Triglycerides to High-Density Lipoprotein Cholesterol Ratio Predicts Chronic Renal Disease in Patients without Diabetes Mellitus (STELLA Study)
Abstract
:1. Highlight
2. Introduction
3. Materials and Methods
3.1. Subjects
3.2. Biochemical Measurements
3.3. Definitions
4. Data Analysis
5. Results
5.1. Correlations
5.2. Comparisons
5.3. Categorical Associations
5.4. Adjusted Models
6. Discussion
7. Limitations
8. Conclusions
Author Contributions
Funding
Ethical Approval
Informed consent
Conflicts of Interest
References
- Reiss, A.B.; Voloshyna, I.; De Leon, J.; Miyawaki, N.; Mattana, J. Cholesterol Metabolism in CKD. Am. J. Kidney Dis. 2015, 66, 1071–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C.O. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, W.F.; Tomassini, J.E.; Neff, D.R. Lipid abnormalities in patients with chronic kidney disease: Implications for the pathophysiology of atherosclerosis. J. Atheroscler. Thromb. 2012, 20, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntner, P.; Coresh, J.; Smith, J.C.; Eckfeldt, J.; Klag, M.J. Plasma lipids and risk of developing renal dysfunction: The Atherosclerosis Risk in Communities Study. Kidney Int. 2000, 58, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Bae, J.C.; Han, J.M.; Kwon, S.; Jee, J.H.; Yu, T.Y.; Lee, M.K.; Kim, J.H. LDL-C/apoB and HDL-C/apoA-1 ratios predict incident chronic kidney disease in a large apparently healthy cohort. Atherosclerosis 2016, 251, 170–176. [Google Scholar] [CrossRef]
- Vaziri, N.D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 2015, 12, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, A.J.; Lyons, T.J.; Zheng, D.; Otvos, J.D.; Lackland, D.T.; McGee, D.; Garvey, W.T.; Klein, R.L. The DCCT/EDIC Research Group Lipoproteins in the DCCT/EDIC cohort: Associations with diabetic nephropathy. Kidney Int. 2003, 64, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Cerezo, I.; Fernández, N.; Romero, B.; Fernández-Carbonero, E.; Hernández-Gallego, R.; Caravaca, F. Prognostic value of apolipoproteins A and B in the clinical course of patients with chronic kidney disease previous to dialysis. Nefrología 2009, 29, 540–547. [Google Scholar]
- Goek, O.-N.; Köttgen, A.; Hoogeveen, R.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol. Dial. Transplant. 2012, 27, 2839–2847. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, J.P.; Gansevoort, R.T.; Bakker, S.J.; Sparks, C.E.; Vart, P.; Dullaart, R.P. Apolipoprotein B Attenuates Albuminuria-Associated Cardiovascular Disease in Prevention of Renal and Vascular Endstage Disease (PREVEND) Participants. J. Am. Soc. Nephrol. 2014, 25, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Yang, W.; Akkina, S.; Alper, A.; Anderson, A.H.; Appel, L.J.; He, J.; Raj, M.S.; Schelling, J.; Strauss, L.; et al. Relation of Serum Lipids and Lipoproteins with Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2014, 9, 1190–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjadj, S.; Duly-Bouhanick, B.; Bekherraz, A.; Bridoux, F.; Gallois, Y.; Mauco, G.; Ebran, J.; Marre, M. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab. 2004, 30, 43–51. [Google Scholar] [CrossRef]
