Ventricular Tachycardia Ablation Guided by Functional Substrate Mapping: Practices and Outcomes
Abstract
:1. Introduction
2. Our Definition of Functional Mapping
3. Techniques for VT Functional Substrate Mapping
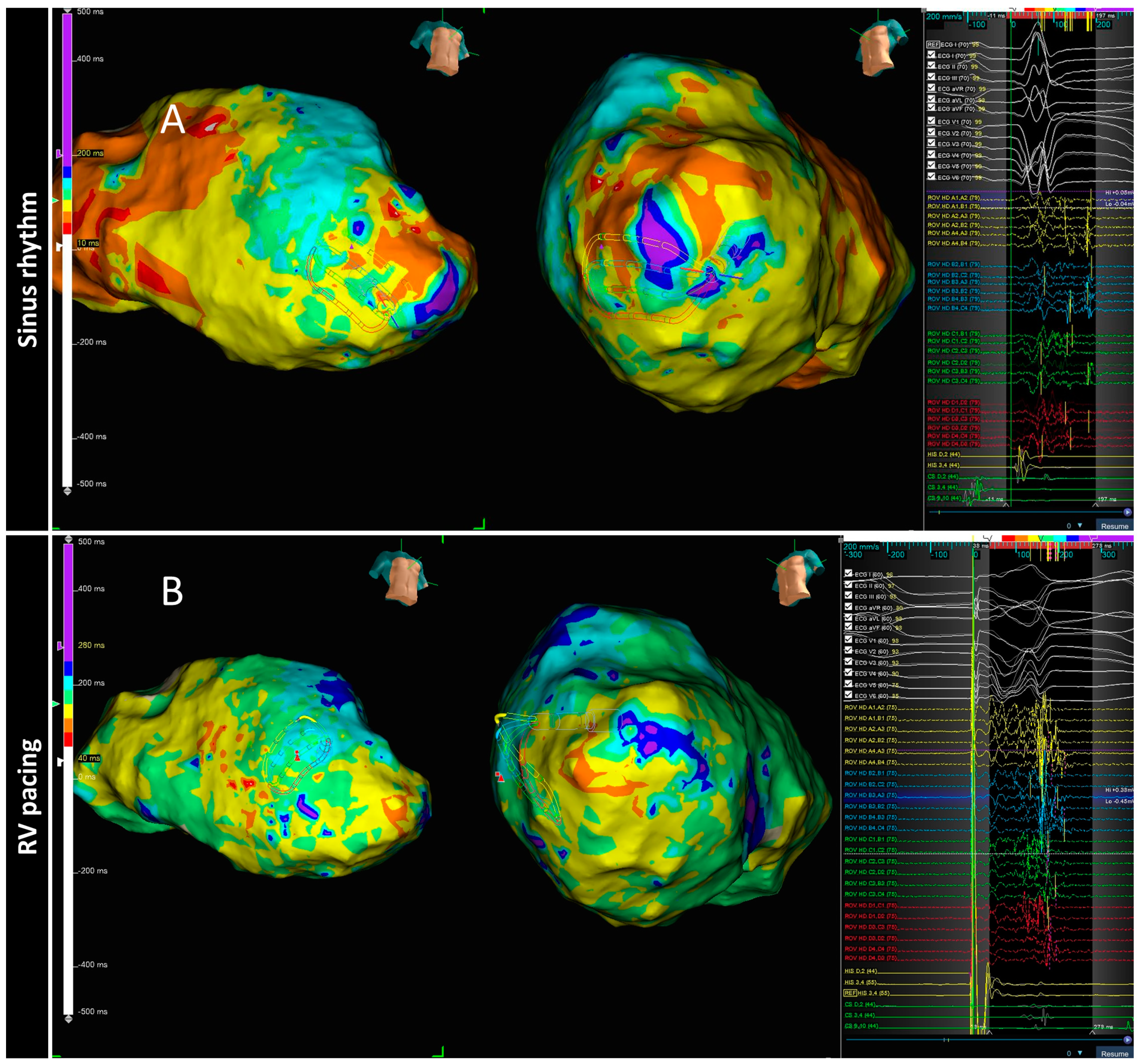
3.1. Decrement Evoked Potential (DEEP) Mapping
3.2. Hidden Slow Conduction (HSC) Mapping
3.3. Evoked Delayed Potential (EDP) Mapping
3.4. Paced Electrogram Feature Analysis (PEFA)
3.5. The Barts Sense Protocol
3.6. PHYSIO-VT
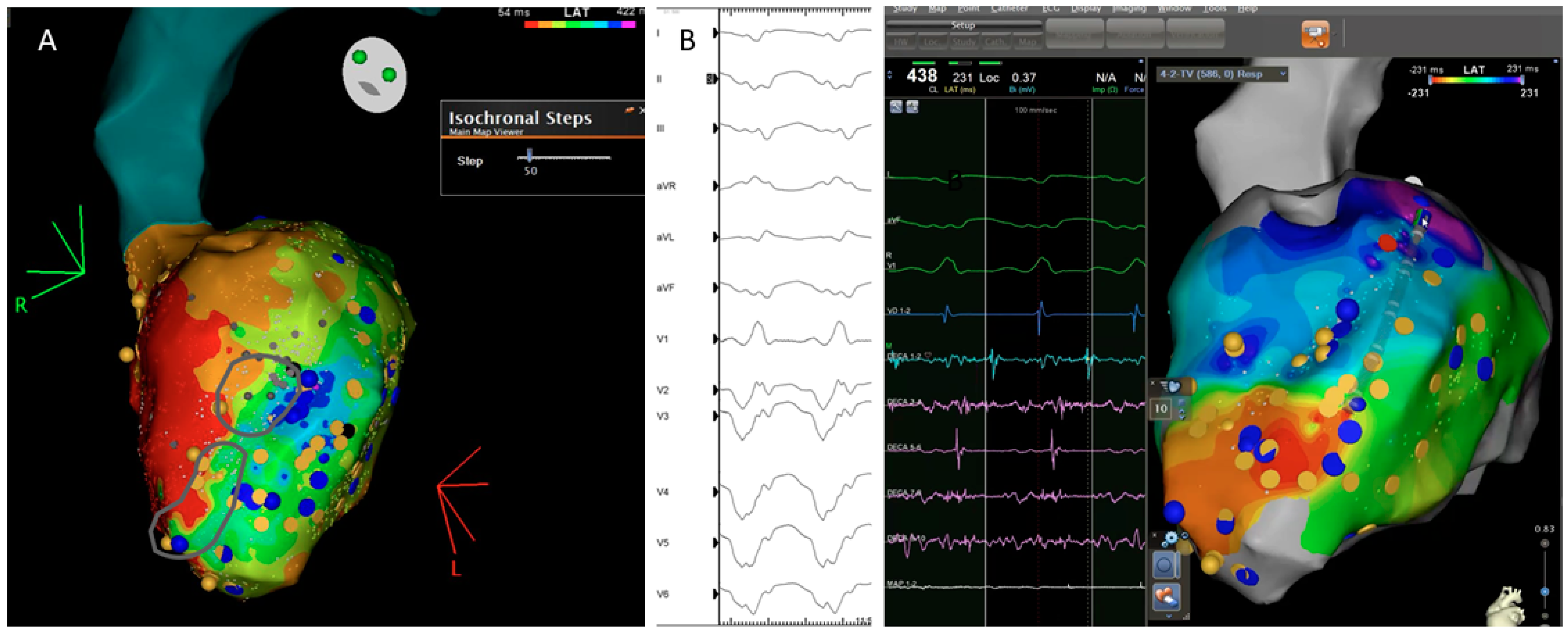
3.7. Isochronal Late Activation Mapping (ILAM)
4. Discussion
- The arrhythmic substrate is dynamic: its electric properties can change with different pacing settings and that help unmask regions that are critical for re-entry.
- Late potentials showing decremental properties (DEEPs) seem to be more likely associated with VT isthmuses.
- A pace protocol based in ventricular extra-stimulus has demonstrated to be able to change the shape and duration of selected EGMs, especially in patients with small scars. Targeting those electrograms that delay from the far field signal could lead to less VT recurrence in the follow up.
- The direction of the wavefront also has an important impact in the arrhythmic substrate characterization and needs careful evaluation in some patients.
- The analysis of the timing of the electrograms, not absolute and individually, but relative to the duration of all the ventricular electrograms and their pattern of propagation, has led to the development of a method for the identification of deceleration zones. These DZs have shown a good correlation with VT isthmus.
