Candida Species Biofilms’ Antifungal Resistance
Abstract
:1. Introduction
2. Candida Biofilms: A Real Problem
2.1. Biofilm Characteristics
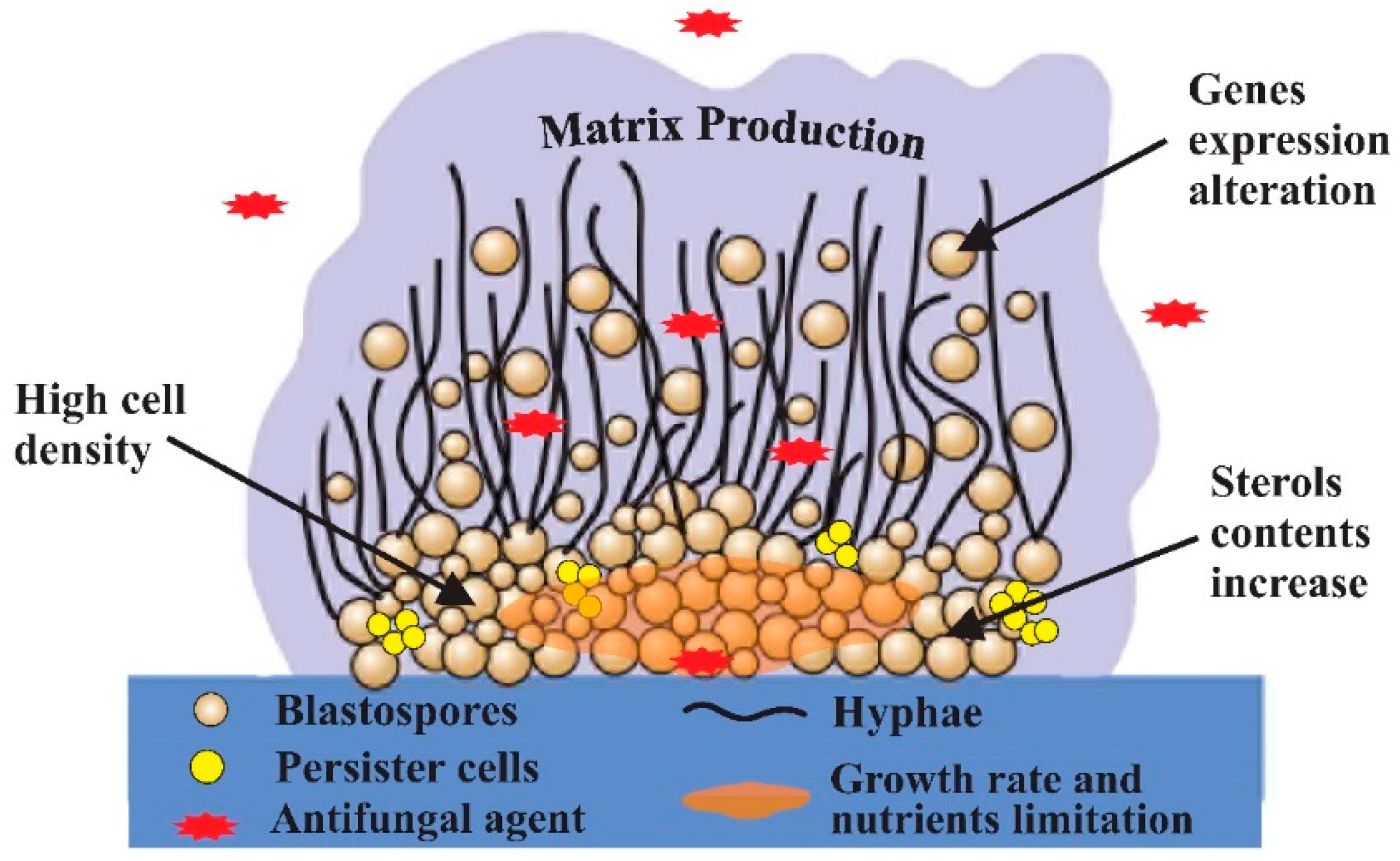
2.2. Candida Biofilms and Resistance Mechanisms
2.2.1. Impact of Candida Cells Density, Nutrient and Growth Limitation
2.2.2. Contribution of the Extracellular Matrix Production
2.2.3. Emergence of Persister Cells
2.2.4. Impact of Sterols Contents and Its Correlation with ERG Genes Expression
2.2.5. Over-Expression of Other Antifungal Targets
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Redding, S.; Dongari-Bagtzoglou, A. Candida glabrata: An emerging oral opportunistic pathogen. J. Dent. Res. 2007, 86, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cuesta, C.; Sarrion-Perez, M.G.; Bagan, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P. Candida Biofilms: Development, Architecture, and Resistance. Microbiol. Spectr. 2015, 3, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Hawser, S.P.; Douglas, L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 1995, 39, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, R.; Robertson, D.P.; Hodge, P.J.; Lappin, D.F.; Ramage, G. Hydrolytic enzyme production is associated with Candida albicans biofilm formation from patients with type 1 diabetes. Mycopathologia 2010, 170, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Ghannoum, M.A. Candida biofilms: Antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 2004, 5, 186–197. [Google Scholar] [PubMed]
- Negri, M.; Silva, S.; Henriques, M.; Oliveira, R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1399–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Jones, R.N.; Messer, S.A.; Edmond, M.B.; Wenzel, R.P. National surveillance of nosocomial blood stream infection due to Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 1998, 31, 327–332. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2015, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Jana, J.; Susanne, S.; James Thomas, W. Medical Biofilms Detection, Prevention, and Control; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar]
- Kolter, R.; Greenberg, E.P. Microbial sciences: The superficial life of microbes. Nature 2006, 441, 300–302. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harbor Perspect. Biol. 2010, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Brooks, E.G.; Cabezas-Olcoz, J.; Sanchez, H.; Zarnowski, R.; Marchillo, K.; Andes, D.R. Rat indwelling urinary catheter model of Candida albicans biofilm infection. Infect. Immun. 2014, 82, 4931–4940. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.P.; Singh-babak, S.D.; Hartooni, N.; Nobile, C.J. Biofilms and Antifungal Resistance. In Antifungals: From Genomics to Resistance and the Development of Novel Agents; Caister Academic Press: Poole, UK, 2015; pp. 71–90. [Google Scholar]
- Johnson, C.C.; Yu, A.; Lee, H.; Fidel, P.L.; Noverr, M.C. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect. Immun. 2012, 80, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Ivor, D.B.; Sanley, J.R.; Adolph, W.K.; Lawrence, J.K. Torulopsis glabrata fungemia—A clinical pathological study. Medicine 1979, 58, 430–440. [Google Scholar]
- Heffner, D.K.; Franklin, W.A. Endocarditis caused by Torulopsis glabrata. Am. J. Clin. Pathol. 1978, 70, 420–423. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, E. Candida-associated denture stomatitis and angular cheilitis. Oral Candidosis. 1990, 156–183. [Google Scholar]