- Chawla, V.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Sarnak, M.J.; Menon, V. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1582–1587. [Google Scholar] [CrossRef] [Green Version]
- Lamprea-Montealegre, J.A.; Staplin, N.; Herrington, W.G.; Haynes, R.; Emberson, J.; Baigent, C.; De Boer, I.H.; SHARP Collaborative Group. Apolipoprotein B, Triglyceride-Rich Lipoproteins, and Risk of Cardiovascular Events in Persons with CKD. Clin. J. Am. Soc. Nephrol. 2019, 15, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. BMI classification. In Global Database on Body Mass Index: An Interactive Surveillance Tool for Monitoring Nutrition Transition; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- International Diabetes Federation; Alberti, G.; Zimmet, P.; Shaw, J.; Grundy, S.M. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006. [Google Scholar]
- Olsen, M.; Greve, S.; Blicher, M.; Kruger, R.; Sehestedt, T.; Rasmussen, S.; Vishram, J.; Boutouyrie, P.; Laurent, S. OS 14-08 Estimated Carotid-Femoral Pulse Wave Velocity Has Similar Predictive Value as Measured Carotid-Femoral Pulse Wave Velocity. J. Hypertens. 2016, 34, e214. [Google Scholar] [CrossRef]
- Reference Values for Arterial Stiffness’ Collaboration; The Reference Values for Arterial Stiffness’ Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Hear. J. 2010, 31, 2338–2350. [CrossRef]
- Stevens, P.E. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Hsu, H.-J.; Hsu, C.-K.; Lee, C.-C.; Hsu, K.-H.; Sun, C.-Y.; Chen, C.-Y.; Chen, Y.-C.; Yu, Y.-C.; Wu, I.-W. Correlation between spot and 24 h proteinuria: Derivation and validation of equation to estimate daily proteinuria. PLoS ONE 2019, 14, e0214614. [Google Scholar] [CrossRef]
- Chonchol, M.; Gnahn, H.; Sander, D. Impact of subclinical carotid atherosclerosis on incident chronic kidney disease in the elderly. Nephrol. Dial. Transplant. 2008, 23, 2593–2598. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-K.; Yang, C.-Y.; Tsai, C.-T.; Chiu, F.-C.; Huang, Y.-T.; Lee, J.-K.; Cheng, C.-L.; Lin, L.-Y.; Lin, J.-W.; Hwang, J.-J.; et al. Association of low glomerular filtration rate and albuminuria with peripheral arterial disease: The National Health and Nutrition Examination Survey, 1999–2004. Atheroscler. 2010, 209, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Xie, D.; Cedillo-Couvert, E.; Charleston, J.; Chen, J.; Deo, R.; Feldman, H.I.; Go, A.S.; He, J.; Horwitz, E.; et al. Lipids, Apolipoproteins, and Risk of Atherosclerotic Cardiovascular Disease in Persons With CKD. Am. J. Kidney Dis. 2019, 73, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, M.H.; Ballantyne, C.M.; Jacobson, T.A.; Bittner, V.; Braun, L.T.; Brown, A.S.; Brown, W.; Cromwell, W.; Goldberg, R.B.; McKenney, J.M.; et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J. Clin. Lipidol. 2011, 5, 338–367. [Google Scholar] [CrossRef]
