Age-Related Conservation in Plant–Soil Feedback Accompanied by Ectomycorrhizal Domination in Temperate Forests in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Biotic Predictors of Vegetation Composition and Diversity
2.3. Soil Physicochemical and Bioinformatic Analyses
2.4. Statistical Analyses
3. Results
3.1. Plant–Soil Attributes
3.2. EcM Fungal Diversity
3.3. EcM Fungal Exploration Types
3.4. Age-Differentiated EcM Fungal Species
4. Discussion
4.1. Conservative Plant–Soil Traits Coupled to EcM Fungal Dominance
4.2. The Impacts of Aging on EcM Fungal Exploration Strategies
4.3. EcM Indicator Species across Forest Successional Stages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Wallwork, A.; Banin, L.F.; Dent, D.H.; Skiba, U.; Sayer, E. Soil carbon storage is related to tree functional composition in naturally regenerating tropical forests. Funct. Ecol. 2022, 36, 3175–3187. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chang, S.X.; Cheng, J.Y.; Liu, X.Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef]
- Yoder, B.J.; Ryan, M.G.; Waring, R.H.; Schoettle, A.W.; Kaufmann, M.R. Evidence of Reduced Photosynthetic Rates in Old Trees. For. Sci. 1994, 40, 513–527. [Google Scholar] [CrossRef]
- Curtis, P.S.; Gough, C.M. Forest aging, disturbance and the carbon cycle. New Phytol. 2018, 219, 1188–1193. [Google Scholar] [CrossRef]
- Hagenbo, A.; Hadden, D.; Clemmensen, K.E.; Grelle, A.; Manzoni, S.; Mölder, M.; Ekblad, A.; Fransson, P.; Vries, F. Carbon use efficiency of mycorrhizal fungal mycelium increases during the growing season but decreases with forest age across a Pinus sylvestris chronosequence. J. Ecol. 2019, 107, 2808–2822. [Google Scholar] [CrossRef]
- Bai, Z.; Ye, J.; Wei, Y.-L.; Yan, S.-K.; Yuan, H.-S. Soil depth-dependent C/N stoichiometry and fungal and bacterial communities along a temperate forest succession gradient. Catena 2021, 207, 105613. [Google Scholar] [CrossRef]
- Mikutta, R.; Turner, S.; Schippers, A.; Gentsch, N.; Meyer-Stuve, S.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Eger, A.; Hempel, G.; et al. Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 2019, 9, 10294. [Google Scholar] [CrossRef]
- Ma, Y.; Filley, T.R.; Szlavecz, K.; McCormick, M.K. Controls on wood and leaf litter incorporation into soil fractions in forests at different successional stages. Soil Biol. Biochem. 2014, 69, 212–222. [Google Scholar] [CrossRef]
- Blaško, R.; Holm Bach, L.; Yarwood, S.A.; Trumbore, S.E.; Högberg, P.; Högberg, M.N. Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol. Biochem. 2015, 91, 200–211. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Saulino, L.; Allevato, E.; Saracino, A.; Mazzoleni, S.; Idbella, M. Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus. Microorganisms 2023, 11, 1394. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, Y.; Yang, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Divergent assemblage patterns and driving forces for bacterial and fungal communities along a glacier forefield chronosequence. Soil Biol. Biochem. 2018, 118, 207–216. [Google Scholar] [CrossRef]
- Cline, L.C.; Zak, D.R. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 2015, 96, 3374–3385. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Taudiere, A.; Munoz, F.; Lesne, A.; Monnet, A.C.; Bellanger, J.M.; Selosse, M.A.; Moreau, P.A.; Richard, F. Beyond ectomycorrhizal bipartite networks: Projected networks demonstrate contrasted patterns between early- and late-successional plants in Corsica. Front. Plant Sci. 2015, 6, 881. [Google Scholar] [CrossRef]
- Wasyliw, J.; Karst, J.; Heijden, M. Shifts in ectomycorrhizal exploration types parallel leaf and fine root area with forest age. J. Ecol. 2020, 108, 2270–2282. [Google Scholar] [CrossRef]