- Van Der Mei, H.C.; Free, R.H.; Elving, G.J.; Van Weissenbruch, R.; Albers, F.W.J.; Busscher, H.J. Effect of probiotic bacteria on prevalence of yeasts in oropharyngeal biofilms on silicone rubber voice prostheses in vitro. J. Med. Microbiol. 2000, 49, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.; Köhler, J.; Kadosh, D.; Lopez-Ribot, J. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Fiori, B.; Trecarichi, E.M.; Posteraro, P.; Losito, A.; De Luca, A.; Sanguinetti, M.; Fadda, G.; Cauda, R.; Posterano, B. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of Amphotericin B lipid formulations and echinocandins. Society 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Taff, H.T.; Nett, J.E.; Andes, D.R. Comparative analysis of Candida biofilm quantitation assays. Med. Mycol. 2012, 50, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Pannausorn, S.; Fernandez, V.; Römling, F. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses 2012, 56, 264–272. [Google Scholar] [CrossRef]
- Valdivieso, M.; Luna, M.; Bodey, G.P.; Rodriguez, V.; Groschel, D. Fungemia due to Torulopsis glabrata in the compromised host. Cancer 1976, 38, 1750–1756. [Google Scholar] [CrossRef]
- Goodman, J.S.; Seibert, D.G.; Reahl, G.E.; Geckler, R.W. Fungal infection of prosthetic joints: A report of two cases. J. Rheumatol. 1983, 10, 494–495. [Google Scholar] [PubMed]
- Cecchin, E.; De Marchi, S.; Panarello, G.; Franceschin, A.; Chiaradia, V.; Santini, G.; Tesio, F. Torulopsis glabrata Peritonitis Complicating Continuous Ambulatory Peritoneal Dialysis: Successful Management With Oral 5-Fluorocytosine. Am. J. Kidney Dis. 1984, 4, 280–284. [Google Scholar] [CrossRef]
- Paige, C.; Pinson, C.W.; Antonovic, R.; Strausbaugh, L.J. Catheter-related thrombophlebitis of the superior vena cava caused by Candida glabrata. West. J. Med. 1987, 147, 333–335. [Google Scholar] [PubMed]
- Komshian, S.V.; Uwaydah, A.K.; Sobel, J.D.; Crane, L.R. Fungemia Caused by Candida Species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Clin. Infect. Dis. 1989, 11, 379–390. [Google Scholar] [CrossRef]
- Walter, E.B.; Gingras, J.L.; McKinney, R.E. Systemic Torulopsis glabrata infection in a neonate. South. Med. J. 1990, 83, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Fortún, J.; Martín-Dávila, P.; Gómez-García de la Pedrosa, E.; Pintado, V.; Cobo, J.; Fresco, G.; Meije, Y.; Ros, L.; Alvarez, M.E.; Luengo, J.; et al. Emerging trends in candidemia: A higher incidence but a similar outcome. J. Infect. 2012, 65, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Vidanes, G.M.; Maguire, S.L.; Guida, A.; Synnott, J.; Andes, D.; Butler, G. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.; Holland, L.; Butler, G.; Gacser, A.; Bliss, J.M. Candida parapsilosis is a significant neonatal pathogen: A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2013, 32, 206–216. [Google Scholar]
- Wada, M.; Baba, H.; Imura, S. Prosthetic knee Candida parapsilosis infection. J. Arthroplast. 1998, 13, 479–482. [Google Scholar] [CrossRef]
- Fox, P.M.; Lee, G.K. Tissue expander with acellular dermal matrix for breast reconstruction infected by an unusual pathogen: Candida parapsilosis. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, e286–e289. [Google Scholar] [CrossRef] [PubMed]
- Younkin, S.; Evarts, C.M.; Steigbigel, R.T. Candida parapsilosis infection of a total hip-joint replacement: Successful reimplantation after treatment with amphotericin B and 5-fluorocytosine. A case report. J. Bone Jt. Surg. Am. 1984, 66, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.J.; Safi, J.; Markus, M.R.P.; Aiello, V.D.; Grinberg, M.; Pomerantzeff, P. Late failure of surgical treatment for bioprosthetic valve endocarditis due to Candida tropicalis. Clin. Infect. Dis. 1996, 22, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Thomas, F.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontol 2000 2011, 55, 87–103. [Google Scholar]
