicrobial Therapies. Mod. Res. Inflamm. 2022, 11, 9–23. [Google Scholar] [CrossRef]
- Genga, K.R.; Russell, J.A. Update of Sepsis in the Intensive Care Unit. J. Innate Immun. 2017, 9, 441–455. [Google Scholar] [CrossRef]
- Lin, G.-L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Dünser, M.W.; Festic, E.; Dondorp, A.; Kissoon, N.; Ganbat, T.; Kwizera, A.; Haniffa, R.; Baker, T.; Schultz, M.J.; Global Intensive Care Working Group of the European Society of Intensive Care Medicine. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012, 38, 557–574. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Klompas, M.; Branson, R.; Eichenwald, E.C.; Greene, L.R.; Howell, M.D.; Lee, G.; Magill, S.S.; Maragakis, L.L.; Priebe, G.P.; Speck, K.; et al. Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals: 2014 Update. Infect. Control. Hosp. Epidemiol. 2014, 35, 915–936. [Google Scholar] [CrossRef]
- Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics 2024, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Vallet, H.; Guidet, B.; Boumendil, A.; De Lange, D.W.; Leaver, S.; Szczeklik, W.; Jung, C.; Sviri, S.; Beil, M.; Flaatten, H. The impact of age-related syndromes on ICU process and outcomes in very old patients. Ann. Intensive Care 2023, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Weinberger, B. T cells, aging and senescence. Exp. Gerontol. 2020, 134, 110887. [Google Scholar] [CrossRef]
- Flaatten, H.; De Lange, D.W.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Boumendil, A.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥80 years). Intensive Care Med. 2017, 43, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Flaatten, H.; Jung, C.; Vallet, H.; Guidet, B. How Does Frailty Affect ICU Outcome? Curr. Anesthesiol. Rep. 2019, 9, 144–150. [Google Scholar] [CrossRef]
- De Biasio, J.C.; Mittel, A.M.; Mueller, A.L.; Ferrante, L.E.; Kim, D.H.; Shaefi, S. Frailty in Critical Care Medicine: A Review. Anesth. Analg. 2020, 130, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, E.; Zambon, A.; Mazzola, P.; Massariello, F.; Galeazzi, M.; D’oro, L.C.; Bonfanti, P.; Bellelli, G. Assessing the mortality risk in older patients hospitalized with a diagnosis of sepsis: The role of frailty and acute organ dysfunction. Aging Clin. Exp. Res. 2022, 34, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Darvall, J.N.; Bellomo, R.; Bailey, M.; Young, P.J.; Rockwood, K.; Pilcher, D. Impact of frailty on persistent critical illness: A population-based cohort study. Intensive Care Med. 2022, 48, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Chen, D.; Xie, X.-H.; Zhang, J.-E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- A Bengoechea, J.; Pessoa, J.S. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2018, 43, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef]
- Sierawska, O.; Małkowska, P.; Taskin, C.; Hrynkiewicz, R.; Mertowska, P.; Grywalska, E.; Korzeniowski, T.; Torres, K.; Surowiecka, A.; Niedźwiedzka-Rystwej, P.; et al. Innate Immune System Response to Burn Damage—Focus on Cytokine Alteration. Int. J. Mol. Sci. 2022, 23, 716. [Google Scholar] [CrossRef]
- Daehn, M.; Sperr, W.; Brune, K.; Graham, M. Senior Care, 2nd ed.; Lulu Press, Inc.: Morrisville, NC, USA, 2010; p. 236. [Google Scholar]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Lalosevic, M.S.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef]
- Chockalingam, A.; Singh, A.; Kathirvel, S. Chapter 12—Healthy aging and quality of life of the elderly. In Principles and Application of Evidence-Based Public Health Practice; Kathirvel, S., Singh, A., Chockalingam, A., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 187–211. [Google Scholar] [CrossRef]
- Iskander, K.N.; Osuchowski, M.F.; Stearns-Kurosawa, D.J.; Kurosawa, S.; Stepien, D.; Valentine, C.; Remick, D.G. Sepsis: Multiple Abnormalities, Heteroge-neous Responses, and Evolving Understanding. Physiol. Rev. 2013, 93, 1247–1288. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Berselli, A.; Fogagnolo, A.; Capuzzo, M.; Ragazzi, R.; Marangoni, E.; Bertacchini, S.; Volta, C.A. Evaluation of a protocol for vancomycin administration in critically patients with and without kidney dysfunction. BMC Anesthesiol. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens. Bioelectron. 2018, 124–125, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Stanwix, P.L.; May, E.F.; Aman, Z.M. Surface-Enhanced Raman Scattering Imaging of Cetylpyridinium Chloride Adsorption to a Solid Surface. Anal. Chem. 2022, 94, 14169–14176. [Google Scholar] [CrossRef]
