Soft-Tissue Material Properties and Mechanogenetics during Cardiovascular Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Traditional Methods
2.2. Novel Noninvasive Methods
3. Results
3.1. Avian Embryonic Development
3.1.1. Ventricles
| Ref. | Vascular Component | Parameter | Type | Stage | Value | Method | |
|---|---|---|---|---|---|---|---|
| HH | CS | ||||||
| Ventricle Looping | |||||||
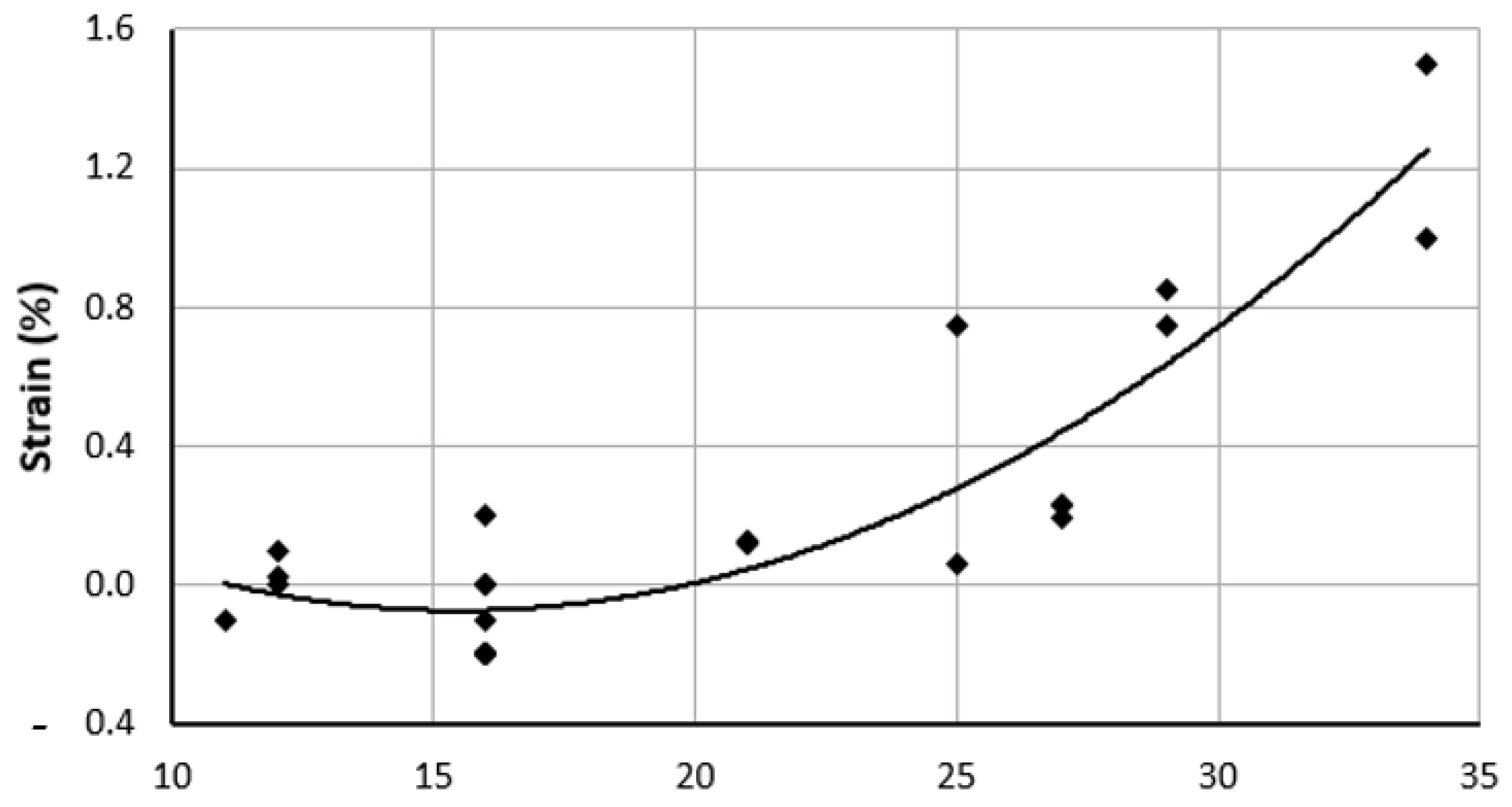
| [43] | ventricle looping | Pressure (kPa) | systolic | 16 | 12.6 | 0.133 | Computational model and cuts |
| diastolic | 0.033 | ||||||
| Stress (kPa) | Cauchy | 2 | |||||
| strain | max | 0 | |||||
| min | −0.2 | ||||||
| bending | −0.2 | ||||||
| Epicardium | |||||||
| [9] | epicardium | strain | max | 16 | 12.6 | 0 | epicardial beads |
| min | −0.2 | ||||||
| bending | −0.2 | ||||||
| stress (kPa) | max | 4 | |||||
| strain | max | −0.1 | |||||
| min | −0.2 | ||||||
| [68] | epicardial | strain | max circ | 11 | 11 | −0.1 | triangular array |
| max inner | 12 | 11 | 0.1 | ||||
| systole bending | 12 | 11 | 0.02 | ||||
| diastole bending | 12 | 11 | 0 | ||||
| Ventricle | |||||||
| [67] | ventricle | strain | max | 16 | 12.6 | 0.2 | MPA |
| [30] | LV | thickness (microns) | 27 | 17.5 | 300 | uniaxial and biaxial testing | |
| 29 | 19 | 400 | |||||
| 31 | 20 | 425 | |||||
| [63] | LV | thickness (microns) | compact layer | 24 | 16 | 30 | Micro-indentation and FEM |
| 29 | 19 | 40 | |||||
| 34 | 21 | 45 | |||||
| RV | 24 | 16 | 40 | ||||
| 29 | 19 | 60 | |||||
| 34 | 21 | 70 | |||||
| [10] | LV | strain | circumferential | 21 | 15 | 0.12 | Beads |
| 27 | 17.5 | 0.23 | |||||
| end diastole | 21 | 15 | 0.12 | ||||
| RV | circumferential | 21 | 15 | 0.13 | |||
| RV | 27 | 17.5 | 0.23 | ||||
| LV | 27 | 17.5 | 0.19 | ||||
| [7] | ventricle | pressure (kPa) | systole max | 21 | 15 | 0.2 | Cuts, theoretical model, and Micro-pressure system |
| diastole max | 21 | 15 | 0.067 | ||||
| [70] | max | 24 | 16 | 0.06 | |||
| [65] | myocardial | circumferential stiffness constant | RV | 27 | 17.5 | 4.3 | Beads |
| LV | 27 | 17.5 | 7.8 | ||||
| [19] | LV | pressure (kPa) | max diastole | 29 | 19 | 0.631 | FEM Servo-pressure |
| max systole | 0.062 | ||||||
| stress (kPa) | von mises | 1 | Cuts | ||||
| strain | von mises | 0.5 | |||||
| [8] | ventricle | stress (kPa) | residual | 12 | 11 | 27.2 | |
| [71] | cardiac jelly | stiffness (N/m) | max | 12 | 11 | 0.00225 | |
| Valve Leaflet | |||||||
| [49] | septal | strain energy density (Pa) | energy density | 25 | 16.5 | 0.3 | FEM |
| mural | 25 | 16.5 | 0.75 | ||||
| septal | 29 | 19 | 0.85 | ||||
| mural | 29 | 19 | 0.75 | ||||
| septal | 34 | 21 | 1.5 | ||||
| mural | 34 | 21 | 1 | ||||
| Atrio Ventricular region | |||||||
| [72] | AV region | modulus (kPa) | effective mod cushion | 17 | 13 | 0.0001 | Micro pipette aspiration |
| 21 | 15 | 0.001 | |||||
| 25 | 16.5 | 0.004 | |||||
| [41] | AV canal | stress (kPa) | Shear min | 24 | 16 | 0.002 | Immunofluorescence |
| Shear min | 28 | 18 | 0.002 | ||||
| Shear min | 30 | 19 | 0.002 | ||||
| Myocardial Wall | |||||||
| [2] | myocardial wall | strain (%) | max | 18 | 13.5 | 70 | Doppler OCT |
| strain rate (1/s) | rate | 5 | |||||
| thickness (mm) | 0.85 | ||||||
| Aorta Dorsal | |||||||
| [4] | aortic (dorsal) | pressure (kPa) | range | 27 | 17.5 | 0–0.180 | LAL and Velocimeter pressure measurement |
| Atrium | |||||||
| [41] | atrium | stress (kPa) | shear min | 24 | 16 | 0.0128 | Stress sensors and Immunofluorescence |
| 28 | 18 | 0.0118 | |||||
| 30 | 0.0128 | ||||||
3.1.2. Heart Valves
3.1.3. Aortic Arch and Vitelline Artery Properties
Geometry
Vascular Function, Pressure-Diameter Loops
Effective Opening Angle and Residual Stress
Stress Distribution and Anisotropy
3.2. Large Animal Models
3.3. Human Embryonic Development
3.4. Small Animal Models
3.5. Mechanogenetic Regulation and Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nye, K.S.; Esplin, M.S.; Monson, K.L. Umbilical cord artery mechanical properties at various gestational ages. Am. J. Perinatol. 2015, 32, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Shi, L.; Yin, X.; Rugonyi, S.; Wang, R.K. Assessment of strain and strain rate in embryonic chick heart in vivo using tissue Doppler optical coherence tomography. Phys. Med. Biol. 2011, 56, 7081–7092. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M.; Rezaee, T.; Hassani, K. Measurement of the circumferential mechanical properties of the umbilical vein: Experimental and numerical analyses. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Lucitti, J.L.; Tobita, K.; Keller, B.B. Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J. Exp. Biol. 2005, 208, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Gjorevski, N.; Nelson, C.M. The mechanics of development: Models and methods for tissue morphogenesis. Birth Defects Res. C Embryo Today 2010, 90, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Forouhar, A.S.; Liebling, M.; Hickerson, A.; Nasiraei-Moghaddam, A.; Tsai, H.J.; Hove, J.R.; Fraser, S.E.; Dickinson, M.E.; Gharib, M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 2006, 312, 751–753. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Taber, L.A.; Clark, E.B. A nonliner poroelastic model for the trabecular embryonic heart. J. Biomech. Eng. 1994, 116, 213–223. [Google Scholar] [CrossRef]
- Yao, J.; Varner, V.D.; Brilli, L.L.; Young, J.M.; Taber, L.A.; Perucchio, R. Viscoelastic material properties of the myocardium and cardiac jelly in the looping chick heart. J. Biomech. Eng. 2012, 134, 024502. [Google Scholar] [CrossRef] [Green Version]