- Rautemaa, R.; Ramage, G. Oral candidosis-clinical challenges of a biofilm disease. Crit. Rev. Microbiol. 2011, 37, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Dongari-Bagtzoglou, A.; Kashleva, H.; Dwivedi, P.; Diaz, P.; Vasilakos, J. Characterization of mucosal Candida albicans biofilms. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Duque, C.; Mariano, F.S.; Peixoto, I.T.; Höfling, J.; Gonçalves, R. Candida spp. in periodontal disease: A brief review. J. Oral Sci. 2010, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M. Presence of mutans Streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Gündüz Arslan, S.; Akpolat, N.; Kama, J.D.; Özer, T.; Hamamci, O. One-year follow-up of the effect of fixed orthodontic treatment on colonization by oral Candida. J. Oral Pathol. Med. 2008, 37, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Canabarro, A.; Valle, C.; Farias, M.R.; Santos, F.B.; Lazera, M.; Wanke, B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J. Periodontal Res. 2012, 48, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; D’Mello, A.; Nobbs, A.H.; Dutton, L.C.; Vickerman, M.M.; Jenkinson, H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009, 77, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Marchillo, K.; Spiegel, C.A.; Andes, D.R. Development and validation of an in vivo Candida albicans biofilm denture model. Infect. Immun. 2010, 78, 3650–3659. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of Candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Coco, B.; Sherry, L.; Bagg, J.; Lappin, D.F. In Vitro Candida albicans Biofilm Induced Proteinase Activity and SAP8 expression correlates with in vivo denture stomatitis severity. Mycopathologia 2012, 174, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S. Microbial biofilm communities in the gastrointestinal tract. J. Clin. Gastroenterol. 2008, 42, S142–S143. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, L.; Sartori, S.; Rossi, M.R.; Bovolenta, R.; Scoponi, M.; Gullini, S.; Abbasciano, V. Degradation of polyurethane gastrostomy devices: What is the role of fungal colonization? Dig. Dis. Sci. 2005, 50, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, K.; Leya, J.; Kruss, D.M.; Mobarhan, S.; Iber, F.L. Intraluminal fungal colonization of gastrostomy tubes. Gastrointest. Endosc. 1993, 39, 413–415. [Google Scholar] [CrossRef]
- Klaerner, H.G.; Uknis, M.E.; Acton, R.D.; Dahlberg, P.S.; Carlone-Jambor, C.; Dunn, D.L. Candida albicans and Escherichia coli are synergistic pathogens during experimental microbial peritonitis. J. Surg. Res. 1997, 70, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Yau, J.Y.Y.; Watt, R.M.; Jin, L.J.; Samaranayake, L.P. Escherichia coli and its lipopolysaccharide modulate in vitro Candida biofilm formation. J. Med. Microbiol. 2009, 58, 1623–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, K.L.; Downward, J.R.E.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Ma, Z.; Zhou, X.; Abdo, Z.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, V.; Matković, L. Determination of ergosterol in cellular fungi by HPLC. A modified technique. J. Argentine Chem. Soc. 2011, 98, 10–15. [Google Scholar]
