Verapamil Inhibits Aspergillus Biofilm, but Antagonizes Voriconazole
Abstract
:1. Introduction
2. Materials and Methods
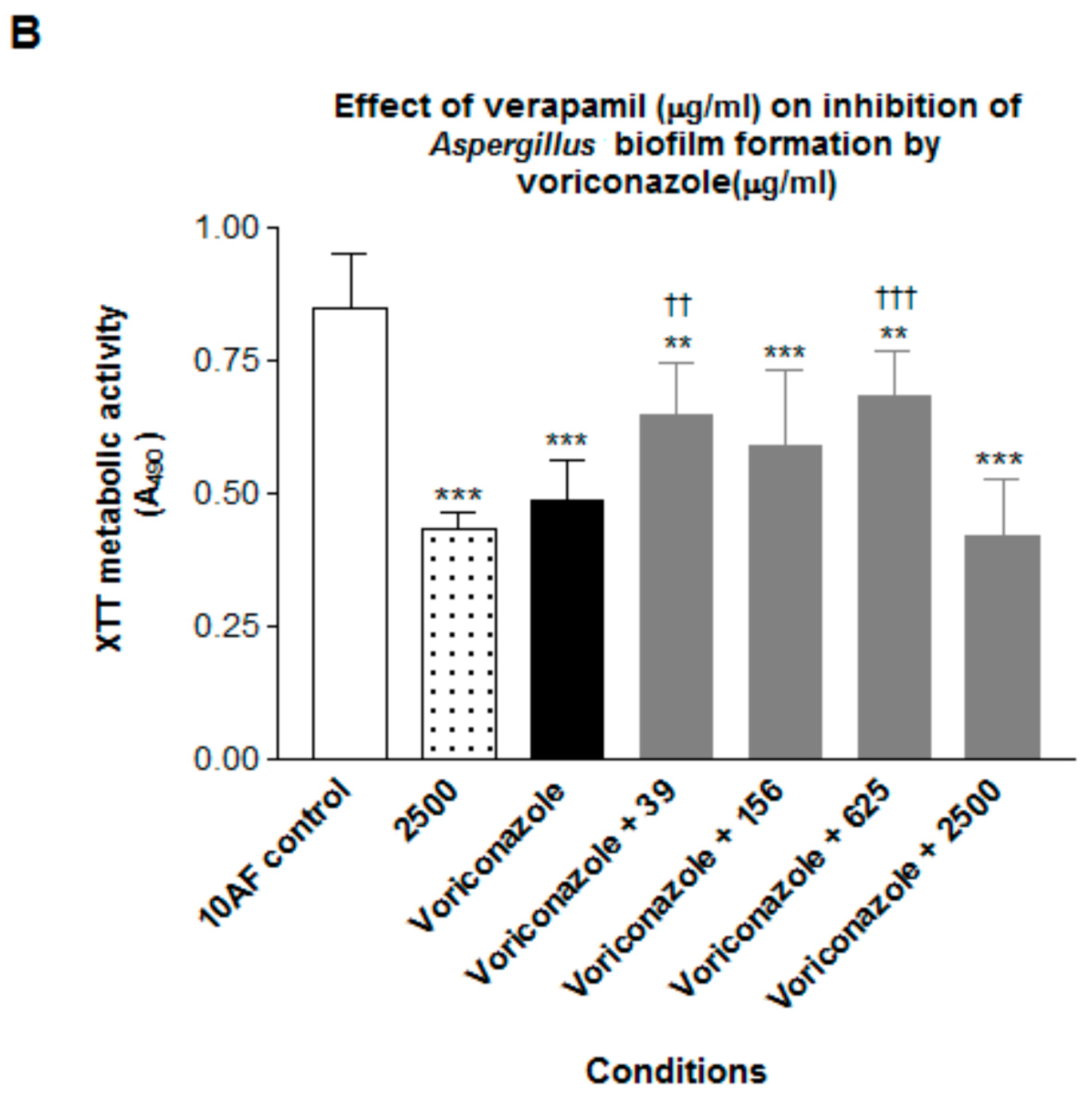
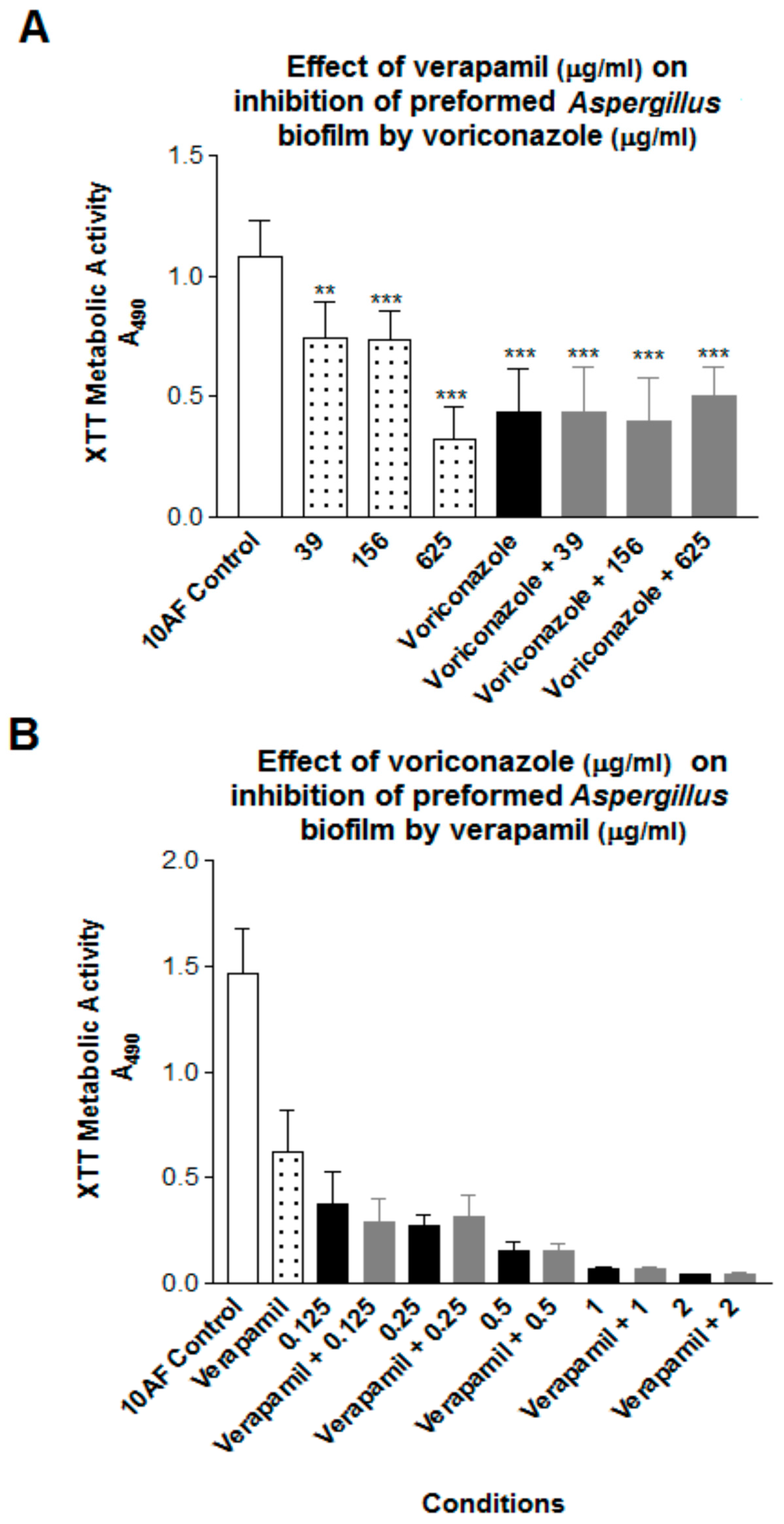
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Q.; Xiao, C.; Zhang, K.; Jia, C.; Ding, X.; Zhang, B.; Wang, Y.; Li, M. The calcium channel blocker verapamil inhibits oxidative stress response in Candida albicans. Mycopathologia 2014, 177, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Shanks, S.; Duncan, V.M.S.; Yang, M.; Mackenzie, K.; Gow, N.A. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 2007, 17, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ding, X.; Zhang, B.; Xu, N.; Jia, C.; Mao, J.; Zhang, B.; Xing, L.; Li, M. Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res. 2014, 14, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Wickes, B.L.; López-Ribot, J.L. Inhibition on Candida albicans biofilm formation using divalent cation chelators (EDTA). Mycopathologia 2007, 164, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ding, X.; Xu, N.; Cheng, X.; Qian, K.; Zhang, B.; Xing, L.; Li, M. In vitro activity of verapamil alone and in combination with fluconazole or tunicamycin against Candida albicans biofilms. Int. J. Antimicrob. Agents 2013, 41, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.; Stevens, D.A. Drugs active against fungi, Pneumocystis and Microsporidia. In Principles and Practice of Infectious, 8th ed.; Bennett, J., Dolin, R., Blaser, M., Eds.; Elsevier Publ.: Philadelphia, PA, USA, 2014. [Google