- Lin, G.; McCormack, M.L.; Ma, C.; Guo, D.F. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 2017, 213, 1440–1451. [Google Scholar] [CrossRef]
- Cheeke, T.E.; Phillips, R.P.; Brzostek, E.R.; Rosling, A.; Bever, J.D.; Fransson, P. Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol. 2017, 214, 432–442. [Google Scholar] [CrossRef]
- Courty, P.E.; Buée, M.; Tech, J.J.T.; Brulé, D.; Colin, Y.; Leveau, J.H.J.; Uroz, S. Impact of soil pedogenesis on the diversity and composition of fungal communities across the California soil chronosequence of Mendocino. Mycorrhiza 2018, 28, 343–356. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Liu, Y.; He, H.; Kou, Y.; Liu, Q. Dominant plant species and soil properties drive differential responses of fungal communities and functions in the soils and roots during secondary forest succession in the subalpine region. Rhizosphere 2022, 21, 100483. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Zhang, H.; Tang, M. Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau. Int. J. Mol. Sci. 2022, 23, 6753. [Google Scholar] [CrossRef]
- Bahram, M.; Netherway, T.; Hildebrand, F.; Pritsch, K.; Drenkhan, R.; Loit, K.; Anslan, S.; Bork, P.; Tedersoo, L. Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol. 2020, 227, 1189–1199. [Google Scholar] [CrossRef]
- Averill, C.; Hawkes, C.V. Ectomycorrhizal fungi slow soil carbon cycling. Ecol. Lett. 2016, 19, 937–947. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Kyaschenko, J.; Varenius, K.; Clemmensen, K.E.; Dahlberg, A.; Karltun, E.; Stendahl, J. A group of ectomycorrhizal fungi restricts organic matter accumulation in boreal forest. Ecol. Lett. 2021, 24, 1341–1351. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Averill, C.; Bhatnagar, J.M.; Dietze, M.C.; Pearse, W.D.; Kivlin, S.N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. USA 2019, 116, 23163–23168. [Google Scholar] [CrossRef]
- Agerer, R. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Mundra, S.; Kauserud, H.; Okland, T.; Nordbakken, J.F.; Ransedokken, Y.; Kjonaas, O.J. Shift in tree species changes the belowground biota of boreal forests. New Phytol. 2022, 234, 2073–2087. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
- Rudawska, M.; Wilgan, R.; Janowski, D.; Iwański, M.; Leski, T. Shifts in taxonomical and functional structure of ectomycorrhizal fungal community of Scots pine (Pinus sylvestris L.) underpinned by partner tree ageing. Pedobiologia 2018, 71, 20–30. [Google Scholar] [CrossRef]
- Hagenbo, A.; Kyaschenko, J.; Clemmensen, K.E.; Lindahl, B.D.; Fransson, P.; Wurzburger, N. Fungal community shifts underpin declining mycelial production and turnover across a Pinus sylvestris chronosequence. J. Ecol. 2018, 106, 490–501. [Google Scholar] [CrossRef]
- Rosenvald, K.; Ostonen, I.; Uri, V.; Varik, M.; Tedersoo, L.; Lõhmus, K. Tree age effect on fine-root and leaf morphology in a silver birch forest chronosequence. Eur. J. For. Res. 2012, 132, 219–230. [Google Scholar] [CrossRef]
- Rees, M.; Condit, R.; Crawley, M.; Pacala, S.; Tilman, D. Long-term studies of vegetation dynamics. Science 2001, 293, 650–655. [Google Scholar] [CrossRef]
- Koizumi, T.; Hattori, M.; Nara, K. Ectomycorrhizal fungal communities in alpine relict forests of Pinus pumila on Mt. Norikura, Japan. Mycorrhiza 2018, 28, 129–145. [Google Scholar] [CrossRef]
- Mucha, J.; Peay, K.G.; Smith, D.P.; Reich, P.B.; Stefanski, A.; Hobbie, S.E. Effect of Simulated Climate Warming on the Ectomycorrhizal Fungal Community of Boreal and Temperate Host Species Growing Near Their Shared Ecotonal Range Limits. Microb. Ecol. 2018, 75, 348–363. [Google Scholar] [CrossRef]