- Kauffman, C.A.; Fisher, J.F.; Sobel, J.D.; Newman, C.A. Candida urinary tract infections—Diagnosis. Clin. Infect. Dis. 2011, 52, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Fisher, J.F.; Kauffman, C.A.; Newman, C.A. Candida urinary tract infections—Epidemiology. Clin. Infect. Dis. 2011, 52, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Denstedt, J.D.; Kang, Y.S.; Lam, D.; Nause, C. Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J. Urol. 1992, 148, 1592–1594. [Google Scholar] [PubMed]
- Harriott, M.M.; Lilly, E.A.; Rodriguez, T.E.; Fidel, P.L.; Noverr, M.C. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 2010, 156, 3635–3644. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Chen, Y.Y.; Hsu, H.S.; Wang, F.D.; Chen, L.Y.; Fung, C.P. A risk factor analysis of healthcare-associated fungal infections in an intensive care unit: A retrospective cohort study. BMC Infect. Dis. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Chassot, F.; Negri, M.F.N.; Svidzinski, A.E.; Donatti, L.; Peralta, R.; Svidzinski, T.; Consolaro, M. Can intrauterine contraceptive devices be a Candida albicans reservoir? Contraception 2008, 77, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Foreman, A.; Jervis-Bardy, J.; Wormald, P.J. Do biofilms contribute to the initiation and recalcitrance of chronic rhinosinusitis? Laryngoscope 2011, 121, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Keir, J.; Pedelty, L.; Swift, A.C. Biofilms in chronic rhinosinusitis: Systematic review and suggestions for future research. J. Laryngol. Otol. 2011, 125, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, F.A.; Georgalas, C.; Fokkens, W.J. The mold conundrum in chronic hyperplastic sinusitis. Curr. Allergy Asthma Rep. 2009, 9, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, F.A.; Georgalas, C.; Fokkens, W.J. Fungus as the cause of chronic rhinosinusitis: The case remains unproven. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, P.; Weber, R. Fungus balls of the paranasal sinuses: A review. Eur. Arch. Oto Rhino Laryngol. 2007, 264, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Karkas, A.; Rtail, R.; Reyt, E.; Timi, N.; Righini, C.A. Sphenoid sinus fungus ball. Eur. Arch. Otorhinolaryngol. 2012, 270, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Marques, L.L.R.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Mowat, E.; Williams, C.; Jones, B.; McChlery, S.; Ramage, G. The characteristics of Aspergillus fumigatus mycetoma development: Is this a biofilm? Med. Mycol. 2009, 47, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Buijssen, K.J.D.A.; van der Laan, B.F.A.M.; van der Mei, H.C.; Atema-Smit, J.; van der Huijssen, P.; Busscher, H.J.; Harmsen, H. Composition and architecture of biofilms on used voice prostheses. Head Neck 2012, 34, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Ell, S.R. Candida ‘the cancer of silastic’. J. Laryngol. Otol. 1996, 110, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; van der Wielen, P.; Cannon, R.D.; Ruske, D.; Dawes, P. Candida albicans binds to saliva proteins selectively adsorbed to silicone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Marie-Soleil, D.; Williamson, D.R.; Albert, M.; Perreault, M.M.; Jiang, X.; Day, A.G.; Heyland, D.K. Impact of Candida species on clinical outcomes in patients with suspected ventilatorassociated pneumonia. Can. Respir. J. 2011, 18, 131–136. [Google Scholar]