- Dizaji, A.N.; Ozek, N.S.; Aysin, F.; Calis, A.; Yilmaz, A.; Yilmaz, M. Combining vancomycin-modified gold nanorod arrays and colloidal nanoparticles as a sandwich model for the discrimination of Gram-positive bacteria and their detection via surface-enhanced Raman spectroscopy (SERS). Analyst 2021, 146, 3642–3653. [Google Scholar] [CrossRef]
- Leonard, H.; Colodner, R.; Halachmi, S.; Segal, E. Recent Advances in the Race to Design a Rapid Diagnostic Test for Antimicrobial Resistance. ACS Sens. 2018, 3, 2202–2217. [Google Scholar] [CrossRef] [PubMed]
- Sturaro, L.L.; Gonoi, T.; Busso-Lopes, A.F.; Tararam, C.A.; Levy, C.E.; Lyra, L.; Trabasso, P.; Schreiber, A.Z.; Kamei, K.; Moretti, M.L. Visible DNA Microarray System as an Adjunctive Molecular Test in Identification of Pathogenic Fungi Directly from a Blood Culture Bottle. J. Clin. Microbiol. 2018, 56, e01908-17. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Fornai, M.; Pacher, P.; Lee, H.T.; Haskó, G. P2X4 receptors, immunity, and sepsis. Curr. Opin. Pharmacol. 2019, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Gesten, F.C.; Phillips, G.S.; Terry, K.M.; Seymour, C.W.; Prescott, H.C.; Friedrich, M.; Iwashyna, T.J.; Osborn, T.; Lemeshow, S. Mortality Changes Associated with Mandated Public Reporting for Sepsis. The Results of the New York State Initiative. Am. J. Respir. Crit. Care Med. 2018, 198, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.T.; Akova, M. The Role of Colistin in the Era of New β-Lactam/β-Lactamase Inhibitor Combinations. Antibiotics 2022, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Dünser, M.W.; Schultz, M.J. (Eds.) Sepsis Management in Resource-Limited Settings. [Internet]; Springer: Cham, Switzerland, 2019. [CrossRef]
- Marques, A.; Torre, C.; Pinto, R.; Sepodes, B.; Rocha, J. Treatment Advances in Sepsis and Septic Shock: Modulating Pro- and Anti-Inflammatory Mechanisms. J. Clin. Med. 2023, 12, 2892. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, E.; D’angerio, M.; Ciobanu, A.L.; Masini, L.; Tartaro, D.L.; Coloretti, I.; Busani, S.; Rubio, I.; Meschiari, M.; Franceschini, E.; et al. Advances and Challenges in Sepsis Management: Modern Tools and Future Directions. Cells 2024, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4, 1–71. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef] [PubMed]
- A Cusumano, J.; Klinker, K.P.; Huttner, A.; Luther, M.K.; A Roberts, J.; LaPlante, K.L. Towards precision medicine: Therapeutic drug monitoring–guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity. Am. J. Health-Syst. Pharm. 2020, 77, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, E. Emerging Infectious Diseases. In Practical Healthcare Epidemiology, 4th ed.; Cambridge University Press: Cambridge, UK, 2019; Volume 25, p. 2161. [Google Scholar] [CrossRef]
- Shukla, P.; Rao, G.M.; Pandey, G.; Sharma, S.; Mittapelly, N.; Shegokar, R.; Mishra, P.R. Therapeutic interventions in sepsis: Current and anticipated pharmacological agents. Br. J. Pharmacol. 2014, 171, 5011–5031. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Lai, C.; Teboul, J.-L. How I personalize fluid therapy in septic shock? Crit. Care 2023, 27, 123. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; McGuire, W.C.; Malhotra, A. Prone Positioning in Acute Respiratory Distress Syndrome. NEJM Evid. 2022, 1, EVIDra2100046. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESGAP (ESCMID Study Group for Antimicrobial stewardshiP). What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- Majumder, A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Hilaire, M.G.-S. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Huang, J.; Cui, C.; Zhou, S.; Chen, M.; Wu, H.; Jin, R.; Chen, X. Impact of multicenter unified enhanced environmental cleaning and disinfection measures on nosocomial infections among patients in intensive care units. