Microenvironment Responsive Modulations in the Fatty Acid Content, Cell Surface Hydrophobicity, and Adhesion of Candida albicans Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Effect of Physical Factors (pH, Temperature and Low Hydrostatic Pressure) on Fatty Acid Content in Candida albicans
2.3. Fatty Acid Analysis Using GC-MS
2.4. Identification of Fatty Acids Using Equivalent Chain Length (ECL) Value
2.5. Evaluation of Cell Surface Hydrophobicity (CSH)
2.6. Evaluation of Adhesion
2.7. Extraction and Estimation of Ergosterol Content
3. Result
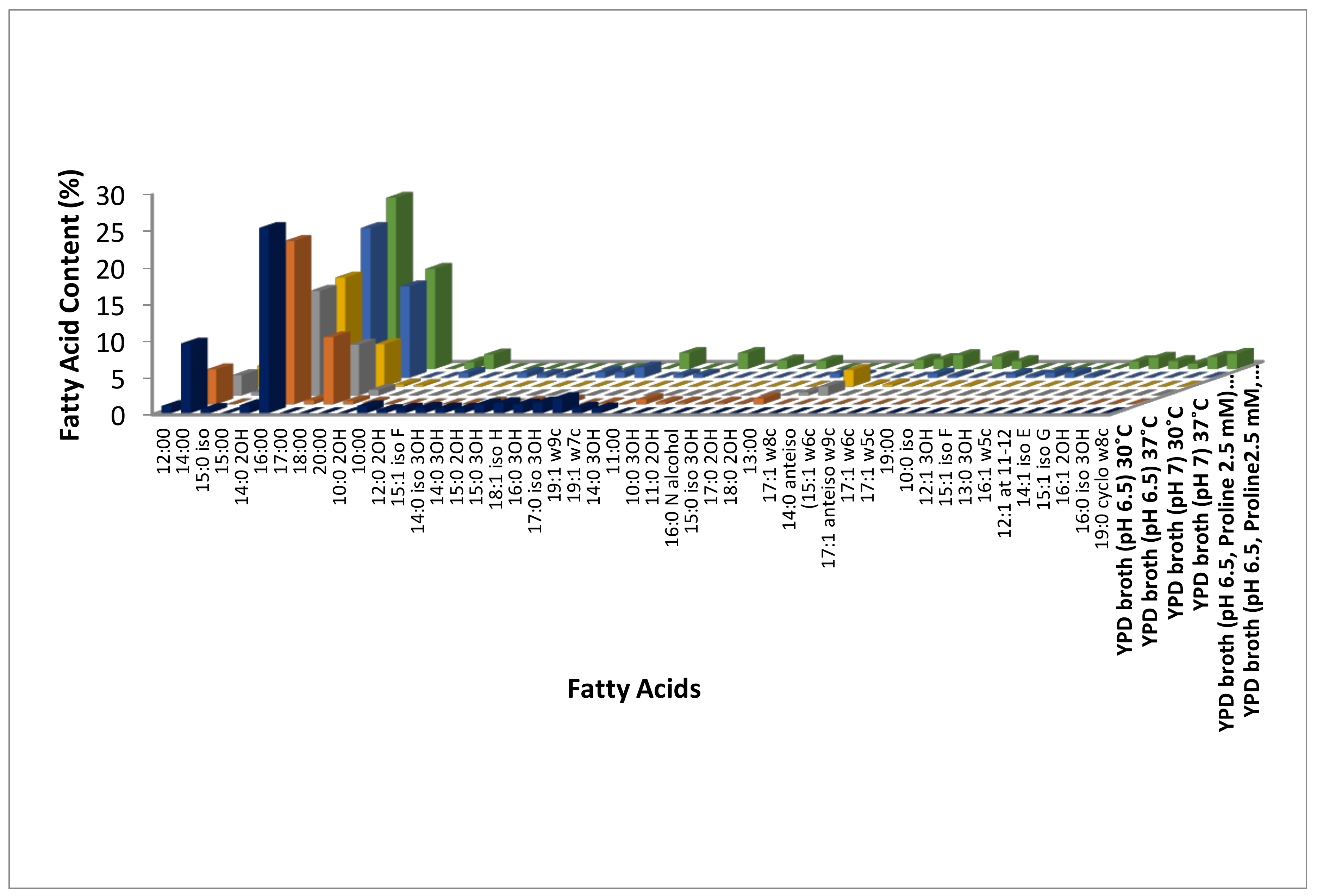
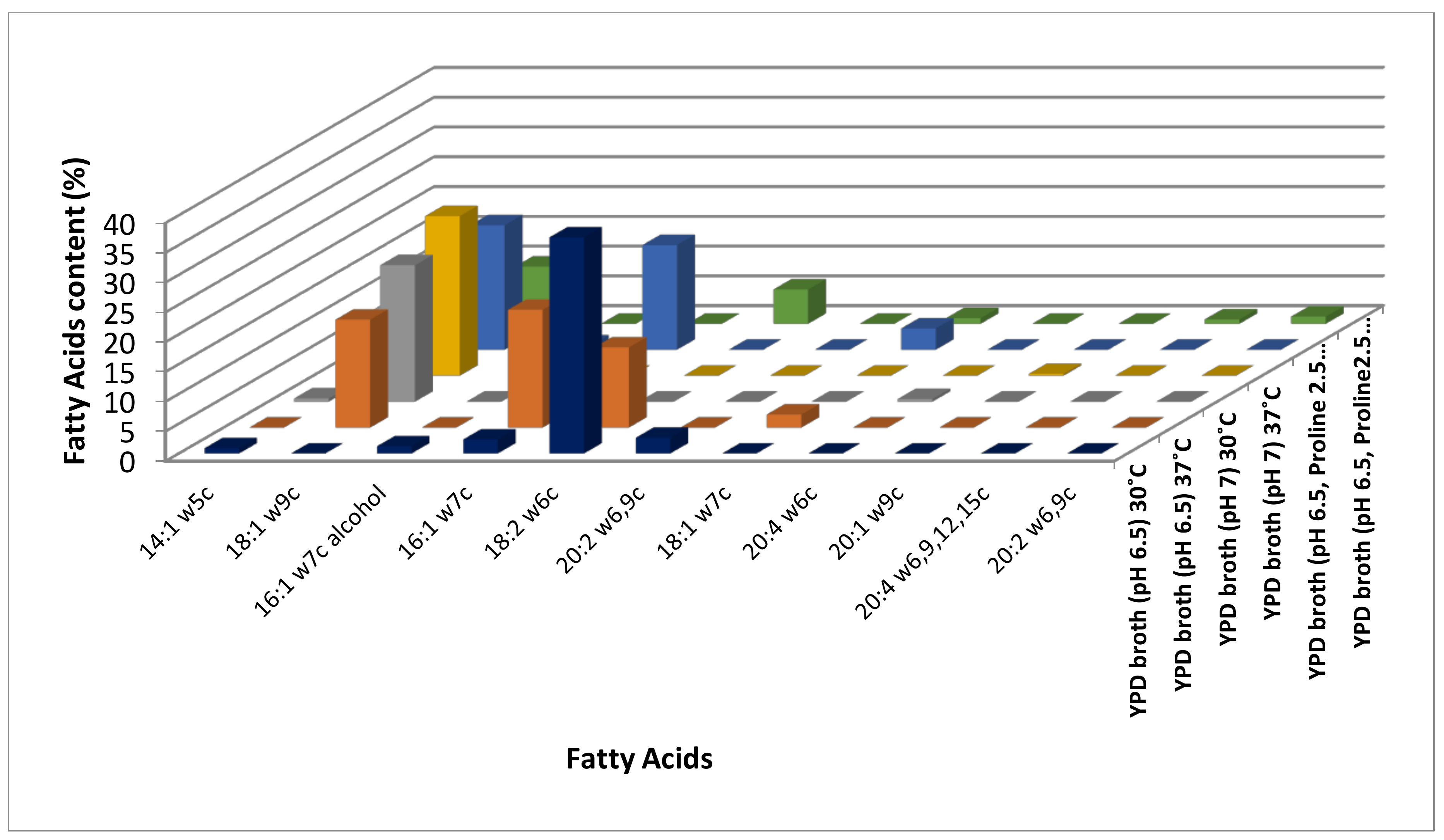
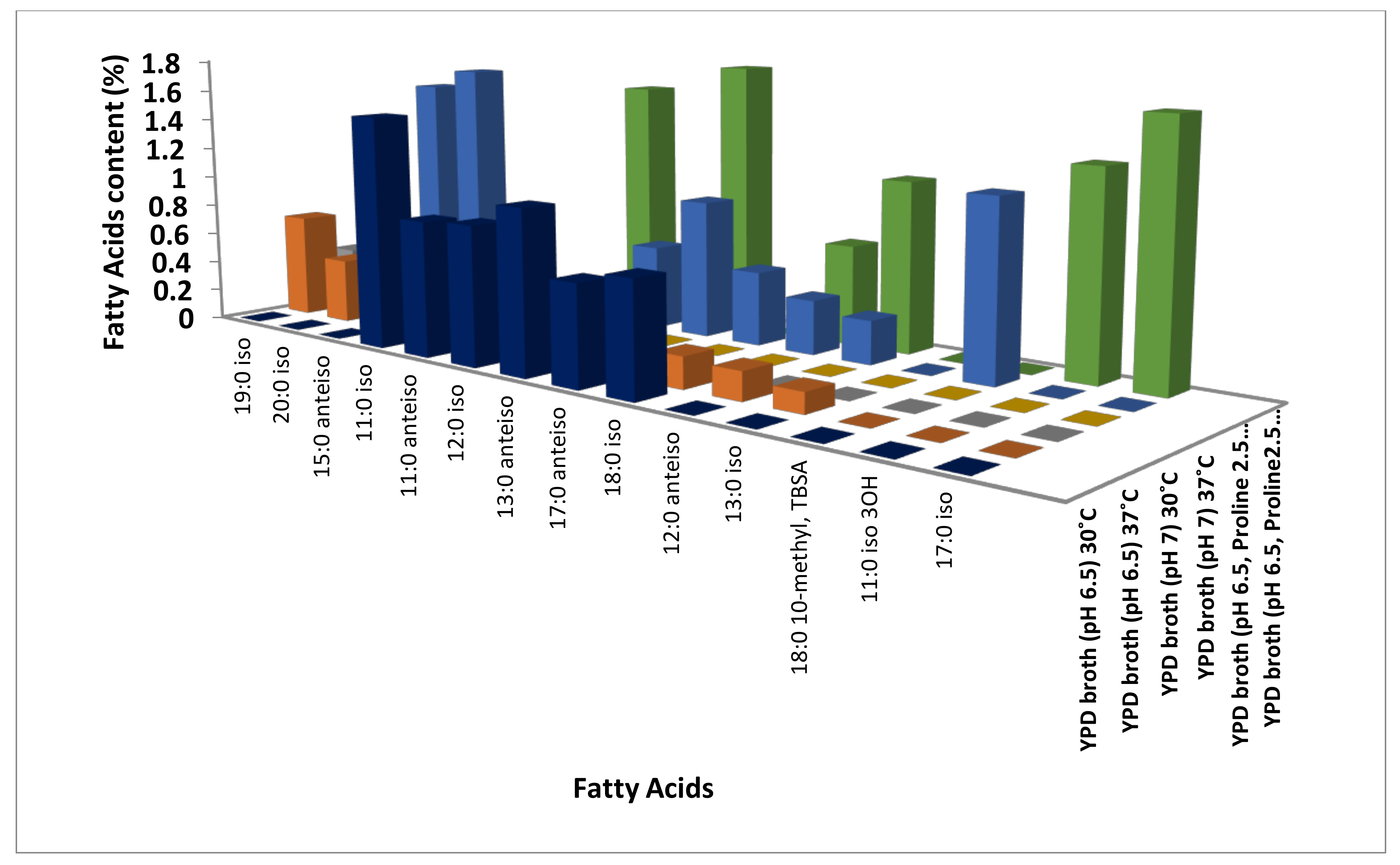
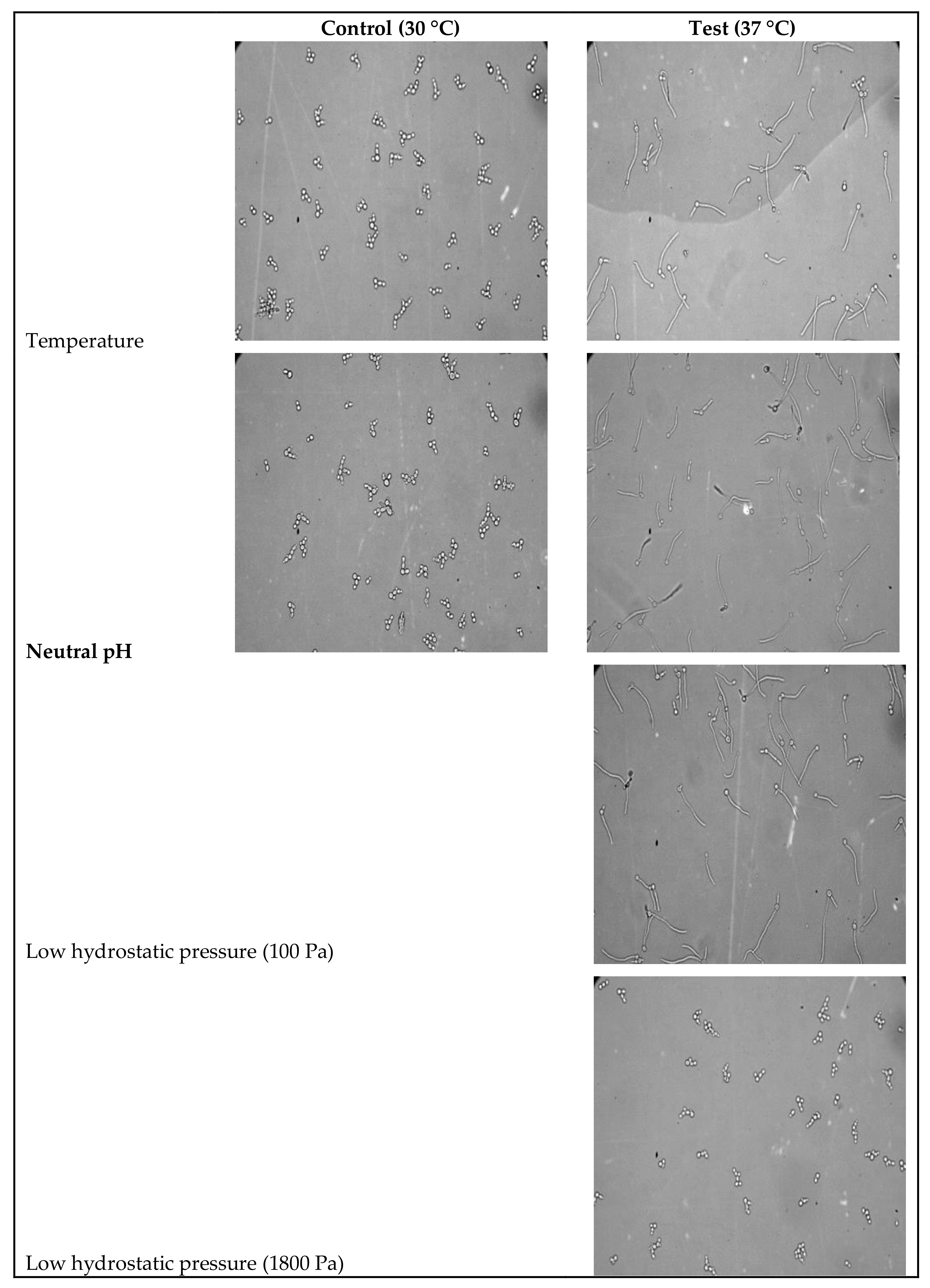
3.1. Identification of Modulations in Fatty Acid Composition in C. albicans (ATCC10231) Cells in Response to Temperature
3.2. Identification of Modulations in Fatty Acid Composition in C. albicans (ATCC10231) Cells in Response to Neutral pH
3.3. Identification of Modulations in Fatty Acid Composition in C. albicans (ATCC10231) Cells in Response to LHP (1800 Pa)
3.4. Modulation of Cell Surface Hydrophobicity (CSH) and Adhesion
3.4.1. CSH
3.4.2. Adhesion
3.5. Ergosterol
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Odds, F.C. Candida species and virulence. ASM News 1994, 60, 313–318. [Google Scholar]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggimann, P.; Bille, J.; Marchetti, O. Diagnosis of invasive candidiasis in the ICU. Ann. Intensive Care 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A.; Clancy, C.J. Candida and Candidiasis; ASM Press: Washington, DC, USA, 2011; pp. 67–86. [Google Scholar]