- Hamet, M.; Pavon, A.; Dalle, F.; Pechinot, A.; Prin, S.; Quenot, J.P.; Charles, P.E. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med. 2012, 38, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Correa, M.; Ludena, Y.; Ramage, G.; Villena, G.K. Recent advances on filamentous fungal biofilms for industrial uses. Appl. Biochem. Biotechnol. 2012, 167, 1235–1253. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infectedwith bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Gutierrez-Correa, M.; Jones, B.; Williams, C. Aspergillus biofilms: Clinical and industrial significance. FEMS Microbiol. Lett. 2011, 324, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mowat, E.; Lang, S.; Williams, C.; McCulloch, E.; Jones, B.; Ramage, G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 2008, 62, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.M.C. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; O’Donoghue, E.; Bennett, K.; Gunaratnam, C.; O’Neil, S.; McElvaney, N.G. Sputum Candida albicans presages FEV decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010, 138, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Delhaes, L.; Monchy, S.; Fréalle, E.; Hubans, C.; Salleron, J.; Leroy, S.; Prevotat, A.; Wallet, F.; Wallaert, B.; Dei-Cas, E.; et al. The airway microbiota in cystic fibrosis: A complex fungal and bacterial community—Implications for therapeutic management. PLoS ONE 2012, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Calfee, M.W.; Farrow, J.M.; Morales, D.K.; Pesci, E.C.; Hogan, D. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.K.; Jacobs, N.J.; Rajamani, S.; Krishnamurthy, M.; Cubillos-Ruiz, J.R.; Hogan, D. Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol. Microbiol. 2010, 78, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Vik, A.; Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004, 54, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; William Costerton, J.; Shirtliff, M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.D.; Duan, K.; Fischer, C.; Parkins, M.D.; Storey, D.G.; Rabin, H.R.; Surette, M.G. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008, 4, e1000184. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.D.; Parkins, M.D.; Rabin, H.R.; Duan, K.; Norgaard, J.C.; Surette, M.G. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2008, 105, 15070–15075. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. In vivo modeling of biofilm-infected wounds: A review. J. Surg. Res. 2012, 178, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surg. Infect. 2009, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Coco, B.J.; Bagg, J.; Cross, L.J.; Jose, A.; Cross, J.; Ramage, G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 2010, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewska, A.; Schwartz, S.; Ruhnke, M. Antifungal treatment strategies in patients with haematological diseases or cancer: From prophylaxis to empirical, pre-emptive and targeted therapy. Mycoses 2012, 55, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-A.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Falkler, W.A.; Meiller, T.F. Fungal Biofilms and Drug Resistance. Emerg. Infect. Dis. 2004, 10, 14–19. [Google Scholar] [CrossRef] [PubMed]
- White, T.C.; Marr, K.A. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Sobel, J.D. Prophylactic antifungal therapy in the intensive care unit. Clin. Infect. Dis. 2001, 32, 1191–1200. [Google Scholar] [PubMed]
- Manzoni, P. Prophylactic fluconazole is effective in preventing fungal colonization and fungal systemic infections in preterm neonates: A single-center, 6-year, retrospective cohort study. Pediatrics 2006, 117, e22–e32. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, P.A. Clinical trials of antifungal prophylaxis among patients in surgical intensive care units: Concepts and considerations. Clin. Infect. Dis. 2004, 39, S193–S199. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Lipman, J.; Dobb, G.J.; Webb, S.A. The use of prophylactic fluconazole in immunocompetent high-risk surgical patients: A meta-analysis. Clin. Infect. Dis. 2006, 9, R710–R717. [Google Scholar]