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Ierodiakonou, D.; Spanias, C.; Mathioudaki, A.; Ioannou, P.; Petrakis, E.C.; Kofteridis, D.P. Doctors’ perceptions, attitudes and practices towards the management of multidrug-resistant organism infections after the implementation of an antimicrobial stewardship programme during the COVID-19 pandemic. Trop. Med. Infect. Dis. 2021, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, G.; Dore, F.; Langer, M.; Garbero, E.; Alagna, L.; Bianchin, A.; Ciceri, R.; Di Paolo, A.; Giani, T.; Giugni, A.; et al. Effectiveness of a Multifaced Antibiotic Stewardship Program: A Pre-Post Study in Seven Italian ICUs. J. Clin. Med. 2022, 11, 4409. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.; Moran, E.; Collyer, B.; McCarthy, N.D.; Green, C.; Keeling, M.J. Informing antimicrobial stewardship with explainable AI. PLoS Digit. Health 2023, 2, e0000162. [Google Scholar] [CrossRef] [PubMed]
- Ya, K.Z.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, E2253806. [Google Scholar] [CrossRef]
- Ibarz, M.; Haas, L.E.M.; Ceccato, A.; Artigas, A. The critically ill older patient with sepsis: A narrative review. Ann. Intensive Care 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Coopersmith, C.M.; De Backer, D.; Deutschman, C.S.; Ferrer, R.; Lat, I.; Machado, F.R.; Martin, G.S.; Martin-Loeches, I.; Nunnally, M.E.; Antonelli, M.; et al. Surviving sepsis campaign: Research priorities for sepsis and septic shock. Intensive Care Med. 2018, 44, 1400–1426. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Hassan-Smith, Z.K.; Greig, C.A.; Lord, J.M.; Jackson, T.A. Acute Sarcopenia Secondary to Hospitalisation—An Emerging Condition Affecting Older Adults. Aging Dis. 2018, 9, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Payne, D.J. Reaction: Alternative Modalities to Address Antibiotic-Resistant Pathogens. Catalysis 2017, 3, 369–372. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Cohen, J. Non-antibiotic strategies for sepsis. Clin. Microbiol. Infect. 2009, 15, 302–307. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis ve septik şok: Mevcut tedavi stratejileri ve yeni yaklaşımlar. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Livigni, S.; Bertolini, G.; Rossi, C.; Ferrari, F.; Giardino, M.; Pozzato, M.; Remuzzi, G. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: A multicenter randomised controlled clinical trial. BMJ Open 2014, 4, 3536. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Borysowski, J. The potential of phage therapy in sepsis. Front. Immunol. 2017, 8, 1783. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic antibodies for infectious disease. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef] [PubMed]

| Aspect | Sepsis | Aging | Interaction |
|---|---|---|---|
| Acute inflammatory response | Triggers dysregulated systemic inflammation | Chronic low-grade inflammation | Exaggerated acute response due to pre-existing chronic inflammation |
| Excessive release of pro-inflammatory cytokines (e.g., IL-6, TNF-α) | Increased production of pro-inflammatory cytokines | Amplified tissue damage and organ dysfunction | |
| Chronic inflammatory response | Leads to persistent inflammation post-recovery | Presence of high inflammation | Chronic inflammation post-sepsis may exacerbate inflammatory response |
| Prolonged immune dysregulation | Immunosenescence (decline in immune function) | Increased susceptibility to recurrent infections | |
| Associated with long-term complications | Contributes to chronic diseases | Higher risk of functional decline and recurrent infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.R.; Balraj, T.A.; Shivashankar, K.K.; Jayaram, T.C.; Prashant, A. Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges. Geriatrics 2024, 9, 45. https://doi.org/10.3390/geriatrics9020045
Kumar NR, Balraj TA, Shivashankar KK, Jayaram TC, Prashant A. Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges. Geriatrics. 2024; 9(2):45. https://doi.org/10.3390/geriatrics9020045
Chicago/Turabian StyleKumar, Nishitha R., Tejashree A. Balraj, Kusuma K. Shivashankar, Tejaswini C. Jayaram, and Akila Prashant. 2024. "Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges" Geriatrics 9, no. 2: 45. https://doi.org/10.3390/geriatrics9020045