4.1. Additional Value as Compared to Historical VT Cohorts
4.2. Functional Substrate and High Density Mapping: Ablate More or Ablate Less?
4.3. Current Practice and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trappe, H.-J.; Brugada, P.; Talajic, M.; Della Bella, P.; Lezaun, R.; Mulleneers, R.; Wellens, H.J. Prognosis of patients with ventricular tachycardia and ventricular fibrillation: Role of the underlying etiology. J. Am. Coll. Cardiol. 1988, 12, 166–174. [Google Scholar] [CrossRef]
- Larsen, G.K.; Evans, J.; Lambert, W.E.; Chen, Y.; Raitt, M.H. Shocks burden and increased mortality in implantable cardioverter-defibrillator patients. Heart Rhythm 2011, 8, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, S.; Yao, Y.; Jiang, J.; Jiang, C.; Xue, Y.; Zhan, X.; Hu, H.; Fu, G.; Gu, K.; et al. Pan-Asia United States PrEvention of Sudden Cardiac Death Catheter Ablation Trial (PAUSE-SCD): Rationale and study design. J. Interv. Card. Electrophysiol. 2019, 57, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bella, P.D.; Baratto, F.; Vergara, P.; Bertocchi, P.; Santamaria, M.; Notarstefano, P.; Calò, L.; Orsida, D.; Tomasi, L.; Piacenti, M.; et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients With an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation 2022, 145, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Arenal, A.; Ávila, P.; Jiménez-Candil, J.; Tercedor, L.; Calvo, D.; Arribas, F.; Fernández-Portales, J.; Merino, J.L.; Hernández-Madrid, A.; Fernández-Avilés, F.J.; et al. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J. Am. Coll. Cardiol. 2022, 79, 1441–1453. [Google Scholar] [CrossRef]
- Biase, L.D.; Burkhardt, J.D.; Lakkireddy, D.; Carbucicchio, C.; Mohanty, S.; Mohanty, P.; Trivedi, C.; Santangeli, P.; Bai, R.; Forleo, G.; et al. Ablation of Stable VTs Versus Substrate Ablation in Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 66, 2872–2882. [Google Scholar] [CrossRef]
- Berruezo, A.; Fernández-Armenta, J.; Andreu, D.; Penela, D.; Herczku, C.; Evertz, R.; Cipolletta, L.; Acosta, J.; Borràs, R.; Arbelo, E.; et al. Scar dechanneling. Circ. Arrhythmia Elec. 2015, 8, 326–336. [Google Scholar] [CrossRef]
- Hsia, H.H.; Callans, D.J.; Marchlinski, F.E. Characterization of Endocardial Electrophysiological Substrate in Patients With Nonischemic Cardiomyopathy and Monomorphic Ventricular Tachycardia. Circulation 2003, 108, 704–710. [Google Scholar] [CrossRef]
- Marchlinski, F.E.; Callans, D.J.; Gottlieb, C.D.; Zado, E. Linear Ablation Lesions for Control of Unmappable Ventricular Tachycardia in Patients with Ischemic and Nonischemic Cardiomyopathy. Circulation 2000, 101, 1288–1296. [Google Scholar] [CrossRef]
- Roca-Luque, I.; Zaraket, F.; Garre, P.; Sanchez-Somonte, P.; Quinto, L.; Borras, R.; Guasch, E.; Arbelo, E.; Tolosana, J.M.; Brugada, J.; et al. Accuracy of standard bipolar amplitude voltage thresholds to identify late potential channels in ventricular tachycardia ablation. J. Interv. Card. Electrophysiol. 2022, 1–11. [Google Scholar] [CrossRef]
- Harada, T.; Stevenson, W.G.; Kocovic, D.Z.; Friedman, P.L. Catheter Ablation of Ventricular Tachycardia After Myocardial Infarction: Relation of Endocardial Sinus Rhythm Late Potentials to the Reentry Circuit. J. Am. Coll. Cardiol. 1997, 30, 1015–1023. [Google Scholar] [CrossRef]
- Jaïs, P.; Maury, P.; Khairy, P.; Sacher, F.; Nault, I.; Komatsu, Y.; Hocini, M.; Forclaz, A.; Jadidi, A.S.; Weerasooryia, R.; et al. Elimination of Local Abnormal Ventricular Activities. Circulation 2012, 125, 2184–2196. [Google Scholar] [CrossRef]