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, T.; et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: The NEMIS prospective multicenter study. Clin. Infect. Dis. 2001, 33, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.T.; Azeredo, J.; Henriques, M. Effects of fluconazole on Candida glabrata biofilms and its relationship with ABC transporter gene expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Silva, S.; Henriques, M. Candida tropicalis biofilm’s matrix-involvement on its resistance to amphotericin B. Diagn. Microbiol. Infect. Dis. 2015, 83, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posteraro, B.; Vella, A.; De Carolis, E.; Sanguinetti, M. Molecular detection of resistance to azole components. Methods Mol. Biol. 2017, 1508, 423–435. [Google Scholar] [PubMed]
- Rodrigues, C.F.; Silva, S.; Azeredo, J.; Henriques, M. Candida glabrata’s recurrent infections: Biofilm formation during Amphotericin B treatment. Lett. Appl. Microbiol. 2016, 63, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Silva, S.; Henriques, M. Effect of voriconazole on Candida tropicalis biofilms: Relation with ERG genes expression. Mycopathologia 2016, 181, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal, P.; Mekala, S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.; Samaranayake, L.P. Biofilm lifestyle of Candida: A mini review. Oral Dis. 2008, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Henriques, M.; Oliveira, R.; Williams, D.; Azeredo, J. In vitro biofilm activity of non-Candida albicans Candida species. Curr. Microbiol. 2010, 61, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie, G.S.; Douglas, L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 1900–1905. [Google Scholar] [PubMed]
- Baillie, G.S.; Douglas, L.J. Iron-limited biofilms of Candida albicans and their susceptibility to Amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 2146–2149. [Google Scholar] [PubMed]
- Dumitru, R.; Hornby, J.M.; Kenneth, W.; Nickerson, K.W. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Kucharíková, S.; Tournu, H.; Lagrou, K.; van Dijck, P.; Bujdakova, H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J. Med. Microbiol. 2011, 60, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K.; Repp, K.K.; Hazen, K.C. Temperature affects the susceptibility of Cryptococcus neoformans biofilms to antifungal agents. Med. Mycol. 2010, 48, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; VandeWalle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Al-fattani, M.A.; Douglas, L.J. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 2012, 55, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Sanchez, H.; Cain, M.T.; Andes, D.R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 2010, 202, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Crawford, K.; Marchillo, K.; Andes, D.R. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 2010, 54, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Sanchez, H.; Cain, M.T.; Ross, K.M.; Andes, D.R. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot. Cell 2011, 10, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Nett, J.E.; Hernday, A.D.; Homann, O.R.; Deneault, J.-S.; Nantel, A.; Andes, D.R.; Johnson, A.D.; Mitchell, A.P. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 2008, 322, 107–131. [Google Scholar] [PubMed]