- Taber, L.A.; Keller, B.B.; Clark, E.B. Cardiac mechanics in the stage-16 chick embryo. J. Biomech. Eng. 1992, 114, 427–434. [Google Scholar] [CrossRef]
- Tobita, K.; Keller, B.B. Right and left ventricular wall deformation patterns in normal and left heart hypoplasia chick embryos. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H959–H969. [Google Scholar] [CrossRef]
- Tobita, K.; Garrison, J.B.; Liu, L.J.; Tinney, J.P.; Keller, B.B. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 283, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Donmazov, S.; Piskin, S.; Pekkan, K. Noninvasive in vivo determination of residual strains and stresses. J. Biomech. Eng. 2015, 137, 061011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasyanov, V.; Moreno-Rodriguez, R.A.; Kalejs, M.; Ozolanta, I.; Stradins, P.; Wen, X.; Yao, H.; Mironov, V. Age-related analysis of structural, biochemical and mechanical properties of the porcine mitral heart valve leaflets. Connect. Tissue Res. 2013, 54, 394–402. [Google Scholar] [CrossRef]
- Zhuan, X.; Luo, X. Residual Stress Estimates from Multi-cut Opening Angles of the Left Ventricle. Cardiovasc. Eng. Technol. 2020, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Namazi, S.S.; Yazdani, J.; Sharif Nia, H. Relation Between Aortic Stiffness Index and Distensibility with Age in Hypertensive Patients. Int. J. Gen. Med. 2020, 13, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, T.; Seo, Y.; Kameda, Y.; Kawamura, R.; Kimura, T.; Shimojo, N.; Xu, D.; Murakoshi, N.; Aonuma, K. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension 2014, 63, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vejdani-Jahromi, M.; Nagle, M.; Trahey, G.E.; Wolf, P.D. Ultrasound shear wave elasticity imaging quantifies coronary perfusion pressure effect on cardiac compliance. IEEE Trans. Med. Imaging 2015, 34, 465–473. [Google Scholar] [CrossRef]
- Walker, J.C.; Ratcliffe, M.B.; Zhang, P.; Wallace, A.W.; Hsu, E.W.; Saloner, D.A.; Guccione, J.M. Magnetic resonance imaging-based finite element stress analysis after linear repair of left ventricular aneurysm. J. Thorac. Cardiovasc. Surg. 2008, 135, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Buffinton, C.M.; Faas, D.; Sedmera, D. Stress and strain adaptation in load-dependent remodeling of the embryonic left ventricle. Biomech. Model. Mechanobiol. 2013, 12, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Stekelenburg-de Vos, S.; Steendijk, P.; Ursem, N.T.; Wladimiroff, J.W.; Poelmann, R.E. Systolic and diastolic ventricular function in the normal and extra-embryonic venous clipped chicken embryo of stage 24: A pressure-volume loop assessment. Ultrasound Obstet. Gynecol. 2007, 30, 325–331. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Hierck, B.P.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004, 230, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, B.C.; Hierck, B.P.; Vrolijk, J.; Baiker, M.; Pourquie, M.J.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005, 96, 1291–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taber, L.A.; Voronov, D.A.; Ramasubramanian, A. The role of mechanical forces in the torsional component of cardiac looping. Ann. N. Y. Acad. Sci. 2010, 1188, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Wessels, A.; Markwald, R. Cardiac morphogenesis and dysmorphogenesis. I. Normal development. Methods Mol. Biol. 2000, 136, 239–259. [Google Scholar] [PubMed]
- Davidson, L.A. Mechanical design in embryos: Mechanical signalling, robustness and developmental defects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20150516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze-Bauer, C.A.J.; Morth, C.; Holzapfel, G.A. Passive Biaxial Mechanical Response of Aged Human Iliac Arteries. J. Biomech. Eng. 2003, 125, 395–406. [Google Scholar] [CrossRef]
- Ferrara, A.; Morganti, S.; Totaro, P.; Mazzola, A.; Auricchio, F. Human dilated ascending aorta: Mechanical characterization via uniaxial tensile tests. J. Mech. Behav. Biomed. Mater. 2016, 53, 257–271. [Google Scholar] [CrossRef]
- Ross, C.; Laurence, D.; Wu, Y.; Lee, C.-H. Biaxial Mechanical Characterizations of Atrioventricular Heart Valves. J. Vis. Exp. 2019, 9, e59170. [Google Scholar] [CrossRef]
- Murdock, K.; Martin, C.; Sun, W. Characterization of mechanical properties of pericardium tissue using planar biaxial tension and flexural deformation. J. Mech. Behav. Biomed. Mater. 2018, 77, 148–156. [Google Scholar] [CrossRef]
- Miller, C.E.; Wong, C.L.; Sedmera, D. Pressure overload alters stress-strain properties of the developing chick heart. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1849–H1856. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, J.; Bird, I.M.; Magness, R.R. Mechanisms of shear stress-induced endothelial nitric-oxide synthase phosphorylation and expression in ovine fetoplacental artery endothelial cells. Biol. Reprod. 2004, 70, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filas, B.A.; Xu, G.; Taber, L.A. Probing regional mechanical properties of embryonic tissue using microindentation and optical coherence tomography. Methods Mol. Biol. 2015, 1189, 3–16. [Google Scholar] [PubMed]
- Smoljkic, M.; Vander Sloten, J.; Segers, P.; Famaey, N. Non-invasive, energy-based assessment of patient-specific material properties of arterial tissue. Biomech. Model. Mechanobiol. 2015, 14, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.D.; Kim, H.Y.; Davidson, L.A. Microscopy tools for quantifying developmental dynamics in Xenopus embryos. Methods Mol. Biol. 2012, 917, 477–493. [Google Scholar]