- Sipsas, N.V.; Lewis, R.E.; Tarrand, J.; Hachem, R.; Rolston, K.V.; Raad, I.I.; Kontoyiannis, D.P. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007). Cancer 2009, 115, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallick, E.M.; Bergeron, A.C.; Jones, S.K., Jr.; Newman, Z.R.; Brothers, K.M.; Creton, R.; Bennett, R.J. Phenotypic plasticity regulates Candida albicans interactions and virulence in the vertebrate host. Front. Microbiol. 2016, 7, 780. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.R.; Daniels, K.J. Plasticity of Candida albicans biofilms. Microbiol. Mol. Biol. Rev. 2016, 80, 565–595. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y. Lipids and dimorphism of Candida albicans and Sporothrixschenckii. In Lipids of Pathogenic Fungi; Prasad, R., Ghannoum, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 219–234. [Google Scholar]
- Finean, J.B.; Michell, R.H. Isolation, composition and general structure of membranes. In New Comprehensive Biochemistry; Blackwell: Oxford, UK, 1981; Volume 1, pp. 1–36. [Google Scholar]
- Mrozik, A.; Piotrowska-Seget, Z.; Labuzek, S. Cytoplasmatic bacterial membrane responses to environmental perturbations. Polish. J. Environ. Stud. 2004, 13, 487–494. [Google Scholar]
- Denich, T.J.; Beaudette, L.A.; Lee, H.; Trevors, J.T. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- Mago, N.; Khuller, G.K. Lipids of Candida albicans: Subcellular distribution and biosynthesis. Microbiology 1990, 136, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, V.; Prasad, R. Comparative lipidomics in clinical isolates of Candida albicans reveal crosstalk between mitochondria, cell wall integrity and azole resistance. PLoS ONE 2012, 7, e39812. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D.; Tronchin, G.; Dubremetz, J.F.; Biguet, J. Ultrastructure of the cell wall of Candida albicans blastospores: Study of its constitutive layers by the use of a cytochemical technique revealing polysaccharides. Ann. Microbiol. 1978, 129, 141–153. [Google Scholar]