- Roca-Luque, I.; Quinto, L.; Sanchez-Somonte, P.; Garre, P.; Alarcón, F.; Zaraket, F.; Vazquez, S.; Prat-Gonzalez, S.; Ortiz-Perez, J.T.; Guasch, E.; et al. Late Potential Abolition in Ventricular Tachycardia Ablation. Am. J. Cardiol. 2022, 174, 53–60. [Google Scholar] [CrossRef]
- Quinto, L.; Sanchez-Somonte, P.; Alarcón, F.; Garre, P.; Castillo, A.; Antonio, R.S.; Borras, R.; Guasch, E.; Arbelo, E.; Tolosana, J.M.; et al. Ventricular tachycardia burden reduction after substrate ablation: Predictors of recurrence. Heart Rhythm 2021, 18, 896–904. [Google Scholar] [CrossRef]
- Anter, E.; Josephson, M.E. Bipolar voltage amplitude: What does it really mean? Heart Rhythm 2016, 13, 326–327. [Google Scholar] [CrossRef]
- Takigawa, M.; Relan, J.; Martin, R.; Kim, S.; Kitamura, T.; Frontera, A.; Cheniti, G.; Vlachos, K.; Massoullié, G.; Martin, C.A.; et al. Effect of bipolar electrode orientation on local electrogram properties. Heart Rhythm 2018, 15, 1853–1861. [Google Scholar] [CrossRef]
- Proietti, R.; Dowd, R.; Gee, L.V.; Yusuf, S.; Panikker, S.; Hayat, S.; Osman, F.; Patel, K.; Salim, H.; Aldhoon, B.; et al. Impact of a high-density grid catheter on long-term outcomes for structural heart disease ventricular tachycardia ablation. J. Interv. Card. Electrophysiol. 2021, 62, 519–529. [Google Scholar] [CrossRef]
- Tschabrunn, C.M.; Roujol, S.; Dorman, N.C.; Nezafat, R.; Josephson, M.E.; Anter, E. High-Resolution Mapping of Ventricular Scar. Circ. Arrhythmia Electrophysiol. 2016, 9, e003841. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, S.; Garre, P.; Sánchez-Somonte, P.; Borras, R.; Quinto, L.; Caixal, G.; Lopez, M.P.; Althoff, T.; Guasch, E.; Arbelo, E.; et al. Orthogonal high density mapping with VT isthmus analysis vs. pure substrate VT ablation: A case-control study. Front. Cardiovasc. Med. 2022, 9, 912335. [Google Scholar] [CrossRef]
- Mountantonakis, S.E.; Park, R.E.; Frankel, D.S.; Hutchinson, M.D.; Dixit, S.; Cooper, J.; Callans, D.; Marchlinski, F.E.; Gerstenfeld, E.P. Relationship Between Voltage Map “Channels” and the Location of Critical Isthmus Sites in Patients with Post-Infarction Cardiomyopathy and Ventricular Tachycardia. J. Am. Coll. Cardiol. 2013, 61, 2088–2095. [Google Scholar] [CrossRef] [Green Version]
- Tung, R.; Vaseghi, M.; Frankel, D.S.; Vergara, P.; Di Biase, L.; Nagashima, K.; Yu, R.; Vangala, S.; Tseng, C.-H.; Choi, E.-K.; et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm 2015, 12, 1997–2007. [Google Scholar] [CrossRef]
- Ghanbari, H.; Baser, K.; Yokokawa, M.; Stevenson, W.; Della Bella, P.; Vergara, P.; Deneke, T.; Kuck, K.-H.; Kottkamp, H.; Fei, S.; et al. Noninducibility in Postinfarction Ventricular Tachycardia as an End Point for Ventricular Tachycardia Ablation and Its Effects on Outcomes. Circ. Arrhythmia Electrophysiol. 2014, 7, 677–683. [Google Scholar] [CrossRef]
- Lammers, W.J.; Kirchhof, C.; Bonke, F.I.; Allessie, M.A. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am. J. Physiol. Heart Circ. Physiol. 1992, 262, H47–H55. [Google Scholar] [CrossRef]
- Segal, O.R.; Chow, A.W.C.; Peters, N.S.; Davies, D.W. Mechanisms that initiate ventricular tachycardia in the infarcted human heart. Heart Rhythm 2010, 7, 57–64. [Google Scholar] [CrossRef]
- Jackson, N.; Gizurarson, S.; Viswanathan, K.; King, B.; Massé, S.; Kusha, M.; Porta-Sanchez, A.; Jacob, J.R.; Khan, F.; Das, M.; et al. Decrement Evoked Potential Mapping: Basis of a Mechanistic Strategy for Ventricular Tachycardia Ablation. Circ. Arrhythmia Electrophysiol. 2015, 8, 1433–1442. [Google Scholar] [CrossRef]
