From Streptococcal Pharyngitis/Tonsillitis to Myocarditis: A Systematic Review
Abstract
:1. Introduction
2. Case Presentation
3. Materials and Methods
4. Results
4.1. Selected Studies
4.2. Patient Characteristics
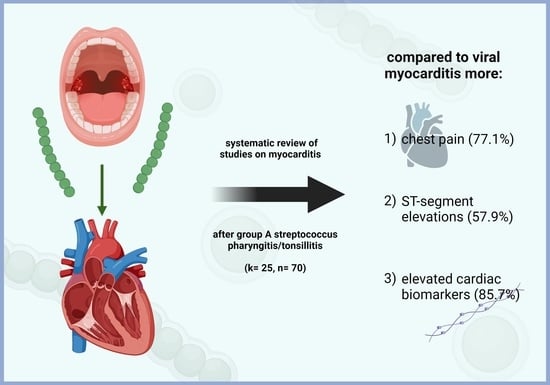
4.3. Clinical Characteristics
4.4. ECG Findings and Cardiac Markers
4.5. Imaging
4.5.1. Transthoracic Echocardiography (TTE)
4.5.2. Cardiac Magnetic Resonance Imaging (CMR)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Efstratiou, A.; Lamagni, T. Epidemiology of Streptococcus pyogenes. In Streptococcus pyogenes Basic Biology to Clinical Manifestations. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343616/ (accessed on 3 February 2022).
- Anderson, J.; Paterek, E. Tonsillitis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544342/ (accessed on 3 February 2022).
- Elamm, C.; Fairweather, D.; Cooper, L.T. Pathogenesis and Diagnosis of Myocarditis. Heart 2012, 98, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, I.; Saphir, O. Myocarditis Associated with Acute Nasopharyngitis and Acute Tonsillitis. Am. Heart J. 1947, 34, 831–851. [Google Scholar] [CrossRef]
- Sika-Paotonu, D.; Beaton, A.; Raghu, A.; Steer, A.; Carapetis, J. Acute Rheumatic Fever and Rheumatic Heart Disease. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425394/ (accessed on 4 February 2022).
- Zühlke, L.J.; Beaton, A.; Engel, M.E.; Hugo-Hamman, C.T.; Karthikeyan, G.; Katzenellenbogen, J.M.; Ntusi, N.; Ralph, A.P.; Saxena, A.; Smeesters, P.R.; et al. Group A Streptococcus, Acute Rheumatic Fever and Rheumatic Heart Disease: Epidemiology and Clinical Considerations. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Talmon, Y.; Gilbey, P.; Fridman, N.; Wishniak, A.; Roguin, N. Acute Myopericarditis Complicating Acute Tonsillitis: Beware the Young Male Patient with Tonsillitis Complaining of Chest Pain. Ann. Otol. Rhinol. Laryngol. 2008, 117, 295–297. [Google Scholar] [CrossRef]
- Karjalainen, J. Streptococcal Tonsillitis and Acute Nonrheumatic Myopericarditis. Chest 1989, 95, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Mavrogeni, S.; Bratis, K.; Kitsiou, A.; Kolovou, G. Streptococcal Tonsillitis and Acute Streptococcal Myocarditis: An Unusual Combination Assessed by Cardiac Magnetic Resonance Imaging and Endomyocardial Biopsy. Ann. Otol. Rhinol. Laryngol. 2012, 121, 604–608. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Neagu, O.; Rodríguez, A.F.; Callon, D.; Andréoletti, L.; Cohen, M.C. Myocarditis Presenting as Sudden Death in Infants and Children: A Single Centre Analysis by ESGFOR Study Group. Pediatric Dev. Pathol. 2021, 24, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kalpakos, T.; Wilgenhof, A.; Michiels, V.; Cosyns, B.; Vermeersch, P. Streptococcal Pharyngitis Associated Myocarditis (SPAM): The Perfect ST-Segment Elevation Myocardial Infarction (STEMI) Mimic in Young Individuals. A Case Series. Acta Cardiol. 2021, 76, 449–454. [Google Scholar] [CrossRef]