- Gendernalik, A.; Zebhi, B.; Ahuja, N.; Garrity, D.; Bark, D., Jr. In Vivo Pressurization of the Zebrafish Embryonic Heart as a Tool to Characterize Tissue Properties During Development. Ann. Biomed. Eng. 2021, 49, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Teal, S.I.; Moore, G.W.; Hutchins, G.M. Development of aortic and mitral valve continuity in the human embryonic heart. Am. J. Anat. 1986, 176, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Kong, C.W.; Chen, S.; Cheng, S.H.; Li, R.A.; Sun, D. Probing the mechanobiological properties of human embryonic stem cells in cardiac differentiation by optical tweezers. J. Biomech. 2012, 45, 123–128. [Google Scholar] [CrossRef]
- Clavero Adell, M.; Ayerza Casas, A.; Jimenez Montanes, L.; Palanca Arias, D.; Lopez Ramon, M.; Alcala Nalvaiz, J.T.; Samper Villagrasa, P. Evolution of strain and strain rate values throughout gestation in healthy fetuses. Int. J. Cardiovasc. Imaging 2020, 36, 59–66. [Google Scholar] [CrossRef]
- Taketazu, M.; Sugimoto, M.; Saiki, H.; Ishido, H.; Masutani, S.; Senzaki, H. Developmental Changes in Aortic Mechanical Properties in Normal Fetuses and Fetuses with Cardiovascular Disease. Pediatr. Neonatol. 2017, 58, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Smoljkic, M.; Verbrugghe, P.; Larsson, M.; Widman, E.; Fehervary, H.; D’Hooge, J.; Vander Sloten, J.; Famaey, N. Comparison of in vivo vs. ex situ obtained material properties of sheep common carotid artery. Med. Eng. Phys. 2018, 55, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Van der Heiden, K.; Groenendijk, B.C.; Hierck, B.P.; Hogers, B.; Koerten, H.K.; Mommaas, A.M.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev. Dyn. 2006, 235, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Juran, C.; McClendon, M.; Eyadiel, C.; McFetridge, P.S. Development of a mechanically tuneable 3D scaffold for vascular reconstruction. J. Biomed. Mater. Res. A 2012, 100, 3480–3489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, I.E.; Taber, L.A. Mechanical effects of looping in the embryonic chick heart. J. Biomech. 1994, 27, 311–321. [Google Scholar] [CrossRef]
- Disney, C.M.; Lee, P.D.; Hoyland, J.A.; Sherratt, M.J.; Bay, B.K. A review of techniques for visualising soft tissue microstructure deformation and quantifying strain Ex Vivo. J. Microsc. 2018, 272, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashkarinia, S.S.; Coban, G.; Ermek, E.; Celik, M.; Pekkan, K. Spatiotemporal remodeling of embryonic aortic arch: Stress distribution, microstructure, and vascular growth in silico. Biomech. Model. Mechanobiol. 2020, 19, 1897–1915. [Google Scholar] [CrossRef]
- Xu, G.; Kemp, P.S.; Hwu, J.A.; Beagley, A.M.; Bayly, P.V.; Taber, L.A. Opening angles and material properties of the early embryonic chick brain. J. Biomech. Eng. 2010, 132, 011005. [Google Scholar] [CrossRef]
- Trier, S.M.; Davidson, L.A. Quantitative microscopy and imaging tools for the mechanical analysis of morphogenesis. Curr. Opin. Genet. Dev. 2011, 21, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Ogden, R.W. Nonlinear Elasticity, Anisotropy, Material Stability and Residual Stresses in Soft Tissue. In Biomechanics of Soft Tissue in Cardiovascular Systems; Holzapfel, G.A., Ogden, R.W., Eds.; Springer: Vienna, Austria, 2003; pp. 65–108. [Google Scholar]
- Buskohl, P.R.; Gould, R.A.; Butcher, J.T. Quantification of embryonic atrioventricular valve biomechanics during morphogenesis. J. Biomech. 2012, 45, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Zamir, E.A.; Taber, L.A. Material Properties and Residual Stress in the Stage 12 Chick Heart During Cardiac Looping. J. Biomech. Eng. 2005, 126, 823–830. [Google Scholar] [CrossRef]
- Ogden, R.W.; Hill, R. Large deformation isotropic elasticity—On the correlation of theory and experiment for incompressible rubberlike solids. Proc. R. Soc. Lond. A Math. Phys. Sci. 1972, 326, 565–584. [Google Scholar] [CrossRef]
- von Dassow, M.; Strother, J.A.; Davidson, L.A. Surprisingly Simple Mechanical Behavior of a Complex Embryonic Tissue. PloS ONE 2011, 5, e15359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1981. [Google Scholar]
- Chuong, C.J.; Fung, Y.C. Three-Dimensional Stress Distribution in Arteries. J. Biomech. Eng. 1983, 105, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Ren, M.; Wiputra, H.; Mojumder, J.; Chan, W.X.; Tulzer, A.; Tulzer, G.; Buist, M.L.; Mattar, C.N.; Lee, L.C.; et al. Biomechanics of Human Fetal Hearts with Critical Aortic Stenosis. Ann. Biomed. Eng. 2021, 49, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.; Peña, E.; Martínez, M.A.; Kuhl, E. Computational modeling of hypertensive growth in the human carotid artery. Comput. Mech. 2014, 53, 1183–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göktepe, S.; Acharya, S.N.S.; Wong, J.; Kuhl, E. Computational modeling of passive myocardium. Int. J. Numer. Methods Biomed. Eng. 2011, 27, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.C.; Genet, M.; Acevedo-Bolton, G.; Ordovas, K.; Guccione, J.M.; Kuhl, E. A computational model that predicts reverse growth in response to mechanical unloading. Biomech. Model. Mechanobiol. 2015, 14, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Taber, L.A. Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech. Model. Mechanobiol. 2008, 7, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Konofagou, E.E.; D’Hooge, J.; Ophir, J. Myocardial elastography—A feasibility study in vivo. Ultrasound Med. Biol. 2002, 28, 475–482. [Google Scholar] [CrossRef]