- Tusscher, K.H.W.J.T.; Noble, D.; Noble, P.J.; Panfilov, A.V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1573–H1589. [Google Scholar] [CrossRef]
- Porta-Sanchez, A.; Jackson, N.; Lukac, P.; Kristiansen, S.B.; Nielsen, J.M.; Gizurarson, S.; Massé, S.; Labos, C.; Viswanathan, K.; King, B.; et al. Multicenter Study of Ischemic Ventricular Tachycardia Ablation with Decrement-Evoked Potential (DEEP) Mapping with Extra Stimulus. JACC Clin. Electrophysiol. 2018, 4, 307–315. [Google Scholar] [CrossRef]
- Acosta, J.; Andreu, D.; Penela, D.; Cabrera, M.; Carlosena, A.; Korshunov, V.; Vassanelli, F.; Borras, R.; Martínez, M.; Fernández-Armenta, J.; et al. Elucidation of hidden slow conduction by double ventricular extrastimuli: A method for further arrhythmic substrate identification in ventricular tachycardia ablation procedures. Europace 2016, 20, 337–346. [Google Scholar] [CrossRef]
- Acosta, J.; Soto-Iglesias, D.; Jáuregui, B.; Armenta, J.F.; Penela, D.; Frutos-López, M.; Arana-Rueda, E.; Pedrote, A.; Mont, L.; Berruezo, A. Long-term outcomes of ventricular tachycardia substrate ablation incorporating hidden slow conduction analysis. Heart Rhythm 2020, 17, 1696–1703. [Google Scholar] [CrossRef]
- Riva, M.D.; Naruse, Y.; Ebert, M.; Androulakis, A.F.A.; Tao, Q.; Watanabe, M.; Wijnmaalen, A.P.; Venlet, J.; Brouwer, C.; Trines, S.A.; et al. Targeting the Hidden Substrate Unmasked by Right Ventricular Extrastimulation Improves Ventricular Tachycardia Ablation Outcome After Myocardial Infarction. JACC Clin. Electrophysiol. 2018, 4, 316–327. [Google Scholar]
- Oduneye, S.O.; Pop, M.; Shurrab, M.; Biswas, L.; Ramanan, V.; Barry, J.; Crystal, E.; A Wright, G. Distribution of abnormal potentials in chronic myocardial infarction using a real time magnetic resonance guided electrophysiology system. J. Cardiovasc. Magn. Reson. 2015, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariat, M.H.; Gupta, D.; E Gul, E.; Glover, B.; Hashemi, J.; Abdollah, H.; Baranchuk, A.; Simpson, C.; A Michael, K.; Redfearn, D.P.; et al. Ventricular substrate identification using close-coupled paced electrogram feature analysis. Europace 2018, 21, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Roelke, M.; Garan, H.; McGovern, B.A.; Ruskin, J.N. Analysis of the initiation of spontaneous monomorphic ventricular tachycardia by stored intracardiac electrograms. J. Am. Coll. Cardiol. 1994, 23, 117–122. [Google Scholar] [CrossRef]
- Saeed, M.; Link, M.S.; Mahapatra, S.; Mouded, M.; Tzeng, D.; Jung, V.; Contreras, R.; Swygman, C.; Homoud, M.; Estes, N.; et al. Analysis of intracardiac electrograms showing monomorphic ventricular tachycardia in patients with implantable cardioverter-defibrillators. Am. J. Cardiol. 2000, 85, 580–587. [Google Scholar] [CrossRef]
- Srinivasan, N.T.; Garcia, J.; Schilling, R.J.; Ahsan, S.; Babu, G.G.; Ang, R.; Dhinoja, M.B.; Hunter, R.J.; Lowe, M.; Chow, A.W.; et al. Multicenter Study of Dynamic High-Density Functional Substrate Mapping Improves Identification of Substrate Targets for Ischemic Ventricular Tachycardia Ablation. JACC Clin. Electrophysiol. 2020, 6, 1783–1793. [Google Scholar] [CrossRef]
- Peters, N.S.; Coromilas, J.; Hanna, M.S.; Josephson, M.E.; Costeas, C.; Wit, A.L. Characteristics of the Temporal and Spatial Excitable Gap in Anisotropic Reentrant Circuits Causing Sustained Ventricular Tachycardia. Circ. Res. 1998, 82, 279–293. [Google Scholar] [CrossRef]
- Rohr, S. Myofibroblasts in diseased hearts: New players in cardiac arrhythmias? Heart Rhythm 2009, 6, 848–856. [Google Scholar] [CrossRef]