- Derbas, L.A.; Samanta, A.; Potla, S.; Younis, M.; Schmidt, L.M.; Saeed, I.M. Separating Acute Rheumatic Fever from Nonrheumatic Streptococcal Myocarditis. Case Rep. Med. 2019, 2019, 4674875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C. 31-Jähriger Mann Mit Fieber, Thoraxschmerz Und Angina Tonsillaris. DMW Dtsch. Med. Wochenschr. 2019, 144, 201–202. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.E.; Coulson, J.D.; Sekar, P.; Resar, J.R.; Nelson McMillan, K. Non-Rheumatic Streptococcal Myocarditis Mimicking Acute Myocardial Infarction in an Adolescent Male. Cardiol. Young 2018, 28, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Puga, L.; Teixeira, R.; Botelho, A.; Lourenço, C.; Gonçalves, L. Acute Non-Rheumatic Myopericarditis: A Rare Complication of Pharyngitis. Eur. J. Case Rep. Intern. Med. 2018, 5, 000987. [Google Scholar] [CrossRef]
- Pourmand, A.; Gelman, D.; Davis, S.; Shokoohi, H. Nonrheumatic Myopericarditis Post Acute Streptococcal Pharyngitis: An Uncommon Cause of Sore Throat with ST Segment Elevation. Am. J. Emerg. Med. 2017, 35, 806.e1–806.e3. [Google Scholar] [CrossRef]
- Sturmberger, T.; Niel, J.; Aichinger, J.; Ebner, C. Acute Myocarditis with Normal Wall Motion Detected with 2D Speckle Tracking Echocardiography. Echo Res. Pract. 2016, 3, K15–K19. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, J.L.; Jurado, M.; Porres-Aguilar, M.; Olivas-Chacon, C.; Porres-Muñoz, M.; Mukherjee, D.; Taveras, J. Acute Nonrheumatic Streptococcal Myocarditis Resembling St-Elevation Acute Myocardial Infarction in a Young Patient. Bayl. Univ. Med. Cent. Proc. 2015, 28, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Chikly, A.; Durst, R.; Lotan, C.; Chen, S. Recurrent Acute Nonrheumatic Streptococcal Myocarditis Mimicking STEMI in a Young Adult. Case Rep. Cardiol. 2014, 2014, 964038. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.; Dooris, M.; Woods, M.L. Non-Rheumatic Streptococcal Myocarditis—Warm Hands, Warm Heart. J. Med. Microbiol. 2013, 62, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, G.A.; Gainor, J.F.; Stamm, L.M.; Weinberg, A.N.; Dec, G.W.; Ruskin, J.N. Acute Nonrheumatic Streptococcal Myocarditis: STEMI Mimic in Young Adults. Am. J. Med. 2012, 125, 1230–1233. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Bar-Ilan, A.; Goland, S.; Somin, M.; Doniger, T.; Basevitz, A.; Unger, R. Perimyocarditis Following Streptococcal Group A Infection: From Clinical Cases to Bioinformatics Analysis. Eur. J. Intern. Med. 2010, 21, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Mokabberi, R.; Shirani, J.; Afsaneh, H.M.; Go, B.D.; Schiavone, W. Streptococcal Pharyngitis-Associated Myocarditis Mimicking Acute STEMI. JACC Cardiovasc. Imaging 2010, 3, 892–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talmon, Y.; Ishai, R.; Samet, A.; Sturman, A.; Roguin, N. Acute Myopericarditis Complicating Acute Tonsillitis: A Prospective Study. Ann. Otol. Rhinol. Laryngol. 2009, 118, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Khavandi, A.; Whitaker, J.; Elkington, A.; Probert, J.; Walker, P.R. Acute Streptococcal Myopericarditis Mimicking Myocardial Infarction. Am. J. Emerg. Med. 2008, 26, 638.e1–638.e2. [Google Scholar] [CrossRef] [PubMed]
- Kochar, M.; López-Candales, A.; Ramani, G.; Rajagopalan, N.; Edelman, K. Unusual Echocardiographic Features Seen in a Case of Giant Cell Myocarditis. Can. J. Cardiol. 2008, 24, 855–856. [Google Scholar] [CrossRef] [Green Version]
- Said, S.A.M.; Severin, W.P.J. Acute Nonrheumatic Myopericarditis Associated with Group A Hemolytic Streptococcal Tonsillitis in a Male ICU-Nurse. Neth. J. Med. 1998, 53, 266–270. [Google Scholar] [CrossRef]