- McQueen, M.J.; Hawken, S.; Wang, X.; Ounpuu, S.; Sniderman, A.; Probstfield, J.; Steyn, K.; Sanderson, J.E.; Hasani, M.; Volkova, E.; et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet 2008, 372, 224–233. [Google Scholar] [CrossRef]
- Sieber, J.; Lindenmeyer, M.T.; Kampe, K.; Campbell, K.N.; Cohen, C.D.; Hopfer, H.; Mundel, P.; Jehle, A.W. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Physiol. 2010, 299, F821–F829. [Google Scholar] [CrossRef] [Green Version]
- Joles, J.A.; Kunter, U.; Janssen, U.; Kriz, W.; Rabelink, T.J.; Koomans, H.A.; Floege, J. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J. Am. Soc. Nephrol. 2000, 11, 669–683. [Google Scholar]
- Enkhmaa, B.; Anuurad, E.; Zhang, Z.; Pearson, T.A.; Berglund, L. Usefulness of Apolipoprotein B/Apolipoprotein A-I Ratio to Predict Coronary Artery Disease Independent of the Metabolic Syndrome in African Americans. Am. J. Cardiol. 2010, 106, 1264–1269. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, R.; Dodesini, A.R.; Lepore, G. Lipids and Renal Disease. J. Am. Soc. Nephrol. 2006, 17, S145–S147. [Google Scholar] [CrossRef]
- Kronenberg, F. Dyslipidemia and nephrotic syndrome: Recent advances. J. Ren. Nutr. 2005, 15, 195–203. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- González-Chávez, A.; Simental-Mendía, L.E.; Elizondo-Argueta, S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cirugía y Cirujanos 2011, 79, 126–131. [Google Scholar] [PubMed]
- Wang, X.; Chen, H.; Shao, X.; Xiong, C.; Hong, G.; Chen, J.; Li, X.; You, X.; Gao, P.; Chen, Y.; et al. Association of Lipid Parameters with the Risk of Chronic Kidney Disease: A Longitudinal Study Based on Populations in Southern China. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, M.; Barazzoni, R.; Guarnieri, G. Inflammation and Insulin Resistance in Uremia. J. Ren. Nutr. 2008, 18, 70–75. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Mean/Median | SD/Interquartile Range |
|---|---|---|
| Age (years) | 67.3 | 15.6 |
| BMI (Kg/m2) | 28 | 26–31 |
| WC (cm) | 101 | 95–110 |
| LDL-C (mg/dL) | 113.9 | 26.05 |
| TG/HDL-C | 3.37 | 1.9 |
| SBP (mmHg) | 146 | 130–155 |
| DBP (mmHg) | 80 | 75–90 |
| MBP (mmHg) | 107.6 | 99.2–112 |
| ePWV (m/s) | 11.77 | 2.7 |
| PP (mmHg) | 63.14 | 16.03 |
| UACR (mg/gr) | 28.7 | 11.2–125 |
| eGFR (ml/min/1.73 m2) | 52.2 | 22.4 |
| Category Variables | n (%) | |
| Gender (males/females) | 97 (53%)/86 (47%) | |
| Hypertension (yes/no) | 137(74.9%)/46(25.1%) | |
| Smoking (yes/no) | 33 (18%)/150 (82%) | |
| Anti-hypertensive medications (yes/no)- beta-blockers - calcium channel blockers - inhibitors of angiotensin II AT1 receptors | 137(74.9%)/46(25.1%) | |
| Primary renal disease | ||
| - hypertensive nephrosclerosis | 115 (62.8%) | |
| - interstitial nephritis | 31 (16.9%) | |
| - polycystic nephropathy | 4 (2.2%) | |
| - other/unknown | 33 (18%) |
| Variables | TG/HDL-C | |
|---|---|---|
| r | p value | |