- Calderone, R.A.; Gow, N.A. Host recognition by Candida species. In Candida and Candidiasis; ASM Press: Washington, DC, USA, 2002; pp. 67–86. [Google Scholar]
- Chander, J. Candidiasis. A Text Book of Medical Mycology; Mehta Publishers: New Delhi, Iadia, 2009; pp. 266–290. [Google Scholar]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A. Role of hydrophobic interactions in microbial adhesion to plastics used in medical devices. In Microbial Cell Surface Hydrophobicity; Doyle, R.J., Rosenberg, M., Eds.; ASM: Washington, DC, USA, 1990; pp. 107–136. [Google Scholar]
- Samaranayake, Y.H.; Samaranayake, L.P.; Yau, J.Y.Y.; Ellepola, A.N.B.; Anil, S.; Yeung, K.W.S. Adhesion and cell-surface-hydrophobicity of sequentially isolated genetic isotypes of Candida albicans in an HIV-infected Southern Chinese cohort. Mycoses 2003, 46, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Wagner, R.C. Introduction to Biological Membranes; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Rapte, P. Low Hydrostatic Pressure (LHP) Modulates Candida albicans Morphogenesis by Affecting Mitochondrial Ultrastructure, Protein Folding and Cell Separation. Ph.D. Thesis, Swami Ramanand Teerth Marathwada University, Nanded, India, 2015. [Google Scholar]
- El Sayed, S.M.; Mahmoud, H.S.; Nabo, M.M.H. Methods of wet cupping therapy (Al-Hijamah): In light of modern medicine and prophetic medicine. Altern. Integr. Med. 2013, 2, 1–16. [Google Scholar]
- Tafheem, S.; Zore, G. Effect of Hydrostatic Pressure on Candida Albicans Morphogenesis. Master’s Dissertation, Swami Ramanand Treerth Marathwada University, Nanded, India, 2013. [Google Scholar]