- Al-Dhaheri, R.S.; Douglas, L.J. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob. Agents Chemother. 2008, 52, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Knot, P.D.; Suci, P.A.; Miller, R.L.; Nelson, R.D.; Tyler, B.J. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and β-1,6-glucan pathway genes. Antimicrob. Agents Chemother. 2006, 50, 3708–3716. [Google Scholar]
- Bink, A.; Vandenbosch, D.; Coenye, T.; Nelis, H.; Cammue, B.P.A.; Thevissen, K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011, 55, 4033–4037. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lincoln, L.; Marchillo, K.; Andes, D. β-1,3 Glucan as a test for central venous catheter biofilm infection. J. Infect. Dis. 2007, 195, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Tanabe, K.; Niimi, M.; Monk, B.C. Candida albicans drug resistance—Another way to cope with stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- White, T.C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate, with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 1997, 41, 1482–1487. [Google Scholar] [PubMed]
- Tscherner, M.; Schwarzmüller, T.; Kuchler, K. Pathogenesis and antifungal drug resistance of the human fungal pathogen Candida glabrata. Pharmaceuticals 2011, 4, 169–186. [Google Scholar] [CrossRef]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.W.; Nickels, J.T.; Edlind, T.D. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2000, 44, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Borecká-Melkusová, S.; Moran, G.P.; Sullivan, D.J.; Kucharíková, S.; Chrovát, D.; Bujdáková, H. The expression of genes involved in the ergosterol biosynthesis pathway in Candida albicans and Candida dubliniensis biofilms exposed to fluconazole. Mycoses 2009, 52, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, T.; Ding, C.; Guida, A.; D’Enfert, C.; Higgins, D.G.; Butler, G. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot. Cell 2009, 8, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Chatzimoschou, A.; Simitsopoulou, M.; Dalakiouridou, M.; Diza-Mataftsi, E.; Tsantali, C.; Roilides, E. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother. 2008, 52, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Morschhäuser, J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1587, 240–248. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.P.; Patterson, T.F.; López-Ribot, J.L. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 2002, 40, 2228–2230. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Barker, K.S.; Znaidi, S.; Schneider, S.; Dierolf, F.; Dunkel, N.; Aïd, M.; Boucher, G.; Rogers, P.D.; Raymond, M.; et al. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Maki, K.; Ikeda, F.; Holmes, A.R.; Lamping, E.; Niimi, M.; Monk, B.C.; Cannon, R.D. Overexpression of Candida albicans CDR1, CDR2, or MDR1 Does Not Produce Significant Changes in Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Silver, P.M.; Oliver, B.G.; White, T.C. Characterization of caspofungin susceptibilities by broth and agar in Candida albicans clinical isolates with characterized mechanisms of azole resistance. Med. Mycol. 2008, 46, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicro. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef]
- Albertson, G.D.; Niimi, M.; Cannon, R.D.; Jenkinson, H.F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 1996, 40, 2835–2841. [Google Scholar] [PubMed]
- Coste, A.; Turner, V.; Ischer, F.; Diogo, D.; Bougnoux, M.-E.; d’Enfert, C.; Berman, J.; Sanglard, D. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, N.; Blass, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the multidrug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Bizerra, F.C.; Nakamura, C.V.; De Poersch, C.; Svidzinski, T.; Quesada, R.; Goldenberg, S.; Krieger, M.; Yamada-Ogatta, S. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008, 8, 442–450. [Google Scholar] [CrossRef] [PubMed]