- Tan, K.; Piao, S.; Peng, C.; Fang, J. Satellite-based estimation of biomass carbon stocks for northeast China’s forests between 1982 and 1999. For. Ecol. Manag. 2007, 240, 114–121. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, T.; Wei, L.; Shu, Y. The spatial distribution of forest carbon sinks and sources in China. Chin. Sci. Bull. 2012, 57, 1699–1707. [Google Scholar] [CrossRef]
- Chen, S.; Lu, N.; Fu, B.; Wang, S.; Deng, L.; Wang, L. Current and future carbon stocks of natural forests in China. For. Ecol. Manag. 2022, 511, 120137. [Google Scholar] [CrossRef]
- Zhang, Y.; Drobyshev, I.; Gao, L.; Zhao, X.; Bergeron, Y. Disturbance and regeneration dynamics of a mixed Korean pine dominated forest on Changbai Mountain, North-Eastern China. Dendrochronologia 2014, 32, 21–31. [Google Scholar] [CrossRef]
- Ye, J.; Cong, L.; Liu, S.; Tian, S.; Sun, H.; Luan, Y.; Bai, Z. Climatic Variability Determines the Biological Diversity and Function of a Mixed Forest in Northeastern China at the Local-Scale. Forests 2023, 14, 98. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Wang, S.P.; Ali, A.; Gazol, A.; Ruiz-Benito, P.; Wang, X.G.; Lin, F.; Ye, J.; Hao, Z.Q.; Loreau, M. Aboveground carbon storage is driven by functional trait composition and stand structural attributes rather than biodiversity in temperate mixed forests recovering from disturbances. Ann. For. Sci. 2018, 75, 67. [Google Scholar] [CrossRef]
- Yuan, Z.; Gazol, A.; Wang, X.; Lin, F.; Ye, J.; Zhang, Z.; Suo, Y.; Kuang, X.; Wang, Y.; Jia, S.; et al. Pattern and dynamics of biomass stock in old growth forests: The role of habitat and tree size. Acta Oecol. 2016, 75, 15–23. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Wang, S.; Gazol, A.; Freckleton, R.; Wang, X.; Lin, F.; Ye, J.; Zhou, L.; Hao, Z.; et al. Abiotic and biotic determinants of coarse woody productivity in temperate mixed forests. Sci. Total Environ. 2018, 630, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuan, Z.; Ali, A.; Sanaei, A.; Mao, Z.; Ding, F.; Zheng, D.; Fang, S.; Jia, Z.; Tao, Z.; et al. Anthropogenic Disturbances Shape Soil Capillary and Saturated Water Retention Indirectly via Plant Functional Traits and Soil Organic Carbon in Temperate Forests. Forests 2021, 12, 1588. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Corrales, A.; Zhu, K.; Yuan, Z.; Lin, F.; Ye, J.; Hao, Z.; Wang, X. Tree mycorrhizal associations mediate soil fertility effects on forest community structure in a temperate forest. New Phytol. 2019, 223, 475–486. [Google Scholar] [CrossRef]
- Subedi, S.; Hogan, J.A.; Ross, M.S.; Sah, J.P.; Baraloto, C. Evidence for trait-based community assembly patterns in hardwood hammock forests. Ecosphere 2019, 10, e02956. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhang, Z.; Liu, H.; Liu, Y.; Feng, Y.; Yang, G.; Ren, C.; Han, X. Linking soil bacterial community assembly with the composition of organic carbon during forest succession. Soil Biol. Biochem. 2022, 173, 108790. [Google Scholar] [CrossRef]
- Tanunchai, B.; Ji, L.; Schroeter, S.A.; Wahdan, S.F.M.; Hossen, S.; Delelegn, Y.; Buscot, F.; Lehnert, A.S.; Alves, E.G.; Hilke, I.; et al. FungalTraits vs. FUNGuild: Comparison of Ecological Functional Assignments of Leaf- and Needle-Associated Fungi Across 12 Temperate Tree Species. Microb. Ecol. 2023, 85, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Escolar, C.; Bardgett, R.D.; Dungait, J.A.; Gozalo, B.; Ochoa, V. Warming reduces the cover and diversity of biocrust-forming mosses and lichens, and increases the physiological stress of soil microbial communities in a semi-arid Pinus halepensis plantation. Front. Microbiol. 2015, 6, 865. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.H.; Lee, D.K. Stand structure and regeneration of Quercus mongolica forests in Korea. For. Ecol. Manag. 1998, 106, 27–34. [Google Scholar] [CrossRef]