Scholar]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Bowyer, P. Voriconazole resistance in Aspergillus fumigatus: Should we be concerned? Clin. Infect. Dis. 2013, 57, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Latgé, J. Aspergillus biofilm in vitro and in vivo. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Stevens, D.A. Cegelski L: Fungal biofilm composition and opportunities in drug discovery. Future Med. Chem. 2016, 8, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Melmon, K.; Morelli, H.; Hoffman, B.B.; Nierenberg, D.W. Melmon and Morrelli’s Clinical Pharmacology: Basic Principles in Therapeutics, 3rd ed.; McGraw-Hill Inc.: New York, NY, USA, 1992. [Google Scholar]
- Denning, D.W.; Stevens, D.A. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 1991, 35, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.G.; Penner, J.C.; Moss, R.B.; Haagensen, J.A.J.; Clemons, K.V.; Spormann, A.M.; Nazik, H.; Cohen, K.; Banaei, N.; Carolino, E.; et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS ONE 2015, 10, e0134692. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Ferreira, J.A.; Joubert, L.M.; Clemons, K.V.; Stevens, D.A.; Cegelski, L. Analysis of the Aspergillus fumigatus biofilm extracellular matrix by solid-state nuclear magnetic resonance spectroscopy. Eukaryot. Cell 2015, 14, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Nazik, H.; Penner, J.C.; Ferreira, J.A.; Haagensen, J.A.J.; Cohen, K.; Spormann, A.M.; Martinez, M.; Chen, V.; Hsu, J.L.; Clemons, K.V.; et al. Effects of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob. Agents Chemother. 