| Candida Species | Biofilm Forming Capacity | Biofilm Characteristics | Refs. |
|---|---|---|---|
| Candida albicans | +++++ | Basal blastospore layer with a dense overlying matrix composed of exopolysaccharides and hyphae. | [27,28] |
| Candida dubliniensis | ++/+++ | Chains of cells with thin extracellular matrix material. | [29,30] |
| Hhigh variability among clinical isolates. | |||
| Candida glabrata | ++/+++ | Forms considerably less biofilm than C. albicans. | [6,28] |
| High in both protein and carbohydrate content. | |||
| Candida krusei | ++++ | Thick multilayered biofilm of pseudohyphal forms embedded within the polymer matrix. | [31] |
| Candida parapsilosis | +++ | Clumped blastospores and less volume. | [23,27,28,30,32] |
| Large amounts of carbohydrate with less protein. | |||
| High variability among clinical isolates. | |||
| Candida tropicalis | +++ | Chains of cells with thin, but large, amounts of extracellular matrix material. | [24,28] |
| Low amounts of carbohydrate and protein. |
| Biofilm | Condition/Disease | Most Common Candida Species | Refs. |
|---|---|---|---|
| Medical Devices | Endocarditis | Candida albicans | [14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] |
| Total parenteral nutrition | Candida glabrata | ||
| Prosthetic joints | Candida tropicalis | ||
| Peritoneal dialysis | Candida parapsilosis | ||
| Cannulation | |||
| Ventriculoperitoneal shunts | |||
| Prosthetic knees | |||
| Hip joints | |||
| Breast implants | |||
| Bioprosthetic heart valves | |||
| Catheter-related disease: urinary catheter, central venous catheter, intravenous catheter | |||
| Oral | Caries | Candida albicans | [10,24,33,34,35,36,37,38,39,40,41,42,43,44,45,46] |
| Periodontal disease | Candida glabrata | ||
| Endodontic infection | Candida dubliniensis Candida tropicalis | ||
| Several mucosal infections | Candida krusei | ||
| Candida parapsilosis | |||
| Gastrointestinal (GI) and Urinary Tract | Feeding tubes for enteral nutrition | Candida albicans | [47,48,49,50,51,52,53,54,55,56,57,58,59] |
| Ulcerative colitis | Candida tropicalis | ||
| GI candidiasis | |||
| Pyelonephritis | |||
| Cystitis | |||
| Prostatitis | |||
| Intrauterine contraceptives | |||
| Upper Airways | Rhinosinusitis | Candida albicans | [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| Ventilator-associated | Candida glabrata | ||
| Pneumonia | Candida krusei | ||
| Lower Airways | Cystic Fibrosis | Candida albicans | [86,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| Allergic bronchopulmonary diseases | |||
| Wounds | Diabetic foot ulcer | Candida albicans | [100,101,102,103] |
| Non-healing surgical wounds | Candida glabrata | ||
| Chronic wound infections | |||
| Pressure ulcers | |||
| Venous leg ulcers |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. https://doi.org/10.3390/jof3010008
Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M. Candida Species Biofilms’ Antifungal Resistance. Journal of Fungi. 2017; 3(1):8. https://doi.org/10.3390/jof3010008
Chicago/Turabian StyleSilva, Sónia, Célia F. Rodrigues, Daniela Araújo, Maria Elisa Rodrigues, and Mariana Henriques. 2017. "Candida Species Biofilms’ Antifungal Resistance" Journal of Fungi 3, no. 1: 8. https://doi.org/10.3390/jof3010008
APA StyleSilva, S., Rodrigues, C. F., Araújo, D., Rodrigues, M. E., & Henriques, M. (2017). Candida Species Biofilms’ Antifungal Resistance. Journal of Fungi, 3(1), 8. https://doi.org/10.3390/jof3010008