- Ren, H.; Gao, G.; Ma, Y.; Li, Z.; Wang, S.; Gu, J. Shift of root nitrogen-acquisition strategy with tree age is mediated by root functional traits along the collaboration gradient of the root economics space. Tree Physiol. 2023, 43, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, C.; Lu, D.; Wang, G.G.; Zheng, X.; Cao, J.; Zhang, J. Regeneration and succession: A 50-year gap dynamic in temperate secondary forests, Northeast China. For. Ecol. Manag. 2021, 484, 118943. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Choung, Y. Distribution, abundance, and effect on plant species diversity of Sasa borealis in Korean forests. J. Ecol. Environ. 2018, 42, 9. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Shin, Y.; Choung, Y. Spatial pattern and association of tree species in a mixed Abies holophylla-broadleaved deciduous forest in Odaesan National Park. J. Plant Biol. 2012, 55, 242–250. [Google Scholar] [CrossRef]
- Dolezal, J.; Song, J.S.; Altman, J.; Janecek, S.; Cerny, T.; Srutek, M.; Kolbek, J. Tree growth and competition in a post-logging Quercus mongolica forest on Mt. Sobaek, South Korea. Ecol. Res. 2008, 24, 281–290. [Google Scholar] [CrossRef]
- Johnson, D.J.; Clay, K.; Phillips, R.P. Mycorrhizal associations and the spatial structure of an old-growth forest community. Oecologia 2018, 186, 195–204. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Birch, J.D.; Lutz, J.A.; Turner, B.L.; Karst, J. Divergent, age-associated fungal communities of Pinus flexilis and Pinus longaeva. For. Ecol. Manag. 2021, 494, 119277. [Google Scholar] [CrossRef]
- Luo, S.; Phillips, R.P.; Jo, I.; Fei, S.; Liang, J.; Schmid, B.; Eisenhauer, N. Higher productivity in forests with mixed mycorrhizal strategies. Nat. Commun. 2023, 14, 1377. [Google Scholar] [CrossRef]
- Anthony, M.A.; Crowther, T.W.; van der Linde, S.; Suz, L.M.; Bidartondo, M.I.; Cox, F.; Schaub, M.; Rautio, P.; Ferretti, M.; Vesterdal, L.; et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022, 16, 1327–1336. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Crous, K.Y.; De Kauwe, M.G.; Verryckt, L.T.; Goll, D.; Zaehle, S.; Bloomfield, K.J.; Ciais, P.; Cernusak, L.A.; Domingues, T.F.; et al. Convergence in phosphorus constraints to photosynthesis in forests around the world. Nat. Commun. 2022, 13, 5005. [Google Scholar] [CrossRef]
- Khokon, A.M.; Janz, D.; Polle, A. Ectomycorrhizal diversity, taxon-specific traits and root N uptake in temperate beech forests. New Phytol. 2023, 239, 739–751. [Google Scholar] [CrossRef]
- Jorgensen, K.; Clemmensen, K.E.; Wallander, H.; Lindahl, B.D. Do ectomycorrhizal exploration types reflect mycelial foraging strategies? New Phytol. 2023, 237, 576–584. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Suz, L.M. Do Ectomycorrhizal Trees Select Ectomycorrhizal Fungi That Enhance Phosphorus Uptake under Nitrogen Enrichment? Forests 2023, 14, 467. [Google Scholar] [CrossRef]
- Plassard, C.; Louche, J.; Ali, M.A.; Duchemin, M.; Legname, E.; Cloutier-Hurteau, B. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 33–43. [Google Scholar] [CrossRef]