- Fernandes, P.M.B.; Farina, M.; Kurtenbach, E. Effect of hydrostatic pressure on the morphology and ultrastructure of wild-type and trehalose synthase mutant cells of Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2001, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kurzai, O.; Heinz, W.J.; Sullivan, D.J.; Coleman, D.C.; Frosch, M.; Mühlschlegel, F.A. Rapid PCR test for discriminating between Candida albicans and Candida dubliniensis isolates using primers derived from the pH-regulated PHR1and PHR2 genes of C. albicans. J. Clin. Microbiol. 1999, 37, 1587–1590. [Google Scholar] [PubMed]
- Zore, G.B.; Thakre, A.D.; Rathod, V.; Karuppayil, S.M. Evaluation of anti-Candida potential of geranium oil constituents against clinical isolates of Candida albicans differentially sensitive to fluconazole: Inhibition of growth, dimorphism and sensitization. Mycoses 2011, 54, e99–e109. [Google Scholar] [CrossRef] [PubMed]
- Saporito-Irwin, S.M.; Birse, C.E.; Sypherd, P.S.; Fonzi, W.A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell Biol. 1995, 15, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Maserti, B.; Podda, A.; Giorgetti, L.; Del Carratore, R.; Chevret, D.; Migheli, Q. Proteome changes during yeast-like and pseudohyphal growth in the biofilm-forming yeast Pichiafermentans. Amino Acids 2015, 47, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 101; MIDI Inc.: Newark, NJ, USA, 1990. [Google Scholar]
- Hazen, K.C.; Hazen, B.W. A polystyrene microsphere assay for detecting surface hydrophobicity variations within Candida albicans populations. J. Microbiol. Methods 1987, 6, 289–299. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Panagoda, G.J.; Ellepola, A.N.B.; Samaranayake, L.P. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses 2001, 44, 29–35. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Meurman, J.H.; Kari, K.; Rautemaa, R.; Samaranayake, L.P. In vitro adhesion of Candida species to denture base materials. Mycoses 2006, 49, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [PubMed]