- Davey, M.G.; Tickle, C. The chicken as a model for embryonic development. Cytogenet. Genome Res. 2007, 117, 231–239. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Rychterova, V.; Hu, N.; Clark, E.B. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. Off. Publ. Am. Assoc. Anat. 1999, 254, 238–252. [Google Scholar] [CrossRef]
- Al Naieb, S.; Happel, C.M.; Yelbuz, T.M. A detailed atlas of chick heart development in vivo. Ann. Anat. 2013, 195, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Tobita, K.; Schroder, E.A.; Tinney, J.P.; Garrison, J.B.; Keller, B.B. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2386–H2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamir, E.A.; Taber, L.A. On the effects of residual stress in microindentation tests of soft tissue structures. J. Biomech. Eng. 2004, 126, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.E.; Vanni, M.A.; Taber, L.A.; Keller, B.B. Passive stress-strain measurements in the stage-16 and stage-18 embryonic chick heart. J. Biomech. Eng. 1997, 119, 445–451. [Google Scholar] [CrossRef]
- Alford, P.W.; Taber, L.A. Regional epicardial strain in the embryonic chick heart during the early looping stages. J. Biomech. 2003, 36, 1135–1141. [Google Scholar] [CrossRef]
- Taber, L.A.; Chabert, S. Theoretical and experimental study of growth and remodeling in the developing heart. Biomech. Model. Mechanobiol. 2002, 1, 29–43. [Google Scholar] [CrossRef]
- Zimmerman, F.J.; Hughes, S.F.; Cuneo, B.; Benson, D.W. The effect of cardiac cycle length on ventricular end-diastolic pressure and maximum time derivative of pressure in the stage 24 chick embryo. Pediatr. Res. 1991, 29, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Zamir, E.A.; Srinivasan, V.; Perucchio, R.; Taber, L.A. Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 2003, 31, 1327–1336. [Google Scholar] [CrossRef]
- Butcher, J.T.; McQuinn, T.C.; Sedmera, D.; Turner, D.; Markwald, R.R. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ. Res. 2007, 100, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dur, O.; Patrick, M.J.; Tinney, J.P.; Tobita, K.; Keller, B.B.; Pekkan, K. Aortic arch morphogenesis and flow modeling in the chick embryo. Ann. Biomed. Eng. 2009, 37, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Goktas, S.; Karakaya, C.; Cakiroglu, A.I.; Karahuseyinoglu, S.; Lashkarinia, S.S.; Ermek, E.; Pekkan, K. Microstructure of early embryonic aortic arch and its reversibility following mechanically altered hemodynamic load release. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1208–H1218. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, S.E.; Menon, P.G.; Kowalski, W.J.; Shekhar, A.; Yalcin, H.C.; Nishimura, N.; Schaffer, C.B.; Butcher, J.T.; Pekkan, K. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech. Model. Mechanobiol. 2015, 14, 735–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, C.H.; Liu, X.; Pekkan, K. Characterization of the vessel geometry, flow mechanics and wall shear stress in the great arteries of wildtype prenatal mouse. PLoS ONE 2014, 9, e86878. [Google Scholar] [CrossRef] [Green Version]
- Eghtesady, P.; Michelfelder, E.; Altaye, M.; Ballard, E.; Hirsh, R.; Beekman, R.H., 3rd. Revisiting animal models of aortic stenosis in the early gestation fetus. Ann. Thorac. Surg. 2007, 83, 631–639. [Google Scholar] [CrossRef] [PubMed]
- O’Tierney, P.F.; Anderson, D.F.; Faber, J.J.; Louey, S.; Thornburg, K.L.; Giraud, G.D. Reduced systolic pressure load decreases cell-cycle activity in the fetal sheep heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R573–R578. [Google Scholar] [CrossRef] [Green Version]
- Sacks, M.S.; Yoganathan, A.P. Heart valve function: A biomechanical perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1369–1391. [Google Scholar] [CrossRef] [Green Version]
- Jett, S.; Laurence, D.; Kunkel, R.; Babu, A.R.; Kramer, K.; Baumwart, R.; Towner, R.; Wu, Y.; Lee, C.H. An investigation of the anisotropic mechanical properties and anatomical structure of porcine atrioventricular heart valves. J. Mech. Behav. Biomed. Mater. 2018, 87, 155–171. [Google Scholar] [CrossRef]
- Khoiy, K.A.; Pant, A.D.; Amini, R. Quantification of Material Constants for a Phenomenological Constitutive Model of Porcine Tricuspid Valve Leaflets for Simulation Applications. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef]
- Lodder, J.; Verkerke, G.J.; Delemarre, B.J.; Dodou, D. Morphological and mechanical properties of the posterior leaflet chordae tendineae in the mitral valve. Proc. Inst. Mech. Eng. H 2016, 230, 77–84. [Google Scholar] [CrossRef]
- Allison, B.J.; Brain, K.L.; Niu, Y.; Kane, A.D.; Herrera, E.A.; Thakor, A.S.; Botting, K.J.; Cross, C.M.; Itani, N.; Skeffington, K.L.; et al. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J. Physiol. 2016, 594, 1247–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segar, J.L.; Hajduczok, G.; Smith, B.A.; Merrill, D.C.; Robillard, J.E. Ontogeny of baroreflex control of renal sympathetic nerve activity and heart rate. Am. J. Physiol. 1992, 263, H1819–H1826. [Google Scholar] [CrossRef]