- Odriozola, I.; Martinovic, T.; Bahnmann, B.D.; Ryšánek, D.; Mašínová, T.; Sedlák, P.; Merunková, K.; Kohout, P.; Tomšovský, M.; Baldrian, P. Stand age affects fungal community composition in a Central European temperate forest. Fungal Ecol. 2020, 48, 100985. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bialic-Murphy, L.; Zohner, C.M.; Averill, C.; van den Hoogen, J.; Ma, H.; Mo, L.; Smith, G.R.; Acosta, A.T.R.; Aubin, I.; et al. Global relationships in tree functional traits. Nat. Commun. 2022, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.V.; Peay, K.G.; Fukami, T. Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol. Ecol. 2014, 87, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, W.; Wang, J.; Lambers, H.; Yin, H. Extraradical hyphae alleviate nitrogen deposition-induced phosphorus deficiency in ectomycorrhiza-dominated forests. New Phytol. 2023, 239, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, J.; Wang, Q.; Yin, M.; Zhu, X.; Liu, Q.; Zhang, Z.; Yin, H. Soil fertility controls ectomycorrhizal mycelial traits in alpine forests receiving nitrogen deposition. Soil Biol. Biochem. 2021, 161, 108386. [Google Scholar] [CrossRef]
- Behnke-Borowczyk, J.; Korzeniewicz, R.; Lukowski, A.; Baranowska, M.; Jagiello, R.; Bulaj, B.; Hauke-Kowalska, M.; Szmyt, J.; Behnke, J.M.; Robakowski, P.; et al. Variability of Functional Groups of Rhizosphere Fungi of Norway Spruce (Picea abies (L.) H.Karst.) in the Boreal Range: The Wigry National Park, Poland. Int. J. Mol. Sci. 2023, 24, 12628. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Durall, D.M.; MacKenzie, W.H. Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 2009, 19, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Rog, I.; Rosenstock, N.P.; Korner, C.; Klein, T. Share the wealth: Trees with greater ectomycorrhizal species overlap share more carbon. Mol. Ecol. 2020, 29, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, K.; Schoning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Trappe, J.M.; Rizzo, D.M. Genea, Genabea and Gilkeya gen. nov.: Ascomata and ectomycorrhiza formation in a Quercus woodland. Mycologia 2017, 98, 699–716. [Google Scholar] [CrossRef]
- Huang, J.; Nara, K.; Zong, K.; Lian, C. Soil propagule banks of ectomycorrhizal fungi along forest development stages after mining. Microb. Ecol. 2015, 69, 768–777. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, S.Y.; Yoo, S.; Fong, J.J.; Kim, C.S.; Jo, J.W.; Lim, Y.W. Influence of Season and Soil Properties on Fungal Communities of Neighboring Climax Forests (Carpinus cordata and Fraxinus rhynchophylla). Front. Microbiol. 2020, 11, 572706. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Xiang, W.; Mueller, G.M.; Egerton-Warburton, L.M.; Yan, W.; Liu, S. Differences in ectomycorrhizal community assembly between native and exotic pines are reflected in their enzymatic functional capacities. Plant Soil 2019, 446, 179–193. [Google Scholar] [CrossRef]
- Mandolini, E.; Bacher, M.; Peintner, U. Ectomycorrhizal fungal communities of Swiss stone pine (Pinus cembra) depend on climate and tree age in natural forests of the Alps. Plant Soil 2022. [Google Scholar] [CrossRef]
- LeDuc, S.D.; Horton, T.R.; Lilleskov, E.A.; Rothstein, D.E. Ectomycorrhizal fungal succession coincides with shifts in organic nitrogen availability and canopy closure in post-wildfire jack pine forests. Oecologia 2013, 172, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kyaschenko, J.; Clemmensen, K.E.; Karltun, E.; Lindahl, B.D. Below-ground organic matter accumulation along a boreal forest fertility gradient relates to guild interaction within fungal communities. Ecol. Lett. 2017, 20, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Maillard, F.; Kohler, A.; Morin, E.; Hossann, C.; Miyauchi, S.; Ziegler-Devin, I.; Gerant, D.; Angeli, N.; Lipzen, A.; Keymanesh, K.; et al. Functional genomics gives new insights into the ectomycorrhizal degradation of chitin. New Phytol. 2023, 238, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bogar, L.M.; Tavasieff, O.S.; Raab, T.K.; Peay, K.G. Does resource exchange in ectomycorrhizal symbiosis vary with competitive context and nitrogen addition? New Phytol. 2022, 233, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Beiler, K.J.; Durall, D.M.; Simard, S.W.; Maxwell, S.A.; Kretzer, A.M. Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytol. 2010, 185, 543–553. [Google Scholar] [CrossRef]