| Age (years) | 0.116 | 0.1 |
| BMI (Kg/m2) | 0.344 | 0.001 |
| WC (cm) | 0.302 | 0.001 |
| LDL-C (mg/dL) | 0.306 | 0.001 |
| SBP (mmHg) | 0.311 | 0.001 |
| DBP (mmHg) | 0.145 | 0.05 |
| MBP (mmHg) | 0.325 | 0.001 |
| ePWV (m/s) | 0.177 | 0.01 |
| PP (mmHg) | 0.245 | 0.001 |
| eGFR (ml/min/1.73 m2) | −0.336 | 0.001 |
| UACR (mg/gr) | 0.280 | 0.001 |
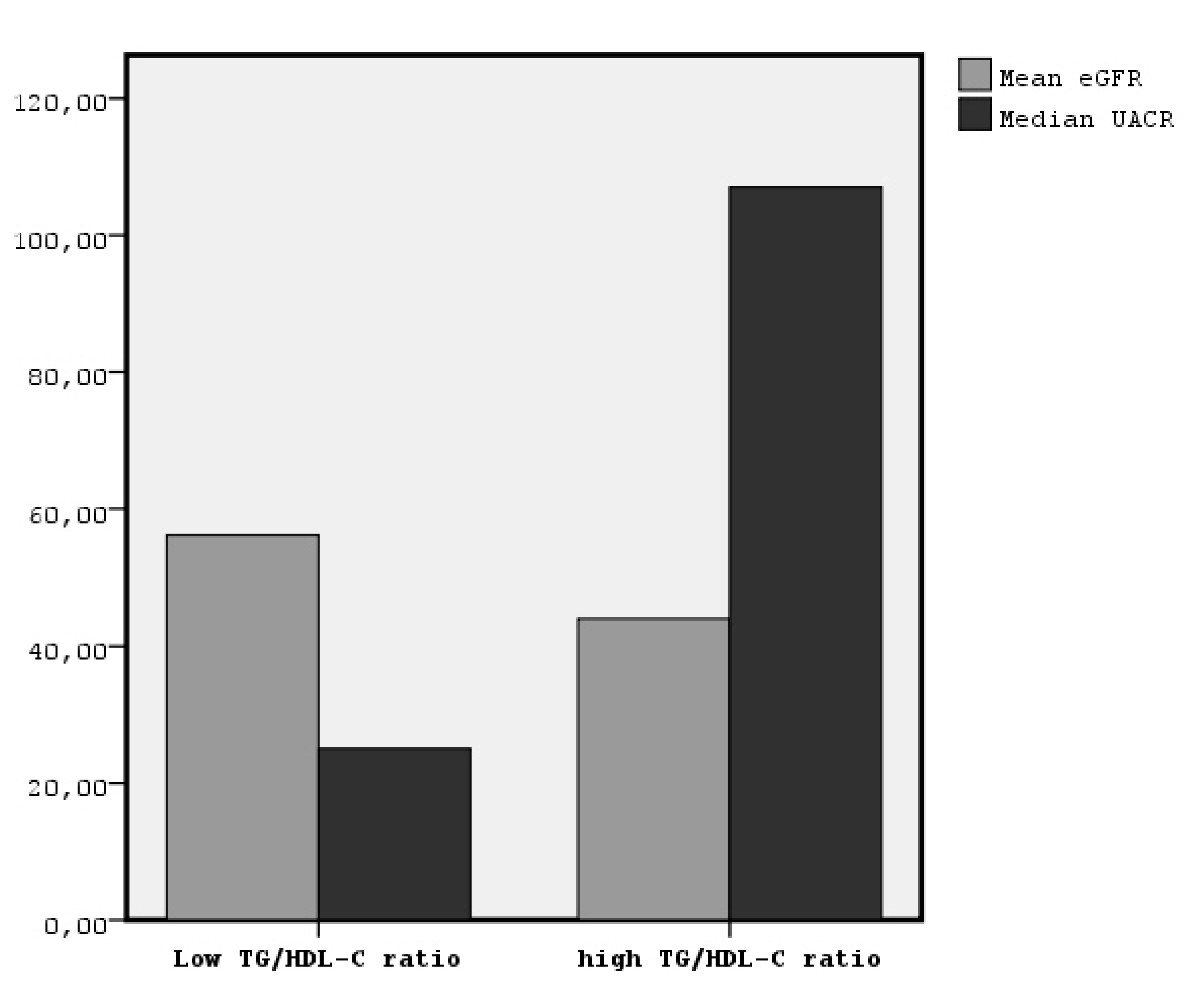
| Characteristic | Patients with TG/HDL-C > 3.41 (n = 81) mean ± SD | Patients with TG/HDL-C < 3.41 (n = 102) mean ± SD | p Value |
|---|---|---|---|
| Age (years) | 70.1 ± 14.8 * | 65.1 ± 15.9 | 0.03 |
| BMI (Kg/m2) | Mean Rank = 108.8 * | 78.7 | 0.001 |
| WC (cm) | Mean Rank = 105.8 * | 81.01 | 0.002 |
| LDL-C (mg/dL) | 119.7 ± 19.2 * | 109.3 ± 29.7 | 0.005 |
| TG/HDL-C | 4.9 ± 1.7 * | 2.1 ± 0.7 | 0.001 |
| SBP (mmHg) | Mean Rank = 107.8 * | 79.4 | 0.001 |
| DBP (mmHg) | Mean Rank = 97.07 | 87.9 | 0.2 |
| MBP (mmHg) | Mean Rank = 107.1 * | 80.0 | 0.001 |
| ePWV (m/s) | 12.3 ± 2.6 * | 11.3 ± 2.7 | 0.006 |
| PP (mmHg) | 67.02 ± 14.3 * | 60.06 ± 16.7 | 0.003 |
| UACR (mg/gr) | Mean Rank = 107.2 * | 80.1 | 0.001 |
| eGFR (ml/min/1.73 m2) | 43.9 ± 20.0 * | 58.8 ± 22.1 | 0.001 |
| Category variables | n (%) | n (%) | |
| Hypertension (yes/no) | 71(51.8%)/10(21.7%) * | 66(48.2%)/36 (78.3%) | 0.001 |
| Smoking (yes/no) | 17 (21%)/64 (79%) * | 16 (15.7%)/86(84.3%) | 0.2 |
| Variables in Model | p-value | Odds Ratio | Confidence Interval |
|---|---|---|---|
| Age (years) | 0.001 | 1.07 | 1.04–1.11 |
| Gender (males/females) | 0.09 | 0.5 | 0.2–1.1 |
| BMI (Kg/m2) | 0.7 | 1.01 | 0.9–1.09 |
| Hypertension (yes/no) | 0.01 | 3.08 | 1.2–7.7 |
| Smoking (yes/no) | 0.2 | 0.6 | 0.2–1.5 |
| TG/HDL-C | 0.001 | 1.5 | 1.2–1.9 |
| Variables in Model | p-value | Odds Ratio | Confidence Interval |
|---|---|---|---|
| Age (years) | 0.002 | 1.03 | 1.01–1.06 |
| Gender (males/females) | 0.4 | 0.7 | 0.4–1.5 |
| BMI (Kg/m2) | 0.9 | 1.002 | 0.9–1.08 |
| Hypertension (yes/no) | 0.08 | 2.1 | 0.9–4.9 |
| Smoking (yes/no) | 0.9 | 1.04 | 0.5–2.4 |
| TG/HDL-C | 0.03 | 1.22 | 1.02–1.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raikou, V.D.; Kyriaki, D.; Gavriil, S. Triglycerides to High-Density Lipoprotein Cholesterol Ratio Predicts Chronic Renal Disease in Patients without Diabetes Mellitus (STELLA Study). J. Cardiovasc. Dev. Dis. 2020, 7, 28. https://doi.org/10.3390/jcdd7030028
Raikou VD, Kyriaki D, Gavriil S. Triglycerides to High-Density Lipoprotein Cholesterol Ratio Predicts Chronic Renal Disease in Patients without Diabetes Mellitus (STELLA Study). Journal of Cardiovascular Development and Disease. 2020; 7(3):28. https://doi.org/10.3390/jcdd7030028
Chicago/Turabian StyleRaikou, Vaia D., Despina Kyriaki, and Sotiris Gavriil. 2020. "Triglycerides to High-Density Lipoprotein Cholesterol Ratio Predicts Chronic Renal Disease in Patients without Diabetes Mellitus (STELLA Study)" Journal of Cardiovascular Development and Disease 7, no. 3: 28. https://doi.org/10.3390/jcdd7030028