- Huhta, H.; Junno, J.; Haapsamo, M.; Erkinaro, T.; Ohtonen, P.; Davis, L.E.; Hohimer, A.R.; Acharya, G.; Rasanen, J. Fetal sheep central haemodynamics and cardiac function during occlusion of the ascending aorta. Exp. Physiol. 2018, 103, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibayan, F.A.; Louey, S.; Jonker, S.; Espinoza, H.; Chattergoon, N.; You, F.; Thornburg, K.L.; Giraud, G. Increased systolic load causes adverse remodeling of fetal aortic and mitral valves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1490–R1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, J.H.; Landauer, M.; Tian, Q.; Phernetton, T.M. Cardiovascular responses to forskolin in the ovine fetus. J. Dev. Physiol. 1989, 11, 7–10. [Google Scholar] [PubMed]
- Ishiwata, T.; Nakazawa, M.; Pu, W.T.; Tevosian, S.G.; Izumo, S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ. Res. 2003, 93, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, A.; Gendernalik, A.; Garrity, D.; Bark, D. Valveless pumping behavior of the simulated embryonic heart tube as a function of contractile patterns and myocardial stiffness. Biomech. Model. Mechanobiol. 2021, 20, 2001–2012. [Google Scholar] [CrossRef]
- Chelliah, A.; Dham, N.; Frank, L.H.; Donofrio, M.; Krishnan, A. Myocardial strain can be measured from first trimester fetal echocardiography using velocity vector imaging. Prenat. Diagn. 2016, 36, 483–488. [Google Scholar] [CrossRef]
- van Geemen, D.; Soares, A.L.; Oomen, P.J.; Driessen-Mol, A.; Janssen-van den Broek, M.W.; van den Bogaerdt, A.J.; Bogers, A.J.; Goumans, M.J.; Baaijens, F.P.; Bouten, C.V. Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves. PLoS ONE 2016, 11, e0149020. [Google Scholar] [CrossRef]
- Emery, J.L.; Omens, J.H.; McCulloch, A.D. Strain softening in rat left ventricular myocardium. J. Biomech. Eng. 1997, 119, 6–12. [Google Scholar] [CrossRef]
- Espe, E.K.; Aronsen, J.M.; Nordén, E.S.; Zhang, L.; Sjaastad, I. Regional right ventricular function in rats: A novel magnetic resonance imaging method for measurement of right ventricular strain. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H143–H153. [Google Scholar] [CrossRef] [PubMed]
- Bolte, C.; Whitsett, J.A.; Kalin, T.V.; Kalinichenko, V.V. Transcription Factors Regulating Embryonic Development of Pulmonary Vasculature. Adv. Anat. Embryol. Cell Biol. 2018, 228, 1–20. [Google Scholar] [PubMed]
- Deng, Y.; Ou, Z.; Li, R.; Chen, Z.; Liang, P.; Sun, L. Affected-embryo-based SNP haplotyping with NGS for the preimplantation genetic testing of Marfan syndrome. Syst. Biol. Reprod. Med. 2021, 67, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Robertson, S.P.; Daniel, P.B. TGFBR1 mutations associated with Loeys-Dietz syndrome are inactivating. J. Recept. Signal. Transduct. Res. 2012, 32, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Park, A.C.; Phillips, C.L.; Pfeiffer, F.M.; Roenneburg, D.A.; Kernien, J.F.; Adams, S.M.; Davidson, J.M.; Birk, D.E.; Greenspan, D.S. Homozygosity and Heterozygosity for Null Col5a2 Alleles Produce Embryonic Lethality and a Novel Classic Ehlers-Danlos Syndrome-Related Phenotype. Am. J. Pathol. 2015, 185, 2000–2011. [Google Scholar] [CrossRef]
- Palit, A.; Bhudia, S.K.; Arvanitis, T.N.; Turley, G.A.; Williams, M.A. Computational modelling of left-ventricular diastolic mechanics: Effect of fibre orientation and right-ventricle topology. J. Biomech. 2015, 48, 604–612. [Google Scholar] [CrossRef]
- Kathiriya, I.S.; Srivastava, D. Left-right asymmetry and cardiac looping: Implications for cardiac development and congenital heart disease. Am. J. Med. Genet. 2000, 97, 271–279. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, L.; Gong, X.; Jia, X.; Song, W.; Liu, M.; Fan, Y. Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PLoS ONE 2012, 7, e34960. [Google Scholar]
- Cattaruzza, M.; Guzik, T.J.; Slodowski, W.; Pelvan, A.; Becker, J.; Halle, M.; Buchwald, A.B.; Channon, K.M.; Hecker, M. Shear stress insensitivity of endothelial nitric oxide synthase expression as a genetic risk factor for coronary heart disease. Circ. Res. 2004, 95, 841–847. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Van der Heiden, K.; Hierck, B.P.; Poelmann, R.E. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology 2007, 22, 380–389. [Google Scholar] [CrossRef]
- Egorova, A.D.; Van der Heiden, K.; Van de Pas, S.; Vennemann, P.; Poelma, C.; DeRuiter, M.C.; Goumans, M.J.; Gittenberger-de Groot, A.C.; ten Dijke, P.; Poelmann, R.E.; et al. Tgfbeta/Alk5 signaling is required for shear stress induced klf2 expression in embryonic endothelial cells. Dev. Dyn. 2011, 240, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.D.; Yong, S.K.; Dheen, S.T.; Bay, B.H.; Tay, S.S. Cardiac malformations are associated with altered expression of vascular endothelial growth factor and endothelial nitric oxide synthase genes in embryos of diabetic mice. Exp. Biol. Med. 2008, 233, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Challande, P.; Osborne-Pellegrin, M.; Regnault, V. Genetics and pathophysiology of arterial stiffness. Cardiovasc. Res. 2009, 81, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zheng, J.; Bird, I.M.; Magness, R.R. Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells. Biol. Reprod. 2003, 69, 1053–1059. [Google Scholar]
- Novodvorsky, P.; Chico, T.J. The role of the transcription factor KLF2 in vascular development and disease. Prog. Mol. Biol. Transl. Sci. 2014, 124, 155–188. [Google Scholar]
- Malek, A.M.; Zhang, J.; Jiang, J.; Alper, S.L.; Izumo, S. Endothelin-1 gene suppression by shear stress: Pharmacological evaluation of the role of tyrosine kinase, intracellular calcium, cytoskeleton, and mechanosensitive channels. J. Mol. Cell Cardiol. 1999, 31, 387–399. [Google Scholar] [CrossRef]
- Halabi, C.M.; Broekelmann, T.J.; Lin, M.; Lee, V.S.; Chu, M.L.; Mecham, R.P. Fibulin-4 is essential for maintaining arterial wall integrity in conduit but not muscular arteries. Sci. Adv. 2017, 3, e1602532. [Google Scholar]