- Ectomycorrhizal ecology under primary succession on coastal sand dunes: Interactions involving Pinus contorta, suilloid fungi and deer. New Phytol. 2006, 169, 345–354. [CrossRef]

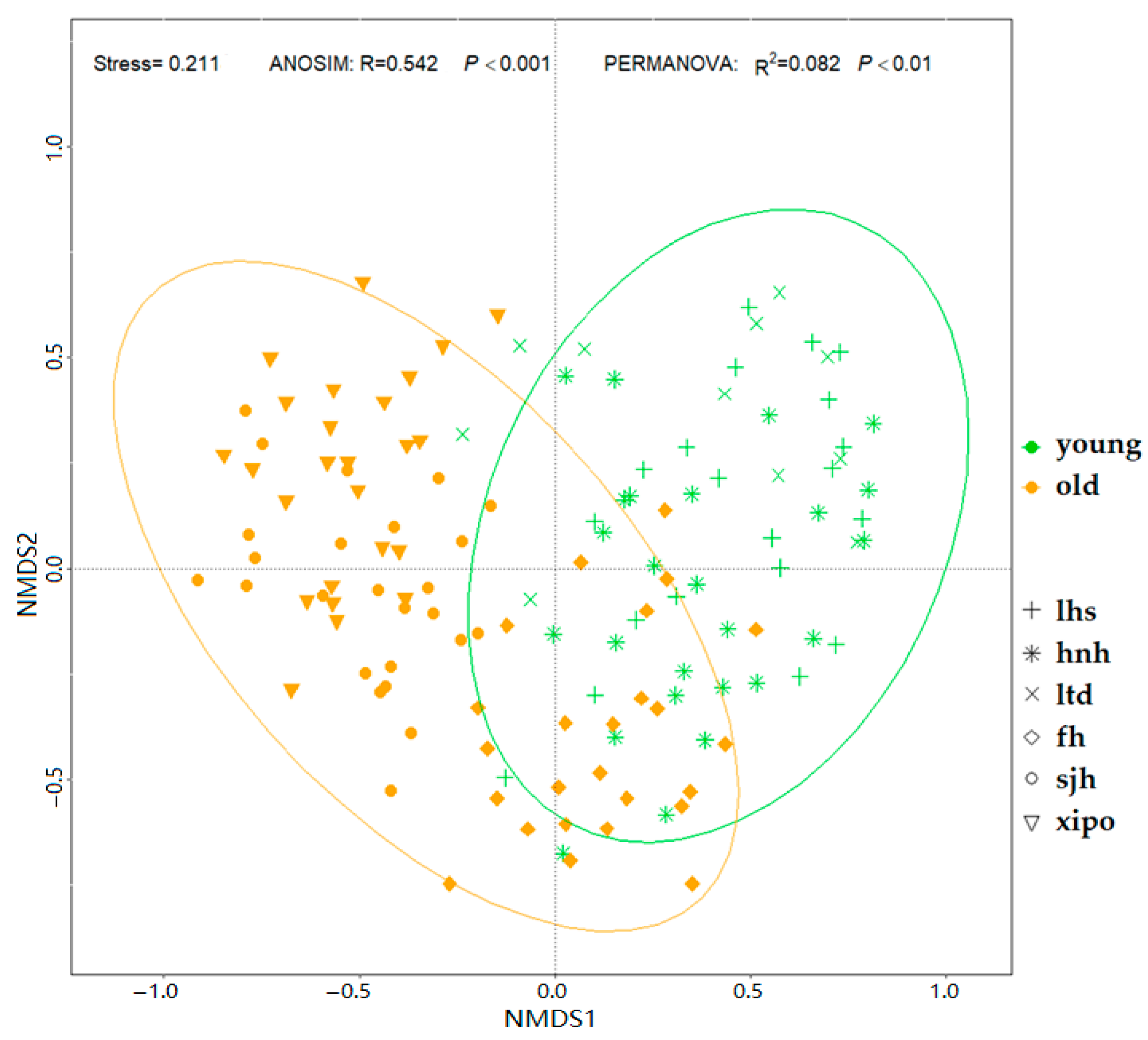
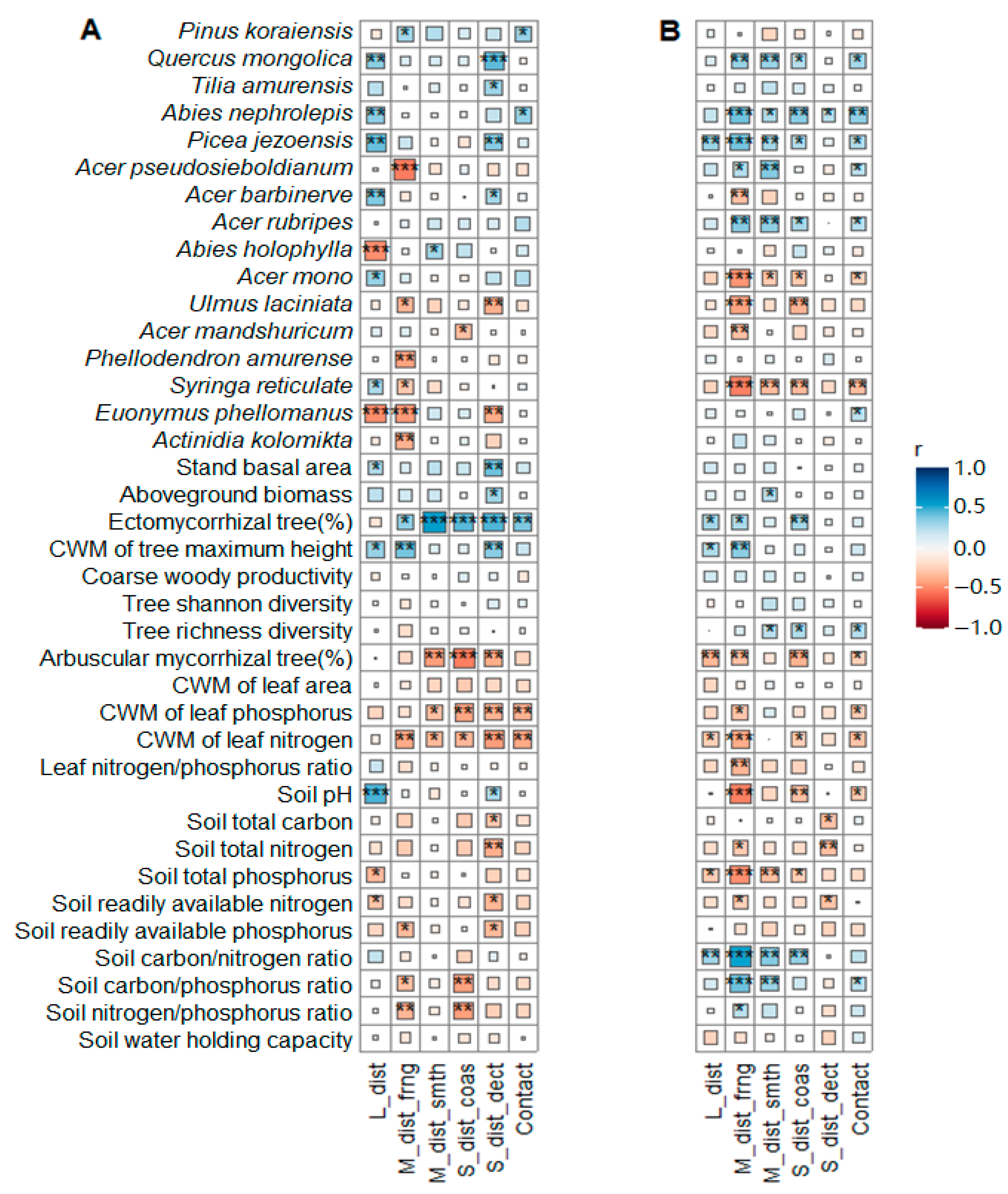
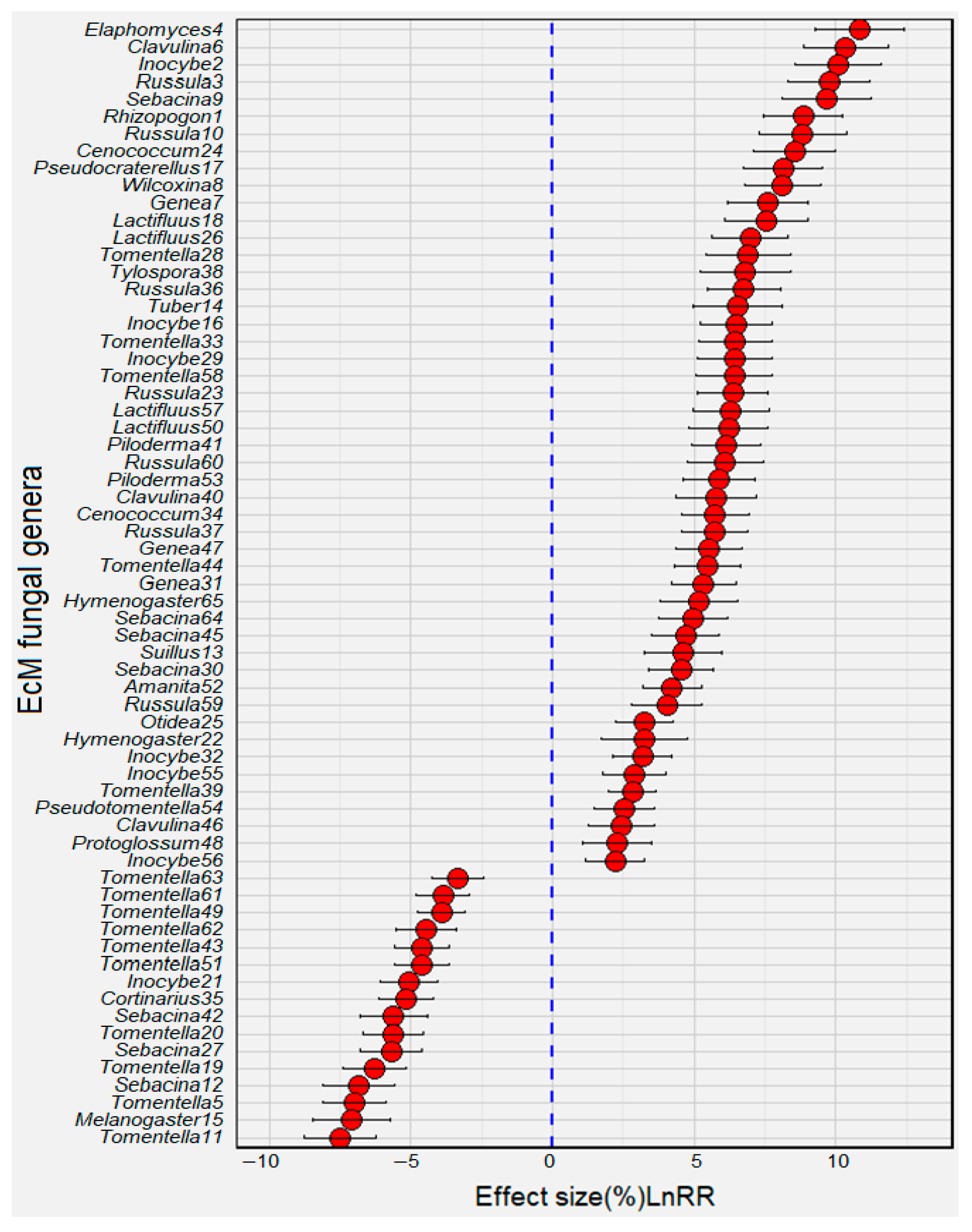
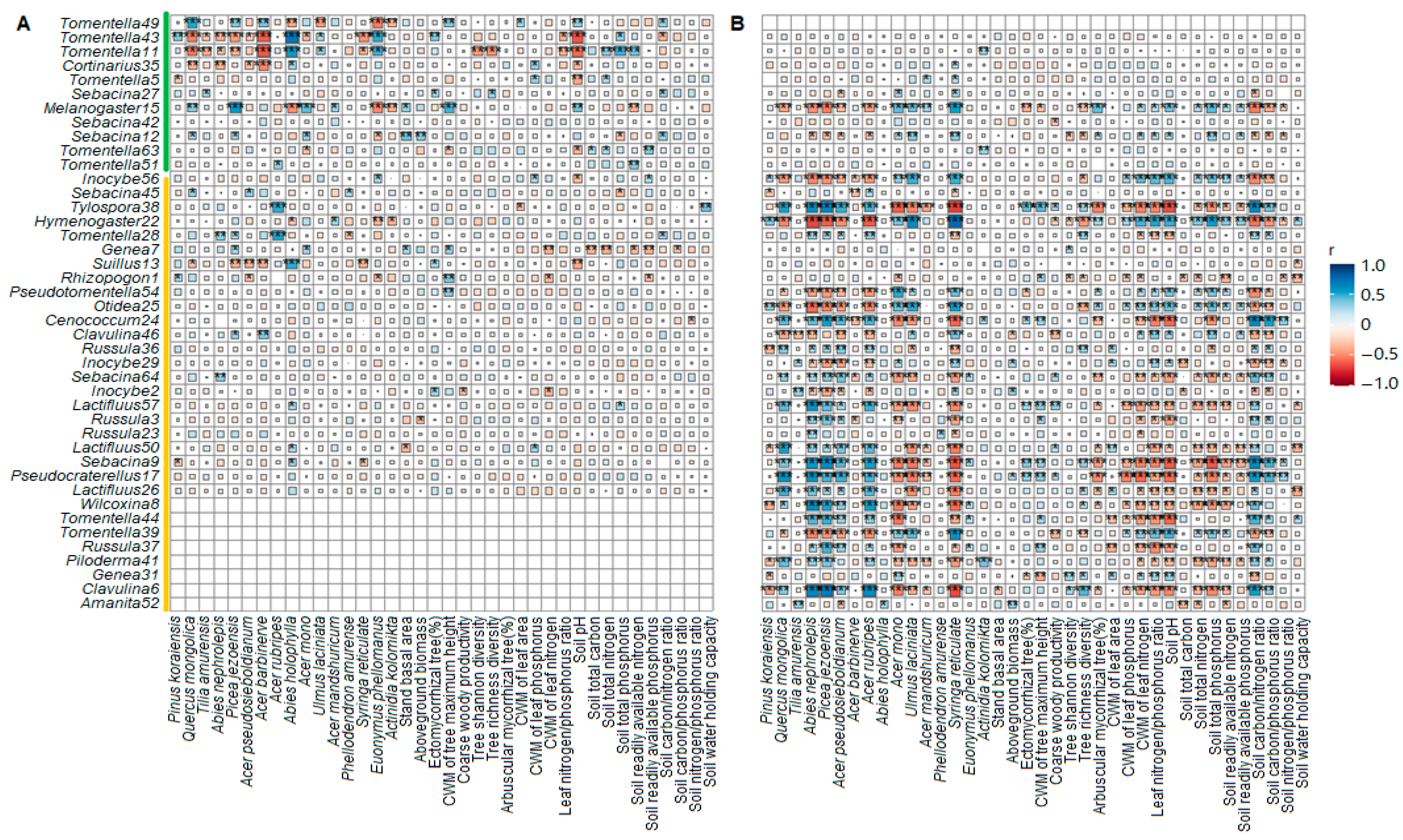
| Alpha-Diversity | Younger Stands lhs (114 y, n = 25) hnh (117 y, n = 25) ltd (122 y, n = 15) | Older Stands fh (251 y, n = 24) sjh (247 y, n = 25) xp (250 y, n = 25) | (O-Y)/Y | ||||
|---|---|---|---|---|---|---|---|
| Shannon | 2.40 ± 0.17 a | 2.22 ± 0.14 a | 1.93 ± 0.20 a | 2.20 ± 0.11 a | 2.30 ± 0.11 a | 2.41 ± 0.12 a | NS |
| Simpson | 0.77 ± 0.04 a | 0.75 ± 0.03 a | 0.67 ± 0.05 a | 0.78 ± 0.03 a | 0.78 ± 0.03 a | 0.78 ± 0.03 a | NS |
| Richness | 63.20 ± 2.79 bc | 71.72 ± 3.54 b | 53.82 ± 5.98 c | 67.40 ± 2.92 bc | 95.32 ± 4.85 a | 100.80 ± 3.29 a | 0.35 *** |
| Chao1 | 92.24 ± 4.83 b | 103.29 ± 5.05 b | 85.19 ± 8.33 b | 88.18 ± 4.08 b | 129.21 ± 6.83 a | 126.74 ± 4.41 a | 0.20 *** |
| Ace | 99.41 ± 5.06 cd | 111.69 ± 5.46 bc | 92.64 ± 7.59 cd | 90.74 ± 4.15 d | 133.27 ± 7.33 a | 128.05 ± 4.33 ab | 0.13 * |
| Pielou evenness | 0.40 ± 0.03 a | 0.36 ± 0.02 a | 0.34 ± 0.03 a | 0.36 ± 0.02 a | 0.35 ± 0.02 a | 0.36 ± 0.02 a | NS |
| Goods’ coverage | 0.95 ± 0.01 c | 0.97 ± 0.01 c | 0.97 ± 0.01 c | 0.99 ± 0.00 b | 1.00 ± 0.00 ab | 1.00 ± 0.00 a | 0.04 *** |
| EcM Types (%) | Younger Stands lhs (114 y, n = 25) hnh (117 y, n = 25) ltd (122 y, n = 15) | Older Stands fh (251 y, n = 24) sjh (247 y, n = 25) xp (250 y, n = 25) | (O-Y)/Y | ||||
|---|---|---|---|---|---|---|---|
| Contact | 5.22 ± 2.67 c | 3.36 ± 1.36 c | 4.65 ± 3.02 c | 9.62 ± 2.17 b | 14.50 ± 2.83 ab | 19.25 ± 2.79 a | 2.38 *** |
| S_dist_coas | 0.07 ± 0.02 c | 0.03 ± 0.01 c | 0.07 ± 0.03 bc | 0.23 ± 0.06 b | 0.46 ± 0.10 a | 0.43 ± 0.07 a | 5.92 *** |
| S_dist_dect | 3.27 ± 1.03 c | 7.32 ± 1.44 bc | 3.51 ± 1.66 c | 12.02 ± 1.99 ab | 16.96 ± 2.43 a | 13.91 ± 2.34 a | 1.79 *** |
| M_dist_frng | 0.40 ± 0.25 bc | 1.17 ± 0.45 b | 0.42 ± 0.34 c | 0.75 ± 0.33 b | 4.47 ± 2.78 a | 4.76 ± 2.20 a | 3.45 *** |
| M_dist_smth | 1.89 ± 0.75 b | 1.81 ± 1.12 b | 2.41 ± 1.16 ab | 2.19 ± 0.51 b | 9.56 ± 2.64 a | 7.51 ± 1.62 ab | 2.29 *** |
| L_dist | 0.03 ± 0.00 c | 0.76 ± 0.44 ab | 0.12 ± 0.04 bc | 0.34 ± 0.16 b | 0.52 ± 0.20 ab | 0.46 ± 0.13 a | 0.17 *** |
| L_dist/M_dist_frng ratio | 0.52 ± 0.13 a | 9.47 ± 7.74 a | 4.08 ± 2.14 a | 1.24 ± 0.48 a | 0.69 ± 0.28 a | 1.92 ± 1.11 a | −0.75 * |
| L_dist/M_dist_smth ratio | 0.06 ± 0.02 b | 5.10 ± 4.58 a | 0.29 ± 0.12 ab | 0.26 ± 0.12 ab | 0.69 ± 0.51 b | 0.15 ± 0.05 b | −0.85 * |
| L_dist/S_dist_dect ratio | 0.04 ± 0.01 a | 0.60 ± 0.46 a | 0.12 ± 0.09 a | 0.13 ± 0.10 a | 0.04 ± 0.02 a | 0.06 ± 0.02 a | NS |
| L_dist/S_dist_coas ratio | 2.28 ± 0.83 b | 41.29 ± 27.30 a | 10.63 ± 4.99 ab | 4.16 ± 1.64 b | 2.28 ± 0.81 b | 1.82 ± 0.58 b | −0.86 ** |
| L_dist/Contact ratio | 0.28 ± 0.09 ab | 2.70 ± 2.13 a | 5.70 ± 4.80 ab | 0.15 ± 0.09 b | 0.07 ± 0.03 b | 0.03 ± 0.01 b | −0.96 *** |
| M_dist_frng/S_dist_dect ratio | 0.16 ± 0.05 ab | 0.30 ± 0.17 ab | 0.08 ± 0.04 b | 0.09 ± 0.03 b | 0.50 ± 0.36 ab | 1.59 ± 1.33 a | NS |
| M_dist_smth/S_dist_dect ratio | 1.65 ± 0.74 a | 0.37 ± 0.18 b | 0.76 ± 0.42 ab | 0.51 ± 0.25 ab | 1.39 ± 0.54 ab | 0.80 ± 0.16 a | NS |