- Gill, M.V.; Klein, N.C.; Cunha, B.A. Nonrheumatic Poststreptococcal Myocarditis. Heart Lung 1995, 24, 425–426. [Google Scholar] [CrossRef]
- Putterman, C.; Caraco, Y.; Shalit, M. Acute Nonrheumatic Perimyocarditis Complicating Streptococcal Tonsillitis. Cardiology 1991, 78, 156–160. [Google Scholar] [CrossRef]
- Caraco, J.; Arnon, R.; Raz, I. Atrioventricular Block Complicating Acute Streptococcal Tonsillitis. Heart 1988, 59, 389–390. [Google Scholar] [CrossRef]
- Acute Myocarditis-StatPearls-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441847/ (accessed on 12 April 2022).
- Hufnagel, G.; Pankuweit, S.; Richter, A.; Schönian, U.; Maisch, B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). Herz 2000, 25, 279–285. [Google Scholar] [CrossRef]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.-M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Buttà, C.; Zappia, L.; Laterra, G.; Roberto, M. Diagnostic and Prognostic Role of Electrocardiogram in Acute Myocarditis: A Comprehensive Review. Ann. Noninvasive Electrocardiol. 2020, 25, e12726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, E.B.; Glancy, D.L. ST-Segment Depression and T-Wave Inversion: Classification, Differential Diagnosis, and Caveats. Clevel. Clin. J. Med. 2011, 78, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Shauer, A.; Gotsman, I.; Keren, A.; Zwas, D.R.; Hellman, Y.; Durst, R.; Admon, D. Acute Viral Myocarditis: Current Concepts in Diagnosis and Treatment. Isr. Med. Assoc. J. 2013, 15, 180–185. [Google Scholar]
- Smith, S.C.; Ladenson, J.H.; Mason, J.W.; Jaffe, A.S. Elevations of Cardiac Troponin I Associated with Myocarditis. Experimental and Clinical Correlates. Circulation 1997, 95, 163–168. [Google Scholar] [CrossRef]
- Angelini, A.; Calzolari, V.; Calabrese, F.; Boffa, G.M.; Maddalena, F.; Chioin, R.; Thiene, G. Myocarditis Mimicking Acute Myocardial Infarction: Role of Endomyocardial Biopsy in the Differential Diagnosis. Heart 2000, 84, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Tijmes, F.; Thavendiranathan, P.; Udell, J.A.; Seidman, M.A.; Hanneman, K. Cardiac MRI Assessment of Nonischemic Myocardial Inflammation: State of the Art Review and Update on Myocarditis Associated with COVID-19 Vaccination. Radiol. Cardiothorac. Imaging 2021, 3, e210252. [Google Scholar] [CrossRef]
- Sanguineti, F.; Garot, P.; Mana, M.; O’h-Ici, D.; Hovasse, T.; Unterseeh, T.; Louvard, Y.; Troussier, X.; Morice, M.C.; Garot, J. Cardiovascular Magnetic Resonance Predictors of Clinical Outcome in Patients with Suspected Acute Myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17, 78. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Morimoto, R.; Ando, R.; Ito, R.; Araki, T.; Mizutani, T.; Kazama, S.; Kimura, Y.; Oishi, H.; Kuwayama, T.; et al. Recurrent Fulminant Non-Rheumatic Streptococcal Myocarditis Proven by Endomyocardial Biopsy and Autopsy. J. Cardiol. Cases, 2022; in press. [Google Scholar] [CrossRef]
- Tandon, R.; Sharma, M.; Chandrashekhar, Y.; Kotb, M.; Yacoub, M.H.; Narula, J. Revisiting the Pathogenesis of Rheumatic Fever and Carditis. Nat. Rev. Cardiol. 2013, 10, 171–177. [Google Scholar] [CrossRef]