- Staiculescu, M.C.; Cocciolone, A.J.; Procknow, J.D.; Kim, J.; Wagenseil, J.E. Comparative gene array analyses of severe elastic fiber defects in late embryonic and newborn mouse aorta. Physiol. Genom. 2018, 50, 988–1001. [Google Scholar] [CrossRef]
- Igoucheva, O.; Alexeev, V.; Halabi, C.M.; Adams, S.M.; Stoilov, I.; Sasaki, T.; Arita, M.; Donahue, A.; Mecham, R.P.; Birk, D.E.; et al. Fibulin-4 E57K Knock-in Mice Recapitulate Cutaneous, Vascular and Skeletal Defects of Recessive Cutis Laxa 1B with both Elastic Fiber and Collagen Fibril Abnormalities. J. Biol. Chem. 2015, 290, 21443–21459. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Procknow, J.D.; Yanagisawa, H.; Wagenseil, J.E. Differences in genetic signaling, and not mechanical properties of the wall, are linked to ascending aortic aneurysms in fibulin-4 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H103–H113. [Google Scholar]
- Stephens, E.H.; Post, A.D.; Laucirica, D.R.; Grande-Allen, K.J. Perinatal changes in mitral and aortic valve structure and composition. Pediatr. Dev. Pathol. 2010, 13, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, M.; Yamamoto, K.; Shimizu, N.; Obi, S.; Kumagaya, S.; Igarashi, T.; Kamiya, A.; Ando, J. Differential gene responses in endothelial cells exposed to a combination of shear stress and cyclic stretch. J. Biotechnol. 2008, 133, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.O.; Jiao, Y.; Phillips, S.J.; Singh, G.; Xu, J.; Balsara, R.; Litvin, J. Alterations in sheep fetal right ventricular tissue with induced hemodynamic pressure overload. Basic Res. Cardiol. 1998, 93, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Cardiac malformations and alteration of TGFbeta signaling system in diabetic embryopathy. Birth Defects Res. B Dev. Reprod. Toxicol. 2010, 89, 97–105. [Google Scholar]
- Renard, M.; Holm, T.; Veith, R.; Callewaert, B.L.; Adès, L.C.; Baspinar, O.; Pickart, A.; Dasouki, M.; Hoyer, J.; Rauch, A.; et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 2010, 18, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Ai, J.; Zhang, R.; Gao, X.; Niu, H.F.; Wang, N.; Xu, Y.; Li, Y.; Ma, N.; Sun, L.H.; Pan, Z.W.; et al. Overexpression of microRNA-1 impairs cardiac contractile function by damaging sarcomere assembly. Cardiovasc. Res. 2012, 95, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Witman, N.; Heigwer, J.; Thaler, B.; Lui, W.O.; Morrison, J.I. miR-128 regulates non-myocyte hyperplasia, deposition of extracellular matrix and Islet1 expression during newt cardiac regeneration. Dev. Biol. 2013, 383, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Bowen, C.J.; Zhou, J.; Sung, D.C.; Butcher, J.T. Cadherin-11 coordinates cellular migration and extracellular matrix remodeling during aortic valve maturation. Dev. Biol. 2015, 407, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.C.; Bowen, C.J.; Vaidya, K.A.; Zhou, J.; Chapurin, N.; Recknagel, A.; Zhou, B.; Chen, J.; Kotlikoff, M.; Butcher, J.T. Cadherin-11 Overexpression Induces Extracellular Matrix Remodeling and Calcification in Mature Aortic Valves. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Lehoux, S.; Tedgui, A. Cellular mechanics and gene expression in blood vessels. J. Biomech. 2003, 36, 631–643. [Google Scholar] [CrossRef]
- Lehoux, S.; Castier, Y.; Tedgui, A. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 2006, 259, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, I.; MacCarrick, G.; Kempers, M.; Meester, J.; Geryl, C.; Rombouts, O.; Peeters, N.; Claes, C.; Boeckx, N.; Sakalihasan, N.; et al. Confirmation of the role of pathogenic SMAD6 variants in bicuspid aortic valve-related aortopathy. Eur. J. Hum. Genet. 2019, 27, 1044–1053. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Huibers, M.M.H.; van Kuik, J.; de Weger, R.A.; Vink, A.; de Jonge, N. The Interleukin-33/ST2 Pathway Is Expressed in the Failing Human Heart and Associated with Pro-fibrotic Remodeling of the Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Veeraveedu, P.T.; Sanada, S.; Okuda, K.; Fu, H.Y.; Matsuzaki, T.; Araki, R.; Yamato, M.; Yasuda, K.; Sakata, Y.; Yoshimoto, T.; et al. Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem. Pharmacol. 2017, 138, 73–80. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, K.M. Genetics of arterial structure and function: Towards new biomarkers for aortic stiffness? Clin. Sci. 2008, 114, 661–677. [Google Scholar]
- Carta, L.; Wagenseil, J.E.; Knutsen, R.H.; Mariko, B.; Faury, G.; Davis, E.C.; Starcher, B.; Mecham, R.P.; Ramirez, F. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2083–2089. [Google Scholar] [CrossRef] [Green Version]
- Wagenseil, J.E.; Mecham, R.P. Elastin in large artery stiffness and hypertension. J. Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Makwana, O.; King, N.M.; Ahles, L.; Selmin, O.; Granzier, H.L.; Runyan, R.B. Exposure to low-dose trichloroethylene alters shear stress gene expression and function in the developing chick heart. Cardiovasc. Toxicol. 2010, 10, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Gould, R.A.; Aziz, H.; Woods, C.E.; Seman-Senderos, M.A.; Sparks, E.; Preuss, C.; Wünnemann, F.; Bedja, D.; Moats, C.R.; McClymont, S.A.; et al. ROBO4 variants predispose individuals to bicuspid aortic valve and thoracic aortic aneurysm. Nat. Genet. 2019, 51, 42–50. [Google Scholar] [CrossRef]