- Anter, E.; Neuzil, P.; Reddy, V.Y.; Petru, J.; Park, K.M.; Sroubek, J.; Leshem, E.; Zimetbaum, P.J.; Buxton, A.E.; Kleber, A.G.; et al. Ablation of Reentry-Vulnerable Zones Determined by Left Ventricular Activation from Multiple Directions: A Novel Approach for Ventricular Tachycardia Ablation: A Multicenter Study (PHYSIO-VT). Circ. Arrhythmia Electrophysiol. Lippincott Williams Wilkins 2020, 13, 539–550. [Google Scholar] [CrossRef]
- Irie, T.; Yu, R.; Bradfield, J.S.; Vaseghi, M.; Buch, E.F.; Ajijola, O.; Macias, C.; Fujimura, O.; Mandapati, R.; Boyle, N.G.; et al. Relationship Between Sinus Rhythm Late Activation Zones and Critical Sites for Scar-Related Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2015, 8, 390–399. [Google Scholar] [CrossRef]
- Aziz, Z.; Shatz, D.; Raiman, M.; Upadhyay, G.A.; Beaser, A.D.; Besser, S.A.; Shatz, N.A.; Fu, Z.; Jiang, R.; Nishimura, T.; et al. Targeted Ablation of Ventricular Tachycardia Guided by Wavefront Discontinuities during Sinus Rhythm: A New Functional Substrate Mapping Strategy. Circulation 2019, 140, 1383–1397. [Google Scholar]
- Arenal, A.; Hernández, J.; Calvo, D.; Ceballos, C.; Atéa, L.; Datino, T.; Atienza, F.; González-Torrecilla, E.; Eidelman, G.; Miracle, A.; et al. Safety, Long-Term Results, and Predictors of Recurrence After Complete Endocardial Ventricular Tachycardia Substrate Ablation in Patients With Previous Myocardial Infarction. Am. J. Cardiol. 2013, 111, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Biase, L.D.; Santangeli, P.; Burkhardt, D.J.; Bai, R.; Mohanty, P.; Carbucicchio, C.; Dello Russo, A.; Casella, M.; Mohanty, S.; Pump, A.; et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, B.; Yokokawa, M.; Good, E.; Crawford, T.; Latchamsetty, R.; Jongnarangsin, K.; Ghanbari, H.; Oral, H.; PelosiJr, F.; Chugh, A.; et al. Characteristics of Intramural Scar in Patients with Nonischemic Cardiomyopathy and Relation to Intramural Ventricular Arrhythmias. Circ. Arrhythmia Electrophysiol. 2013, 6, 891–897. [Google Scholar] [CrossRef]
- Okubo, K.; Frontera, A.; Bisceglia, C.; Paglino, G.; Radinovic, A.; Foppoli, L.; Calore, F.; Della Bella, P. Grid Mapping Catheter for Ventricular Tachycardia Ablation. Circ. Arrhythmia Electrophysiol. 2019, 12, e007500. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Penela, D.; Andreu, D.; Cabrera, M.; Carlosena, A.; Vassanelli, F.; Alarcón, F.; Soto-Iglesias, D.; Korshunov, V.; Borras, R.; et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: A randomized study. Europace 2017, 20, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Strategy | Article | Population | Mapping System | Stimulation Setting | Measurement | Objective | RF Target | RF Time (min) | Results |
|---|---|---|---|---|---|---|---|---|---|
| DEEP | JACKSON 2015 | 6 ischemic. | Intraoperative mapping: custom-made 112 electrode ballon | If LP of fractionated EGM are identify: RV pacing 600 ms + VERP+20 ms | DEEP: delayed local potential after stimulation | To compare DEEP vs. LP mapping to identify VT isthmus (retrospectively) | VT critical sites based on activation mapping | N/A (surgical cryoablation) | DEEP mapping was more specific than LP mapping for identifying VT isthmus. |
| PORTA-SÁNCHEZ 2018 | 20 ischemic. | CARTO: 9 Decanav 6 Pentarray 4 ablation cath. | For all LPs: RV pacing 600 ms + VERP+20 ms | DEEP: S2 local potential delayes or splits > 10 ms compared with S1 | To compare DEEP vs. LP mapping to identify VT isthmus | DEEP area | 30.6 | Specificy of DEEP to detect VT isthmus was better than LPs | |