| Study (First Author, Publication Year) | Number of Patients (n) | Age (Years, Range) | Sex | ECG Findings | Cardiac Markers | Clinical Findings and Further Diagnostic Workup |
|---|---|---|---|---|---|---|
| Neagu, 2021 [12] | 1 | 6 | Female | N/A | N/A | Death within a day after the onset of symptoms (i.e., sore throat, nausea and vomiting) Post-mortem histological features: Diffuse severe myocarditis with involvement of pericardium and AV node |
| Kalpakos, 2021 [13] | 1 | 33 | Male | STE (posterolateral leads) | +(Troponin T, CK, transaminases) | Chest pain, epigastric discomfort and nausea Echocardiography: inferior-lateral hypokinesia Cardiac MRI: myocarditis (inferior-lateral segment of left ventricle) |
| Derbas, 2019 [14] | 1 | 25 | Male | STE (V2-V5) | +(Troponins (NT-pro-BNP)) | Chest pain (pleuritic) as well as dyspnea Echocardiography: global hypokinesia, LVEF 37% Cardiac MRI: left ventricular dysfunction with edema in the apical and mid-anterior wall without late gadolinium enhancement |
| Müller, 2019 [15] | 1 | 31 | Male | N/A | +(Troponin T, pro-NTBNP) | Chest pain Echocardiography: regional wall motion abnormalities of the left ventricle (posteromedial and inferomedial akinesia); moderate mitral valve insufficiency; accessory mitral valve tissue (AMVT) (no clinical correlation between AMVT and myocarditis) |
| O’Brien, 2018 [16] | 1 | 17 | Male | STE (II, III, aVF, V4–V6) | +(Troponin, CK-MB) | Chest pain that worsened when lying down and during deep inspiration as well as additional dyspnea Echocardiography: LVEF 49% Cardiac MRI: transmural delayed enhancement of the mid-septum and apex |
| Silva, 2018 [17] | 1 | 18 | Male | 1st episode: STE (I, II, aVL, V4–V6) 2nd episode: STE (V3–V5), T wave inversion (I, aVL, V3–V4) | +(Troponin I) | Chest pain Cardiac MRI: subepicardial enhancement in the inferolateral wall Additional information: 2nd episode 2 weeks after resolution of initial symptoms |
| Pourmand, 2017 [18] | 1 | 34 | Male | STE (mild; inferior limb leads) | +(CK, CK-MB, Troponin) | Radiating chest pain (to back and left and right arm) Echocardiography: mild pericardial effusion |
| Sturmberger, 2016 [19] | 1 | 26 | Male | Biphasic T waves (II, III, aVF, V1–V4) | +(CK, CK-MB, high-sensitivity Troponin I) | Chest pain Echocardiography: normal findings 2D speckle tracking imaging: decrease of systolic longitudinal strain of the posterior and lateral LV-wall segments Cardiac MRI: subepicardial delayed enhancement at lateral and posterior walls |
| Aguirre, 2015 [20] | 1 | 43 | Male | STE (I, II, aVF, V4–V6) | +(Troponin I, CK-MB) | Chest pain, epigastric pain Cardiac MRI (17 day after discharge): T2 signal intensity involving the subepicardial myocardium |
| Chikly, 2014 [21] | 1 | 37 | Male | STE (II, III, aVF) | +(hs-Troponin T, CPK) | Chest pain Echocardiography: akinesia of the inferior wall Cardiac MRI: T1 hyperintense areas in the inferolateral wall Additional information: This patient had a quite similar cardiac condition 5 years ago (also a few days after a group A streptococcal pharyngitis) |