- Koenig, S.N.; LaHaye, S.; Feller, J.D.; Rowland, P.; Hor, K.N.; Trask, A.J.; Janssen, P.M.; Radtke, F.; Lilly, B.; Garg, V. Notch1 haploinsufficiency causes ascending aortic aneurysms in mice. JCI Insight 2017, 2, e91353. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Lacolley, P. Structural and genetic bases of arterial stiffness. Hypertension 2005, 45, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Puzzi, L.; Borin, D.; Gurha, P.; Lombardi, R.; Martinelli, V.; Weiss, M.; Andolfi, L.; Lazzarino, M.; Mestroni, L.; Marian, A.J.; et al. Knock Down of Plakophillin 2 Dysregulates Adhesion Pathway through Upregulation of miR200b and Alters the Mechanical Properties in Cardiac Cells. Cells 2019, 8, 1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
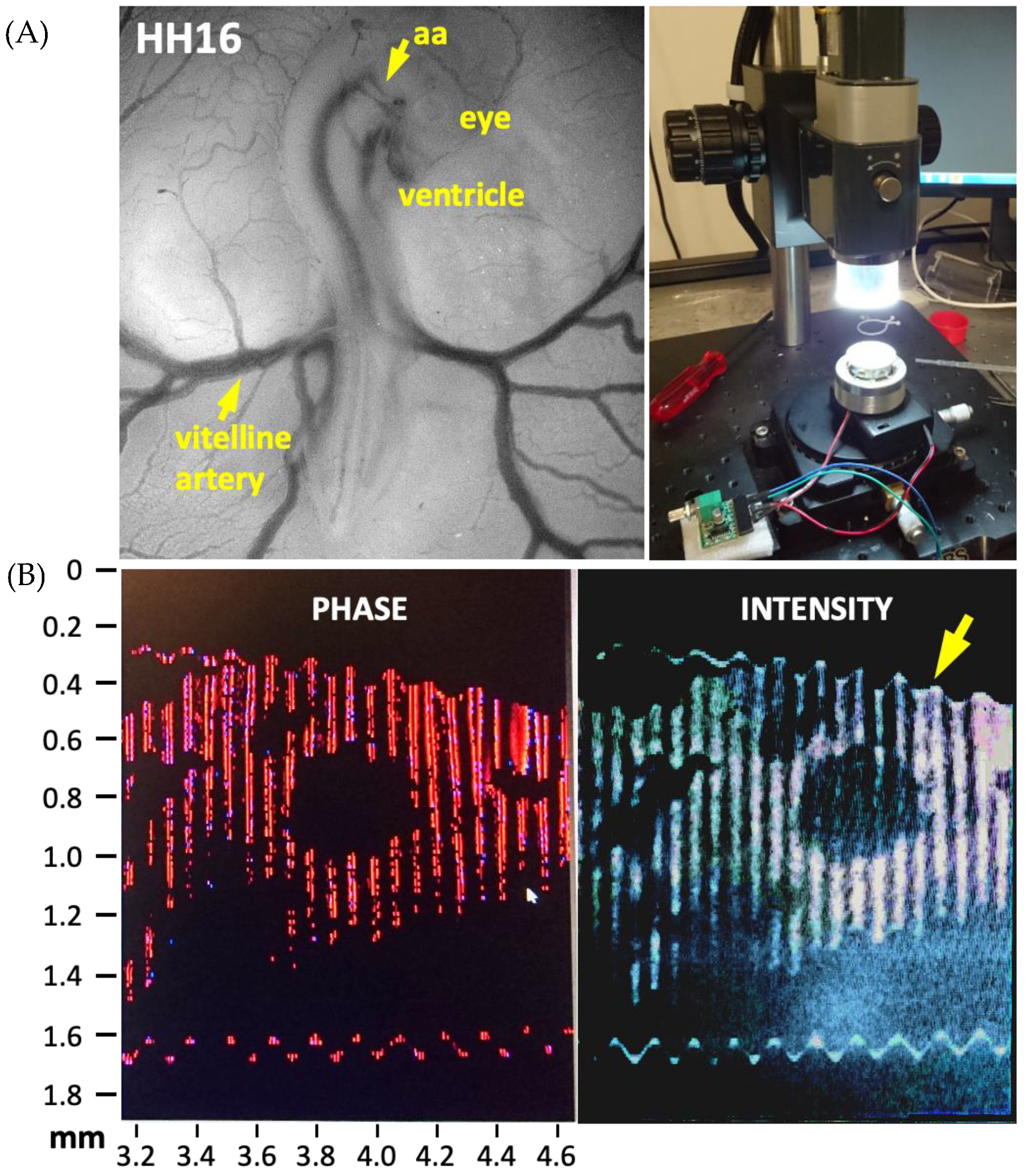


| Mechanical Methods and Numerical Models | Ref. | Visualization Methods | Ref. |
|---|---|---|---|
| Uniaxial/biaxial tensile testing | [30] | Optical coherence tomography | [31,32] |
| Invasive/noninvasive residual stress experiments | [33] | Epifluorescence/fluoroscopy | [34] |
| In vivo pressurization | [35] | Microscopy | [34,36] |
| Optical stretching and optical tweezers | [37] | Magnetic resonance imaging | [18] |
| Finite element modeling (FEM) | [3] | Echocardiograph | [38,39] |
| Cantilever based technologies | [29] | Confocal/two-photon microscopy | [19] |
| Strain energy and Gasser-Ogden-Holzapfel models | [40] | Scanning electron microscopy | [41,42] |
| Cuts | [43] | Histology | [44] |
| Micropipette aspiration with servo-null pressure measurements | [45] | Digital camera | |
| Beads | [9,10] | Radiology | |
| Micro-indentation, atomic force microscopy | [32,46] | Micro computed tomography |
| Vitelline Artery (n = 5) | Aortic Arch (n = 8) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loading | Unloading | Loading | Unloading | ||||||||||
| HH | 16 | 17.5 | 19 | 16 | 17.5 | 19 | 18 | 24 | 18 | 24 | |||
| c | 0.46 ± 0.03 | 0.51 ± 0.04 | 0.58 ± 0.04 | c | 0.43 ± 0.03 | 0.47 ± 0.04 | 0.57 ± 0.04 | c | 0.51 ± 0.03 | 0.61 ± 0.07 | c | 0.48 ± 0.02 | 0.57 ± 0.06 |
| b1 | 4.68 ± 0.14 | 4.45 ± 0.14 | 4.23 ± 0.13 | b1 | 4.49 ± 0.14 | 4.14 ± 0.13 | 4.1 ± 0.12 | b1 | 6.51 ± 0.19 | 5.00 ± 0.25 | b1 | 6.09 ± 0.18 | 4.68 ± 0.24 |
| b2 | 2.81 ± 0.14 | 3.12 ± 0.15 | 3.28 ± 0.16 | b2 | 2.69 ± 0.13 | 2.91 ± 0.14 | 3.18 ± 0.15 | b2 | 2.53 ± 0.07 | 2.13 ± 0.07 | b2 | 2.37 ± 0.07 | 1.99 ± 0.07 |
| b3 | 0.65 ± 0.02 | 0.67 ± 0.02 | 0.65 ± 0.02 | b3 | 0.63 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.02 | b3 | 0.63 ± 0.05 | 0.59 ± 0.06 | b3 | 0.59 ± 0.05 | 0.55 ± 0.05 |
| b4 | 0.37 ± 0.03 | 0.45 ± 0.04 | 0.51 ± 0.04 | b4 | 0.36 ± 0.03 | 0.42 ± 0.03 | 0.50 ± 0.04 | b4 | 0.32 ± 0.02 | 0.27 ± 0.03 | b4 | 0.30 ± 0.02 | 0.26 ± 0.03 |
| b5 | 6.04 ± 0.14 | 7.07 ± 0.17 | 7.86 ± 0.19 | b5 | 5.80 ± 0.14 | 6.58 ± 0.16 | 7.62 ± 0.18 | b5 | 6.40 ± 0.12 | 5.11 ± 0.12 | b5 | 5.99 ± 0.11 | 4.77 ± 0.11 |
| b6 | 1.47 ± 0.03 | 1.76 ± 0.04 | 1.86 ± 0.04 | b6 | 1.41 ± 0.03 | 1.64 ± 0.04 | 1.81 ± 0.04 | b6 | 0.98 ± 0.03 | 0.90 ± 0.03 | b6 | 0.91 ± 0.03 | 0.84 ± 0.03 |
| Porcine | ||||||
|---|---|---|---|---|---|---|
| Ref. | Organ | Parameter | Stage | CS | Value | Method |
| [13] | Mitral valve leaflet | thickness (mm) | Third trimester | - | 0.4 | uniaxial tensile testing |
| stress (kPa) | 7000 | |||||
| strain | 0.35 | |||||
| ultimate stress (kPa) | 0.400 | |||||
| modulus of elasticity (kPa) | 200 | |||||
| Rat | ||||||
| Ref. | Organ | Parameter | Stage | Value | Method | |
| ED | CS | |||||
| [88] | Ventricle | Pulsed Doppler velocimetry and Micro pressure system | ||||
| Ventricle | Systolic pressure (mmHg) | 10.5 | 7 | 3 | ||
| 11.5 | 8 | 5 | ||||
| 12.5 | 9 | 6.5 | ||||
| 13.5 | 10.5 | 8.2 | ||||
| LV | End diastolic area (mm2) | 11.5 | 8 | 0.77 | ||
| RV | 11.5 | 8 | 0.77 | |||
| LV | 12.5 | 9 | 0.8 | |||
| RV | 14.5 | 11.5 | 1.29 | |||
| Cardiac Myocyte | ||||||
| cardiac myocyte | Pressure (N/m) | 2 days | - | 0.75 | ||
| Xenopus | ||||||
| [35] | myocardium | Stiffness (kPa) | 48 hpf | - | 10 | FEM, cannulation, and pressurization |
| [89] | Heart tube | circumferential stress (kPa) | 24–30 hpf | - | 7 | Computational |
| Ref. | Organ | Parameter | Type | Stage (Weeks) | Value | Method |
|---|---|---|---|---|---|---|
| Myocardium | ||||||
| [90] | myocardium | strain (%) | RV global | 1st trimester | 14.4 | Uniaxial tensile testing, FEM, and ECG |
| LV global | 13.8 | |||||
| RV regional | 13.9 | |||||
| LV regional | 13 | |||||
| Ventricle | ||||||
| [38] | LV | strain (%) | global | 16–21 | 28.6 | ECG |
| LV | 22–27 | 27.47 | ||||
| LV | 28–38 | 26.61 | ||||
| RV | 16–21 | 27.79 | ||||
| RV | 22–27 | 26.48 | ||||
| RV | 28–38 | 24.72 | ||||
| LV | systolic | 21 | −15 | |||
| LV | 28 | −25 | ||||
| LV | 34 | −35 | ||||
| umbilical vein | ||||||
| [3] | umbilical vein | strain | derived from true stress and/or strain | - | 4.1 | FEM and uniaxial tensile testing |
| elastic modulus (MPa) | 4.5 | |||||
| stress (kPa) | max | 6000 | ||||
| strain | max | 0.9 | ||||
| umbilical artery | ||||||
| [1] | umbilical artery | stiffness (kPa) | 25 | 57.89 | uniaxial tensile testing and scanning electron microscope | |
| 26–30 | 55.51 | |||||
| 31–35 | 76.53 | |||||
| 36–40 | 80.83 | |||||
| strain | 25 | 1.33 | ||||
| 26–30 | 1.52 | |||||
| 31–35 | 1.39 | |||||
| 36–40 | 1.41 | |||||
| [42] | burst pressure (kPa) | - | 200 | |||
| strength (N) | suture retention | - | 1.75 | |||
| stress (kPa) | - | 3500 | ||||
| strength (N) | longitudinal tensile | - | 21 | |||
| strength (N) | radial tensile | - | 8 | |||
| Aorta | ||||||