- Buffo, J.; Herman, M.A.; Soll, D.R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia 1984, 85, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Los, D.A. Membrane fluidity and temperature perception. Plant Physiol. 1997, 115, 875. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Regulation of enzymatic activity and gene expression by membrane fluidity. Sci. Signal. STKE 2000, 2000, pe1. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.S.; Carlisle, P.L.; Kadosh, D. Coevolution of morphology and virulence in Candida species. Eukaryot. Cell 2011, 10, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candidatropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Chaffin, W.L.; López-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martínez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Costa, A.C.B.P.; Silva, M.P.; Back-Brito, G.N.; Jorge, A.O.C. Candida albicans and virulence factors that increases its pathogenicity. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Microbiology Series; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; Volume 2, pp. 631–636. [Google Scholar]
- Šajbidor, J. Effect of some environmental factors on the content and composition of microbial membrane lipids. Crit. Rev. Biotech. 1997, 17, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Vagelos, P.R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1972, 265, 25–60. [Google Scholar] [CrossRef]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Nat. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Keweloh, H.; Diefenbach, R.; Rehm, H.J. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch. Microbiol. 1991, 157, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R.; Williams, E.E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid. Res. 1990, 29, 167–227. [Google Scholar] [CrossRef]
- Khaware, R.K.; Koul, A.; Prasad, R. High membrane fluidity is related to NaCl stress in Candida membranefaciens. Biochem. Mol. Biol. Int. 1995, 35, 875–880. [Google Scholar] [PubMed]
- Braganza, L.F.; Worcester, D.L. Structural changes in lipid bilayers and biological membranes caused by hydrostatic pressure. Biochemistry 1986, 25, 7484–7488. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 47–68. [Google Scholar] [CrossRef]
- Raut, J.; Rathod, V.; Karuppayil, S.M. Cell surface hydrophobicity and adhesion: A study on fifty clinical isolates of Candida albicans. Nippon IshinkinGakkaiZasshi 2010, 51, 131–136. [Google Scholar] [CrossRef]
- Hazen, K.C. Cell surface hydrophobicity of medically important fungi, especially Candida species. In Microbial Cell Surface Hydrophobicity; ASM Press: Washington, DC, USA, 1990; pp. 249–295. [Google Scholar]
- Hazen, K.C.; Plotkin, B.J.; Klimas, D.M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect. Immun. 1986, 54, 269–271. [Google Scholar] [PubMed]
- Klotz, S.A.; Penn, R.L. Multiple mechanisms may contribute to the adherence of Candida yeasts to living cells. Curr. Microbiol. 1987, 16, 119–122. [Google Scholar] [CrossRef]
- Ener, B.; Douglas, L.J. Correlation between cell-surface hydrophobicity of Candida albicans and adhesion to buccal epithelial cells. FEMS Microbiol. Lett. 1992, 99, 37–42. [Google Scholar] [CrossRef]
- Klotz, S.A.; Drutz, D.J.; Zajic, J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [PubMed]