| M_dist_frng/S_dist_coas ratio | 7.74 ± 2.09 a | 29.36 ± 7.22 a | 10.39 ± 5.83 a | 6.02 ± 1.46 a | 18.09 ± 10.46 a | 15.71 ± 5.26 a | NS |
| M_dist_smth/S_dist_coas ratio | 58.50 ± 14.06 a | 52.96 ± 17.56 a | 242.37 ± 202.31 a | 30.31 ± 10.28 a | 45.30 ± 13.54 a | 29.59 ± 6.69 a | NS |
| M_dist_frng/Contact ratio | 12.80 ± 11.91 ab | 3.60 ± 2.64 a | 0.99 ± 0.37 ab | 0.17 ± 0.04 b | 3.37 ± 2.97 ab | 0.47 ± 0.23 ab | −0.79 ** |
| M_dist_smth/Contact ratio | 0.71 ± 0.34 b | 2.79 ± 1.07 ab | 11.27 ± 6.29 a | 9.58 ± 4.04 ab | 4.01 ± 2.61 ab | 0.65 ± 0.17 ab | −0.75 *** |
| EcM Fungal Species (%) | Younger Stands lhs (114 y, n = 25) hnh (117 y, n = 25) ltd (122 y, n = 15) | Older Stands fh (251 y, n = 24) sjh (247 y, n = 25) xp (250 y, n = 25) | (O-Y)/Y | ||||
|---|---|---|---|---|---|---|---|
| EcM18570.Tomentella49_mds | 0 b | 0.0044 ± 0.0012 a | 0 b | 0 b | 0 b | 0 b | -- *** |
| EcM347.Tomentella43_mds | 0.0529 ± 0.0228 a | 0 b | 0 b | 0 b | 0.0002 ± 0.0002 b | 0 b | −0.9971 *** |
| EcM15392.Tomentella11_mds | 1.1771 ± 0.7551 a | 0.0025 ± 0.0009 b | 0.1035 ± 0.1010 b | 0.0007 ± 0.0007 b | 0.0119 ± 0.0119 b | 0.0002 ± 0.0002 b | −0.9904 *** |
| EcM8957.Cortinarius35_mdf | 0.0126 ± 0.0066 a | 0.0012 ± 0.0005 b | 0 c | 0.0002 ± 0.0002 bc | 0 c | 0 c | −0.9889 *** |
| EcM8395.Tomentella5_mds | 0.0220 ± 0.0128 a | 0.0022 ± 0.0008 bc | 0.0038 ± 0.0013 ab | 0.0003 ± 0.0003 cd | 0 d | 0 d | −0.9883 *** |
| EcM18492.Sebacina27_sdd | 0.0042 ± 0.0012 a | 0.0037 ± 0.0014 ab | 0.0027 ± 0.0012 ab | 0.0005 ± 0.0003 bc | 0.0002 ± 0.0002 c | 0 c | −0.9390 *** |
| EcM19972.Melanogaster15_L | 0.0023 ± 0.0010 bc | 0.0673 ± 0.0228 a | 0.0027 ± 0.0012 bc | 0.0062 ± 0.0018 b | 0.0002 ± 0.0002 c | 0 c | −0.9324 *** |
| EcM11405.Sebacina42_sdd | 0.0105 ± 0.0043 a | 0.1205 ± 0.0638 a | 0.0506 ± 0.0339 ab | 0.0144 ± 0.0106 ab | 0.0002 ± 0.0002 b | 0 b | −0.9281 *** |
| EcM16292.Sebacina12_sdd | 0.0174 ± 0.0098 bc | 0.0634 ± 0.0220 a | 0.0806 ± 0.0611 ab | 0.0146 ± 0.0102 bc | 0 c | 0 c | −0.9036 *** |
| EcM17198.Tomentella63_mds | 0.0031 ± 0.0011 a | 0.0005 ± 0.0003 ab | 0.0065 ± 0.0032 a | 0.0005 ± 0.0005 b | 0.0002 ± 0.0002 b | 0.0002 ± 0.0002 b | −0.8933 *** |
| EcM17285.Tomentella51_mds | 0.0042 ± 0.0018 a | 0.0020 ± 0.0007 a | 0.0034 ± 0.0019 a | 0.0018 ± 0.0018 ab | 0 b | 0 b | −0.7997 *** |
| EcM5007.Inocybe56_sdd | 0.0799 ± 0.0784 b | 0 b | 0 b | 0.2271 ± 0.0971 a | 0 b | 0.0003 ± 0.0003 b | 1.6563 * |
| EcM1607.Sebacina45_sdd | 0 b | 0.0003 ± 0.0003 b | 0.0350 ± 0.0346 ab | 0.0027 ± 0.0016 ab | 0.0104 ± 0.0045 a | 0.0618 ± 0.0336 a | 2.5506 *** |
| EcM21424.Tylospora38_sdd | 0.0013 ± 0.0009 cd | 0.0097 ± 0.0050 cd | 0.4655 ± 0.2663 c | 0 d | 0.4739 ± 0.2129 b | 1.3498 ± 0.5243 a | 5.3177 *** |
| EcM16453.Hymenogaster22_sdd | 0.0063 ± 0.0050 bc | 0.1819 ± 0.1715 b | 0.0004 ± 0.0004 bc | 2.2268 ± 0.7221 a | 0.0003 ± 0.0003 c | 0.0033 ± 0.0012 bc | 7.9012 * |
| EcM12941.Tomentella28_mds | 0.0002 ± 0.0002 c | 0.0025 ± 0.0010 c | 0.0346 ± 0.0244 bc | 0.0395 ± 0.0218 bc | 0.0740 ± 0.0299 ab | 0.2308 ± 0.0731 a | 13.354 *** |
| EcM18546.Genea7_sdc | 0.0008 ± 0.0004 b | 0.0028 ± 0.0014 b | 0.0004 ± 0.0004 b | 0.0141 ± 0.0040 a | 0.0333 ± 0.0165 a | 0.0236 ± 0.0098 a | 13.390 *** |
| EcM12535.Suillus13_L | 0.0086 ± 0.0019 ab | 0.0027 ± 0.0008 b | 0.0126 ± 0.0113 b | 0.2850 ± 0.1577 a | 0.0567 ± 0.0210 a | 0.0571 ± 0.0303 a | 18.770 *** |
| EcM23155.Rhizopogon1_L | 0.0010 ± 0.0005 b | 0.0028 ± 0.0007 b | 0.0004 ± 0.0004 b | 0.0161 ± 0.0048 a | 0.0701 ± 0.0547 a | 0.0594 ± 0.0338 a | 27.243 *** |
| EcM13213.Pseudotomentella54_mds | 0.0002 ± 0.0002 b | 0.0012 ± 0.0009 b | 0 b | 0.0487 ± 0.0231 a | 0.0037 ± 0.0027 b | 0 b | 28.213 * |