| [39] | aorta | Stiffness index | aortic compliance | 25 | 0.7 | Doppler flow profile and ECG |
| 30 | 0.5 | |||||
| 35 | 0.25 | |||||
| Valves | ||||||
| [91] | aortic valve | Elastic Modulus (kPa) | 21 | 4–5 | Micro indentation | |
| Pulmonary valve | 3–4 | |||||
| human embryonic stem cells | ||||||
| [37] | hESC | cardiomyocyte (kPa) | membrane stress | - | 0.0013 | optical stretching |
| elastic modulus | 0.0056 | |||||
| membrane stress | 0.0005 | |||||
| elastic modulus | 0.014 | |||||
| Fetal Malformation | Change in Material Property | Ref. |
|---|---|---|
| Fibrotic infarction | Doubled ventricular strain and increased myocardial stress. | [14] |
| Conotruncal defects | Increased ventricular pressure | [19] |
| pulmonary congestion | Higher longitudinal strain compared to the circumferential direction | [94] |
| Hypertensive heart | Increased stiffness | [15] |
| Reduced strains in LV | [16] | |
| Reduced systolic strains | ||
| Hypertrophy | Increased ventricular wall thickness and stiffness | [17] |
| aneurysm | Higher end diastolic stresses and cross-fiber stresses | [18] |
| Marfan syndrome | Enlargement and weakening of heart muscles | [95] |
| Loeys-Dietz syndrome | Enlargement of aorta | [96] |
| Ehlers-Danlos syndrome | Reduced elasticity, strength, and stiffness of the aortic vessels | [97] |
| Fibrotic infarction | Decreased ventricular pressure with compact and thinner myocardium | [19] |
| Gene | Organ | Defect | Mechanosensitive | Indirect Alterations | Mechanical Properties Altered | Ref. | GP |
|---|---|---|---|---|---|---|---|
| Fibulin 4 coded by EFEMP2 gene | Large conduit arterial walls in mice | Ascending Aortic aneurysms, loose skin, bent forelimb, tortuous artery, and pulmonary emphysema | Interacts with elastin directly | - | Alters elastin, binds to calcium | [109] | KI |
| [110] | KO | ||||||
| [111] | KI | ||||||
| [112] | KO | ||||||
| Endothelin 1 (ET1) | Human umbilical vein endothelial cells | Reacts directly to shear stress and cyclic stretch | Shear stress and cyclic stretch | [114] | WT | ||
| Elastin coded by Eln | Mouse aortic walls | Arterial stenosis, hypertension | Direct interaction | - | Alters elastic fibers, thickening and arterial tortuosity | [110] | KO |
| [128] | M | ||||||
| [129] | KO | ||||||
| miR-1 | Cardiac contractile function in mice | Damage in sarcomere assembly | - | Targets UTRs of MYLK3, CALM1, and CALM2 | Affects structural remodeling of the heart | [118] | KO |
| VEGF | Endothelial cells | Matrix stiffens | - | In turn effects MMP activity | Stiffness, intima | [104,130] | M |
| Cadherin-11 | ECM in aorta in mice | Cardiac dysfunction in valves | - | Reduced Sox9 activity, β1 integrin expression, and RhoA-GTP | Increases thickness and alters stress fibers, causes calcification | [120] | KO |
| [121] | KI | ||||||
| Fibirillin-1 | Mice arteries | Mutation causes Narrowing | Affects arterial diameter | [128] | M | ||
| NOS-3, KLF-2, ET-1 (can be altered by changing trichloroethylene doses) [131] | Chick, bovine, mice embryonic cardiovascular system | Shear stress induced | KLF2 indirectly activated by TGFβ | Activated by shear stress | [104] | M | |
| [101,106] | WT | ||||||
| TGFβ | Embryonic endothelial cells (human) | Cardiac malformations | Activated by shear activities | Can be affected by fibulin deficiency | Directly activated by shear stress | [103,116,117] | WT, M |
| ROBO4 | Bicuspid aortic valve and thoracic aortic | CHD and aneurysm | [132] | KO | |||
| Notch1 | Mice aorta | Ascending Aortic Aneurysm | [133] | KO | |||
| AGTR1, ACE, AGT, CYP11B2, ADD1 | Human artery, vascular | CHD | Indirect association | Elasticity | [105] | R | |
| MMP3, MMP9, M235T | Human artery, mice vascular | CHD | Indirect association | Stiffness and impedance | [105,134] | R | |
| NFκB | Vascular response in mice | Direct | Activated by shear stress | [123] | R | ||
| MAP Kinase | Blood vessels | Shear activated or stretch activated | Shear stress and stretch | [8] | |||
| MEK, PI-3K | Ovine fetoplacental artery endothelial cells | Activated by eNOS (indirectly activated by stress) | Shear stress | [31] | WT | ||
| SMAD6 | Thoracic aorta and bicuspid valve in humans | Thoracic aortic aneurysm | Indirect | Thickness | [124] | M | |
| PKP2 | Cardiac cells inMice | Indirect, affects miR200b first | Knockdown causes reduced stress and work of detachment, increases plasticity index | [135] | KO | ||
| IL33 | Myocardium in mice and humans | Failing heart | Induced by mechanical stress | Stress | [126] | KO | |
| [125] | WT | ||||||
| miR-128 | Cardiac ECM | Hyperplasia | Regulates hyperplasia and Islet1 | [119] | KO | ||
| RAAS | Cardiac vessels | Vascular hypertrophy | Regulates stiffness | Stiffness | [127] | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, H.B.; Dogru, S.; Lashkarinia, S.S.; Pekkan, K. Soft-Tissue Material Properties and Mechanogenetics during Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2022, 9, 64. https://doi.org/10.3390/jcdd9020064
Siddiqui HB, Dogru S, Lashkarinia SS, Pekkan K. Soft-Tissue Material Properties and Mechanogenetics during Cardiovascular Development. Journal of Cardiovascular Development and Disease. 2022; 9(2):64. https://doi.org/10.3390/jcdd9020064
Chicago/Turabian StyleSiddiqui, Hummaira Banu, Sedat Dogru, Seyedeh Samaneh Lashkarinia, and Kerem Pekkan. 2022. "Soft-Tissue Material Properties and Mechanogenetics during Cardiovascular Development" Journal of Cardiovascular Development and Disease 9, no. 2: 64. https://doi.org/10.3390/jcdd9020064