| Chaudhuri, 2013 [22] | 2 | Mean 23.5 (18–29) | Male | STE in both patients | +(Troponin I) | Chest pain (patient 1 left-sided; patient 2 positional and precordial) Echocardiography: LVEF 48% and akinesia in the region of the apex in 1 patient and LVEF 52% and hypokinesia of the apex as well as mild pericardial effusion in the other one Cardiac MRI (in one patient): myocardial edema as well as subepicardial and intramural late gadolinium enhancement |
| Mavrogeni, 2012 [10] | 17 | Median 23 (18–29) | Male | Inverted T waves (V2–V6 and/or II, III, aVF in 15 patients) Nonspecific ST changes (in 2 patients) | + in 8 patients (CK-MB and Troponin I) − in 9 patients | 15 patients with severe chest pain, atypical chest discomfort experienced by 2 patients Cardiac MRI: 13 patients with normal LV function; impaired LV function in 4 patients (median LVEF 49.5%—significantly reduced EF compared to other patients); T2 enhancement in 16 patients; EGE values increased in 16 patients; LGE in 13 patients |
| Upadhyay, 2012 [23] | 5 | Mean 32.6 (22–47) | Male | STE (inferior: 2 patients; lateral: 1; inferolateral: 1; diffuse: 1) | +(Troponin T; in 1 patient N/A) | Chest pain |
| Malnick, 2010 [24] | 4 | Mean 30.5 (29–32) | Male | STE in 3 patients (I, aVL, V2–V6; inferior wall; minimal ST elevations in I and II); “pronounced T waves” in 1 patient (lateral leads) | +(in all patients Troponin, in one also AST, LDH, CPK) | Chest pain (2 radiating; in 1 patient to left arm, in other patient also scapular pain with radiation to left arm and shoulder); palpitations in one patient; pericardial friction rub in one patient; syncope in one patient |
| Mokabberi, 2010 [25] | 8 | Mean? (20–35) | 7 Male, 1 Female | STE (5 anterolateral, 3 inferior) | +(CK in 7, CK-MB in 8, Troponin T in 8) | Chest pain (non-pleuritic) Echocardiography: regional wall motion abnormality in all patients; mitral regurgitation in 4 patients (trivial-to-mild) Cardiac MRI (performed in 7 patients): subepicardial LGE in all patients; LVEF slightly reduced in 4 patients |
| Talmon, 2009 [26] | 2 | Mean 48.5 (35–62) | 1 Male, 1 Female | ST-T changes in both patients | +(Troponin I) in one patient | Pericardial rub in one patient and chest pain in the other patient |
| Khavandi, 2008 [27] | 1 | 25 | Male | STE (I, aVL) | +(Troponin I) | Radiating chest pain (to arms) Cardiac catheterization: apical hypokinesia (contrary to echocardiography which did not show any visible regional wall motion abnormality); elevated LVEDP (20 mmHg) |
| Kochar, 2008 [28] | 1 | 18 | Male | Left axis deviation; Q waves (III, aVF) | +(BNP and Troponin I) | Syncopal episode; worsening dyspnea during physical activity; hypotension; rales (base of the lungs); jugular vein distention Echocardiography (initial): globally hypokinetic LV; EF 20–25% Echocardiography (few days later): worsening of EF (10–15%); contrast throughout LV cavity (consistent with low flow state) Echocardiography (2 weeks after discharge): large and mobile homogenous echodensities (suggesting clots); resolution of contrast seen in previous echocardiography Biopsy: giant cell myocarditis |
| Talmon, 2008 [8] | 2 (other 9 only suspected) | Mean 24.5 (17–32) | Male | Negative T waves in 1 patient; STE in other patient | +(CK, LDH, Troponin I) | Chest pain in both patients; loud second heart sound in 1 patient Echocardiography: mild pericardial effusion in 1 patient |