| HIDDEN SUBSTRATE | ACOSTA 2015 | 37 patients: 75.7% ischemic. | CARTO | Identify potential HSC-EGM (>3 deflections and <133 ms) and double extra VERP+60 and VERP+40 to 20 ms | HSC-EGM: potential HSC-EGM that delays after stimulation | To analyses characteristics of patients with HSC-EGM | CCs (scar dechanneling) and HSC-EGM | Interv. group: 17.41 Hist. cohort: 23.11 | Patients with HSC-EGM: More frequently ischaemic, smaller low voltage area, low number of LPs Location of HSC-EGM: EAM: 18.2% scar area vs. CMR: 92% scar area |
| DE RIVA 2018 | 60 ischemic. | CARTO | RV pacing 500 MS + single extra VERP+50 ms | EDP: low amplitude (<1.5) near field potentials with conduction delay > 10 ms or block. | To compare patients with hidden vs. not hidden substrate | EDPs | Interv. group: 15 Matched cohort: 13 | Hidden substrate group: Better FEVI, smaller scar and dense scar, higher 12 m VT free survival | |
| PEFA | REDFEARN 2018 | (1) 14 ischemic. and 5 healthy controls (2) 10 ischemic | Ensite Precision | RV pacing 600 ms(x6) + VERP 150 RV pacing 600 ms(x6) + VERP 100 RV pacing 600 ms(x6) + VERP 50 | 4 types of response related to latency and EGM duration | (1) To compare different EGM responses after stimulation protocol (2) To validate PEFA method | (1) Operators were blinded to PEFA (2) Type I and II | Interv group: 39.47 Cohort: 39.88 | (1) Type I and II responses: most frequently at VT termination sites (2) PEFA approach reduced VT inducibility |
| BARTS | SRINIVA-SAN 2018 | 30 ischemic. | Ensite Precision | Sinus rhythm (SR)(x5) + VERP 20 ms (SP) | Annotation of LP and LAVAs | To compare LP/LAVA with VT isthmus in two different maps: SR and SP | Total LPs and LAVAs | 32 | LP/LAVAs observed during SP were able to identify regions critical for VT ablation with a greater accuracy than SR mapping |
| PHYSIO VT | ANTER 2020 | 85 ischemic. | RHYTMIA 92.8% CARTO 7.2% | -SR and RV Pacing 600 ms and LV Pacing 600 ms | Area of activation maps (isochronal maps of 10 ms steps) | To compare areas of activation slowing and critical VT isthmus in three different maps (SR, RV and LV) | Acumulative area of activation slowing | 27.7 | The direction of LV activation is influenced by the magnitude and location of activation slowing: SR Mapping identify 66.2% of the entire area of activation slowing. RV and LV unmask an additional 33% |
| ILAMS | AZIZ 2019 | 120 patients: 50% ischemic | Ensite Precision | Annotation of last deflection and division of the total activation window in 8 equal isochrones | Deceleration zones (DZ): 3 isochrones in less than 1 cm. | To correlate DZ with VT isthmus | Primary DZs | 29 | DZs identify during SR are strongly predictive of critical sites for reentry. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Calvo, S.; Roca-Luque, I.; Porta-Sánchez, A. Ventricular Tachycardia Ablation Guided by Functional Substrate Mapping: Practices and Outcomes. J. Cardiovasc. Dev. Dis. 2022, 9, 288. https://doi.org/10.3390/jcdd9090288
Vázquez-Calvo S, Roca-Luque I, Porta-Sánchez A. Ventricular Tachycardia Ablation Guided by Functional Substrate Mapping: Practices and Outcomes. Journal of Cardiovascular Development and Disease. 2022; 9(9):288. https://doi.org/10.3390/jcdd9090288
Chicago/Turabian StyleVázquez-Calvo, Sara, Ivo Roca-Luque, and Andreu Porta-Sánchez. 2022. "Ventricular Tachycardia Ablation Guided by Functional Substrate Mapping: Practices and Outcomes" Journal of Cardiovascular Development and Disease 9, no. 9: 288. https://doi.org/10.3390/jcdd9090288