2015, 59, 6514–6520. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; approved standard. CLSI document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Stevens, D.A.; Aristizibal, B.H. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 and UR-9751, and comparison with fluconazole. Diagn. Microbiol. Infect. Dis. 1997, 29, 103–106. [Google Scholar] [CrossRef]
- Mowat, E.; Lang, S.; Williams, C.; McCulloch, E.; Jones, B.; Ramage, G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 2008, 62, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Mowat, E.; McCulloch, E.; Lappin, D.F.; Jones, B.; Lang, S.; Majithiya, J.B.; Warn, P.; Williams, C.; Ramage, G. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 2011, 55, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.M.C. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Mishra, G.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur. J. Pharm. Sci. 2009, 36, 580–590. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazik, H.; Choudhary, V.; Stevens, D.A. Verapamil Inhibits Aspergillus Biofilm, but Antagonizes Voriconazole. J. Fungi 2017, 3, 50. https://doi.org/10.3390/jof3030050
Nazik H, Choudhary V, Stevens DA. Verapamil Inhibits Aspergillus Biofilm, but Antagonizes Voriconazole. Journal of Fungi. 2017; 3(3):50. https://doi.org/10.3390/jof3030050
Chicago/Turabian StyleNazik, Hasan, Varun Choudhary, and David A. Stevens. 2017. "Verapamil Inhibits Aspergillus Biofilm, but Antagonizes Voriconazole" Journal of Fungi 3, no. 3: 50. https://doi.org/10.3390/jof3030050