| Said, 1998 [29] | 1 | 38 | Male | STE (II, III, aVF, V5–V6); peaked T waves (precordial leads); non-sustained ventricular tachycardia | +(CK, CK-MB, AST, ALT, LDH) | Chest pain that worsened with respiration; dizziness, nausea and diaphoresis Echocardiography: slightly increased LVEDD (59 mm); diffuse hypokinesia; reduced LVEF (39%); mild mitral regurgitation; mild pericardial effusion |
| Gill, 1995 [30] | 1 | 16 | Male | STE (II, III, aVF); T wave inversions (V1–V2) | +(CK and LDH) | Radiating chest pain (to left arm) as well as nausea and vomiting Left ventricular angiography: myopathic left ventricle with normal coronary arteries; EF 25% Echocardiography: slightly enlarged left ventricle (with reduced EF) Biopsy of right ventricle: lymphocytic myopericarditis |
| Putterman, 1991 [31] | 1 | 20 | Male | STE (I, II, aVL, aVF, V4–V6); T wave inversions (V1–V3) | +(CK, CK-MB, AST, LDH) | Radiating chest pain (to left arm) |
| Karjalainen, 1989 [9] | 3 (2 case reports and 1 prospective study) | Mean 20.5 (20–21) 3rd patient’s age N/A | Male | Patient 1: STE (I, II, aVL, V3–V6 + peaked T waves) Patient 2: STE (II, III, aVF, V3–V6) Patient 3: convex ST segment (I, V3–V6) with biphasic T waves; negative T waves (II, III, aVF) | +(CK and CK-MB) 3rd patient N/A | Clinical characteristics: Patient 1: left-sided chest pain (non-radiating) Patient 2: chest pain radiating to the left arm, nausea, loud S3 as well as an S4 heart sound Patient 3: did not experience any cardiac symptoms Echocardiography: Patient 1: LVEDD 58 mm, hypokinesia of the apex (anterior wall) Patient 2: LVEDD 49 mm, hypokinesia of the inferior wall of the LV, mild pericardial effusion Other imaging: Patient 1: positive myocardial scan (99mTc) Patient 2: moderate enlargement of the left heart as well as mild pulmonary venous congestion on chest X-ray |
| Caraco, 1988 [32] | 1 | 38 | Female | Alternating second-/third-degree AV block | −(CK normal and other cardiac markers N/A) | Irregular pulse (60 bpm); 2/6 systolic murmur at base of the heart |
| Gore, 1947 [5] | 11 | Mean 24 (18–33) | Male | Abnormal ECG in 1 patient | N/A | Clinical characteristics: cyanosis present in 5, dyspnea/orthopnea in 5, Cheyne–Stokes respiration in 1, cardiac arrhythmia/irregularity in 3, unexpected death in 5, bronchopneumonia in 7 (of these, 2 interstitial), serous effusions in 6, and pulmonary edema in 3 patients Histology (in brackets estimated severity): diffuse type in 5 cases (1 mild, 3 moderate, 1 marked), mixed type in 2 cases (2 moderate), and interstitial type in 4 patients (1 mild, 3 moderate) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmutzler, L.; Mirna, M.; Hoppe, U.C.; Lichtenauer, M. From Streptococcal Pharyngitis/Tonsillitis to Myocarditis: A Systematic Review. J. Cardiovasc. Dev. Dis. 2022, 9, 170. https://doi.org/10.3390/jcdd9060170
Schmutzler L, Mirna M, Hoppe UC, Lichtenauer M. From Streptococcal Pharyngitis/Tonsillitis to Myocarditis: A Systematic Review. Journal of Cardiovascular Development and Disease. 2022; 9(6):170. https://doi.org/10.3390/jcdd9060170
Chicago/Turabian StyleSchmutzler, Lukas, Moritz Mirna, Uta C. Hoppe, and Michael Lichtenauer. 2022. "From Streptococcal Pharyngitis/Tonsillitis to Myocarditis: A Systematic Review" Journal of Cardiovascular Development and Disease 9, no. 6: 170. https://doi.org/10.3390/jcdd9060170