- Guthrie, C. Guide to yeast genetics and molecular and cell biology. Methods Enzymol. 2002, 350, 18–19. [Google Scholar]
| Growth Medium | Morphology, Cell Surface Hydrophobicity, Adhesion and Ergosterol Content | % ± S.D. | |
|---|---|---|---|
| 30 °C | 37 °C | ||
| YPD broth (pH 6.5) | Hyphae | 08.0 ± 1.0 | 65.0 ± 2.51 |
| CSH | 26.36 ± 1.57 | 22.87 ± 0.50 | |
| Adhesion | 56.0 ± 3.0 | 23.67 ± 2.08 | |
| Ergosterol | 0.066 ± 0.003 | 0.027 ± 0.002 | |
| YPD broth (pH 7.0) | Hyphae | 10.0 ± 1.0 | 85.0 ± 2.0 |
| CSH | 11.71 ± 0.56 | 6.6 ± 0.48 | |
| Adhesion | 68.0 ± 4.0 | 30.6 ± 1.52 | |
| Ergosterol | 0.04 ± 0.002 | 0.008 ± 0.002 | |
| YPD broth (pH 6.5, Proline2.5 mM) | Hyphae | n/a. | 85.0 ± 1.52 |
| CSH | 10.3 ± 0.39 | ||
| Adhesion | 51.0 ± 1.0 | ||
| Ergosterol | 0.013 ± 0.010 | ||
| YPD broth (pH 6.5, Proline2.5 mM, LHP-1800 Pa) | Hyphae | n/a. | 10.0 ± 0.57 |
| CSH | 5.97 ± 0.07 | ||
| Adhesion | 20.66 ± 1.52 | ||
| Ergosterol | 0.018 ± 0.005 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiradhone, A.B.; Ingle, S.S.; Zore, G.B. Microenvironment Responsive Modulations in the Fatty Acid Content, Cell Surface Hydrophobicity, and Adhesion of Candida albicans Cells. J. Fungi 2018, 4, 47. https://doi.org/10.3390/jof4020047
Shiradhone AB, Ingle SS, Zore GB. Microenvironment Responsive Modulations in the Fatty Acid Content, Cell Surface Hydrophobicity, and Adhesion of Candida albicans Cells. Journal of Fungi. 2018; 4(2):47. https://doi.org/10.3390/jof4020047
Chicago/Turabian StyleShiradhone, Asha Bhujangrao, Sujata S. Ingle, and Gajanan B. Zore. 2018. "Microenvironment Responsive Modulations in the Fatty Acid Content, Cell Surface Hydrophobicity, and Adhesion of Candida albicans Cells" Journal of Fungi 4, no. 2: 47. https://doi.org/10.3390/jof4020047