| EcM17847.Otidea25_sdc | 0.0017 ± 0.0017 b | 0 b | 0 b | 0.1026 ± 0.0396 a | 0 b | 0.0002 ± 0.0002 b | 56.307 ** |
| EcM20682.Cenococcum24_sdc | 0 c | 0.0022 ± 0.0012 c | 0.0004 ± 0.0004 c | 0.0015 ± 0.0010 c | 0.1123 ± 0.0552 b | 0.2003 ± 0.0642 a | 99.107 *** |
| EcM12198.Clavulina46_C | 0 b | 0.0042 ± 0.0025 ab | 0.0004 ± 0.0004 b | 0.6249 ± 0.2894 a | 0.0005 ± 0.0004 b | 0.0136 ± 0.0125 b | NS |
| EcM5233.Russula36_C | 0.0046 ± 0.0046 b | 0 b | 0 b | 0.0062 ± 0.0051 b | 0.1426 ± 0.0738 a | 0.5200 ± 0.4635 a | 134.62 *** |
| EcM18248.Inocybe29_sdd | 0 b | 0.0013 ± 0.0009 b | 0 b | 0.2408 ± 0.1243 a | 0.1948 ± 0.1099 a | 0.0072 ± 0.0059 b | 245.96 *** |
| EcM14251.Sebacina64_sdd | 0 b | 0.0003 ± 0.0002 b | 0 b | 0 b | 0.0549 ± 0.0329 a | 0.1630 ± 0.0656 a | 485.08 *** |
| EcM17329.Inocybe2_sdd | 0.0008 ± 0.0007 c | 0.0020 ± 0.0017 c | 0 c | 1.3903 ± 0.5021 a | 0.4446 ± 0.2207 ab | 0.0351 ± 0.0217 b | 520.50 *** |
| EcM10960.Lactifluus57_mds | 0.0046 ± 0.0032 b | 0 b | 0 b | 0 b | 2.0139 ± 1.3653 a | 1.0093 ± 0.6139 a | 612.12 *** |
| EcM11301.Russula3_C | 0.004 ± 0.003 cd | 0 d | 0 d | 0.0649 ± 0.0405 bc | 0.0619 ± 0.0375 ab | 0.2107 ± 0.0823 a | 752.01 *** |
| EcM15576.Russula23_C | 0 b | 0.0002 ± 0.0002 b | 0 b | 0.0202 ± 0.0164 ab | 0.0308 ± 0.0210 a | 0.1279 ± 0.0658 a | 797.19 *** |
| EcM10920.Lactifluus50_mds | 0.0023 ± 0.0017 b | 0.0002 ± 0.0002 b | 0 b | 0 b | 2.0095 ± 1.3214 a | 0.2624 ± 0.1404 a | 843.73 *** |
| EcM10378.Sebacina9_sdd | 0.0008 ± 0.0005 c | 0.0002 ± 0.0002 c | 0 c | 0.0002 ± 0.0002 c | 0.6563 ± 0.2533 b | 0.8736 ± 0.2801 a | 1364.4 *** |
| EcM10234.Pseudocraterellus17_sdd | 0.0002 ± 0.0002 b | 0 b | 0 b | 0 b | 0.2504 ± 0.1733 a | 0.2015 ± 0.1003 a | 2015 *** |
| EcM10122.Lactifluus26_mds | 0.0002 ± 0.0002 c | 0 c | 0 c | 0 c | 0.3946 ± 0.2117 a | 0.3635 ± 0.2125 b | 3381.4 *** |
| EcM16609.Wilcoxina8_sdc | 0 b | 0 b | 0 b | 0 b | 0.0758 ± 0.0287 a | 0.0592 ± 0.0207 a | -- *** |
| EcM16354.Tomentella44_mds | 0 b | 0 b | 0 b | 0.0002 ± 0.0002 b | 0.0008 ± 0.0005 b | 0.2678 ± 0.1013 a | -- *** |
| EcM17223.Tomentella39_mds | 0 b | 0 b | 0 b | 0.0142 ± 0.0080 a | 0 b | 0.0002 ± 0.0002 b | -- *** |
| EcM17687.Russula37_C | 0 b | 0 b | 0 b | 0.0013 ± 0.0008 b | 0.0005 ± 0.0004 b | 0.1561 ± 0.0785 a | -- *** |
| EcM22874.Piloderma41_mdf | 0 b | 0 b | 0 b | 0.0003 ± 0.0003 b | 0.1884 ± 0.1180 a | 0.0515 ± 0.0178 a | -- *** |
| EcM18178.Genea31_sdc | 0 c | 0 c | 0 c | 0.0328 ± 0.0145 b | 0.0695 ± 0.0353 a | 0 c | -- *** |
| EcM11003.Clavulina6_C | 0 b | 0 b | 0 b | 0 b | 0.3262 ± 0.1570 a | 0.9691 ± 0.3412 a | -- *** |
| EcM16726.Amanita52_C_mds | 0 b | 0 b | 0 b | 0.0370 ± 0.0154 a | 0.0639 ± 0.0486 ab | 0.0030 ± 0.0019 ab | -- *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Ye, J.; Liu, S.-F.; Sun, H.-H.; Yuan, Z.-Q.; Mao, Z.-K.; Fang, S.; Long, S.-F.; Wang, X.-G. Age-Related Conservation in Plant–Soil Feedback Accompanied by Ectomycorrhizal Domination in Temperate Forests in Northeast China. J. Fungi 2024, 10, 310. https://doi.org/10.3390/jof10050310
Bai Z, Ye J, Liu S-F, Sun H-H, Yuan Z-Q, Mao Z-K, Fang S, Long S-F, Wang X-G. Age-Related Conservation in Plant–Soil Feedback Accompanied by Ectomycorrhizal Domination in Temperate Forests in Northeast China. Journal of Fungi. 2024; 10(5):310. https://doi.org/10.3390/jof10050310
Chicago/Turabian StyleBai, Zhen, Ji Ye, Shu-Fang Liu, Hai-Hong Sun, Zuo-Qiang Yuan, Zi-Kun Mao, Shuai Fang, Shao-Fen Long, and Xu-Gao Wang. 2024. "Age-Related Conservation in Plant–Soil Feedback Accompanied by Ectomycorrhizal Domination in Temperate Forests in Northeast China" Journal of Fungi 10, no. 5: 310. https://doi.org/10.3390/jof10050310






