Pseudogymnoascus destructans: Causative Agent of White-Nose Syndrome in Bats Is Inhibited by Safe Volatile Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fungal Strains and Media
2.3. Preparation of Fungal Inocula
2.4. Volatile Exposures
2.5. Subculture of VOC Treated P. destructans
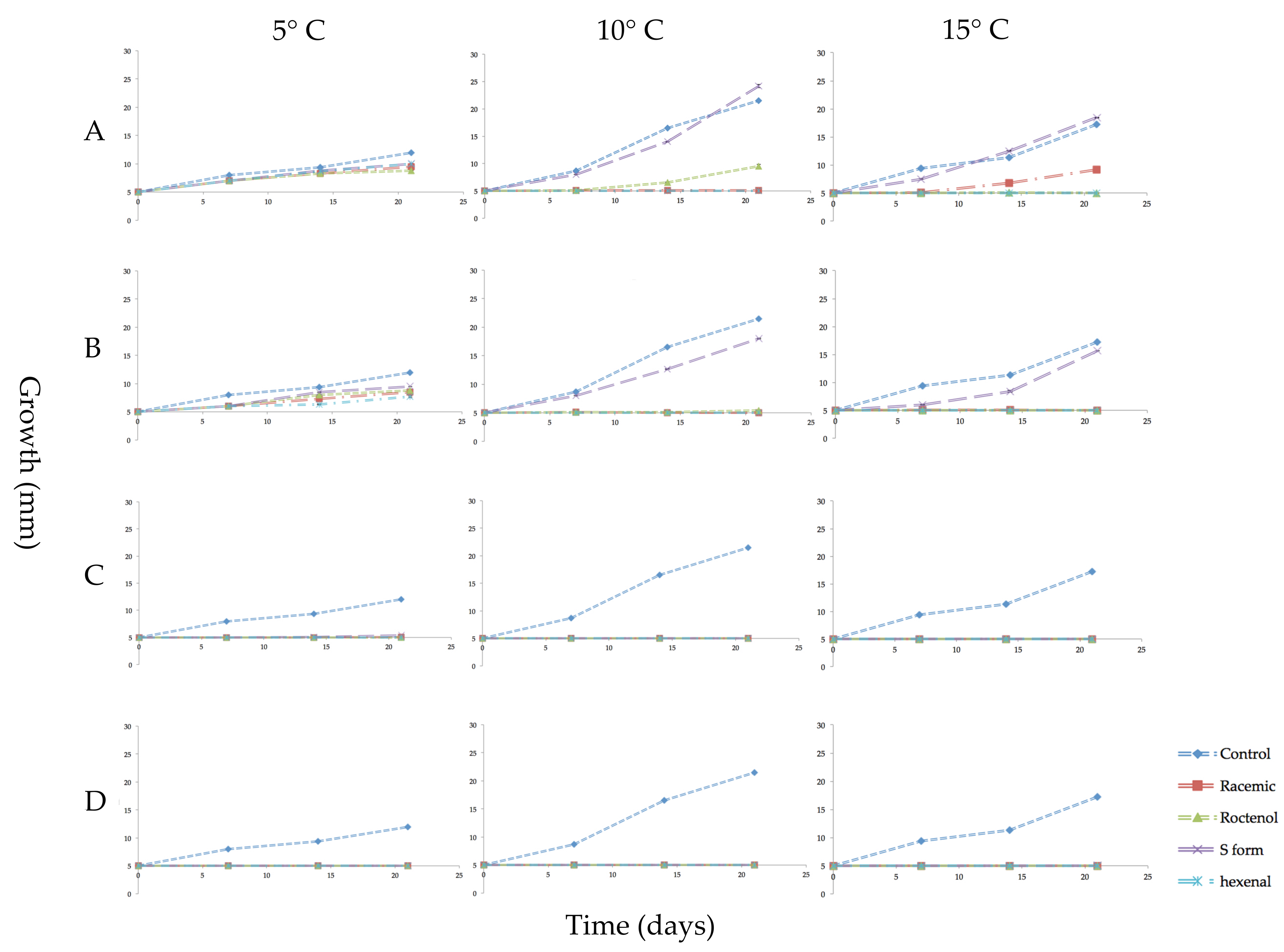
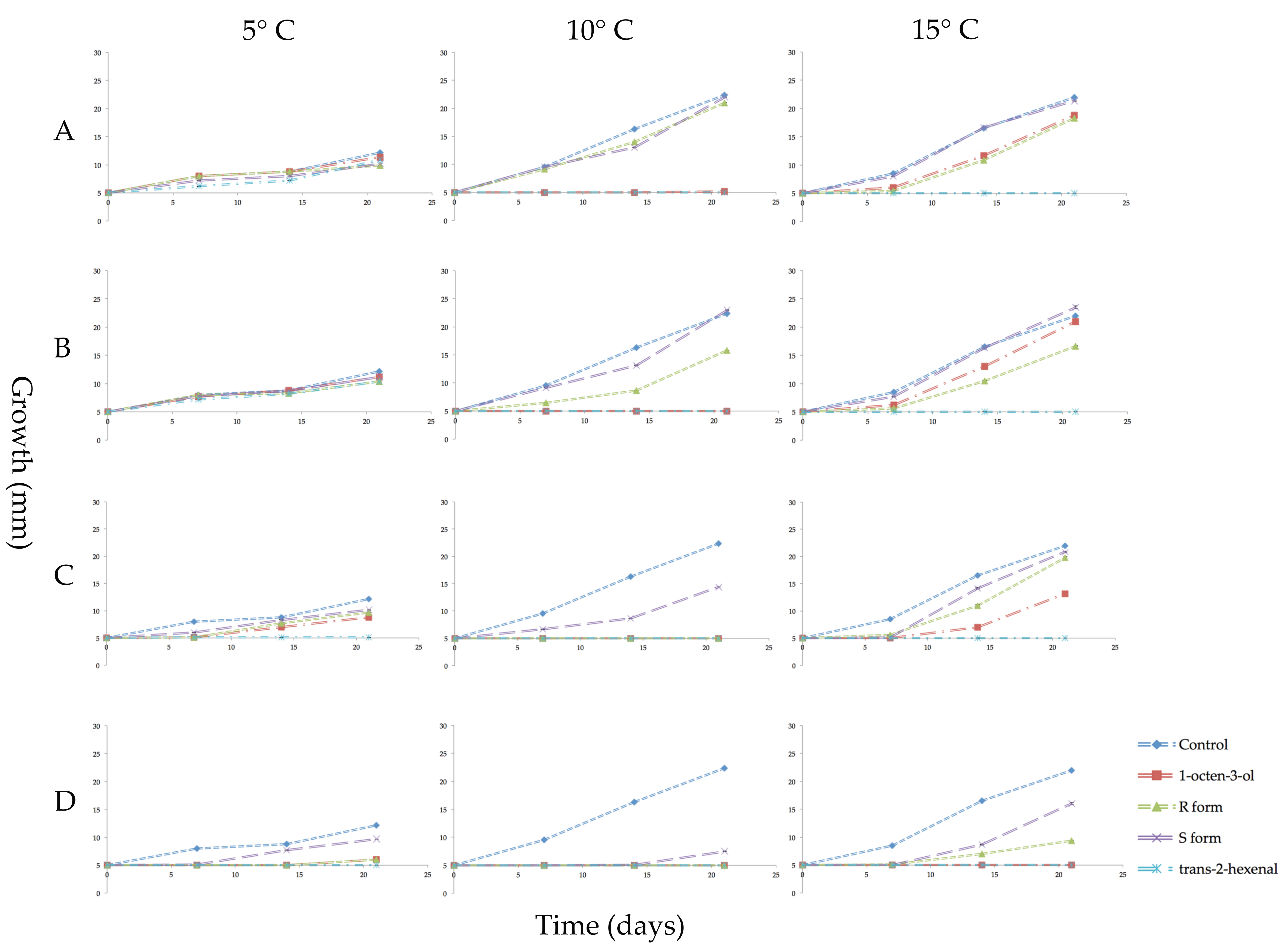
3. Results
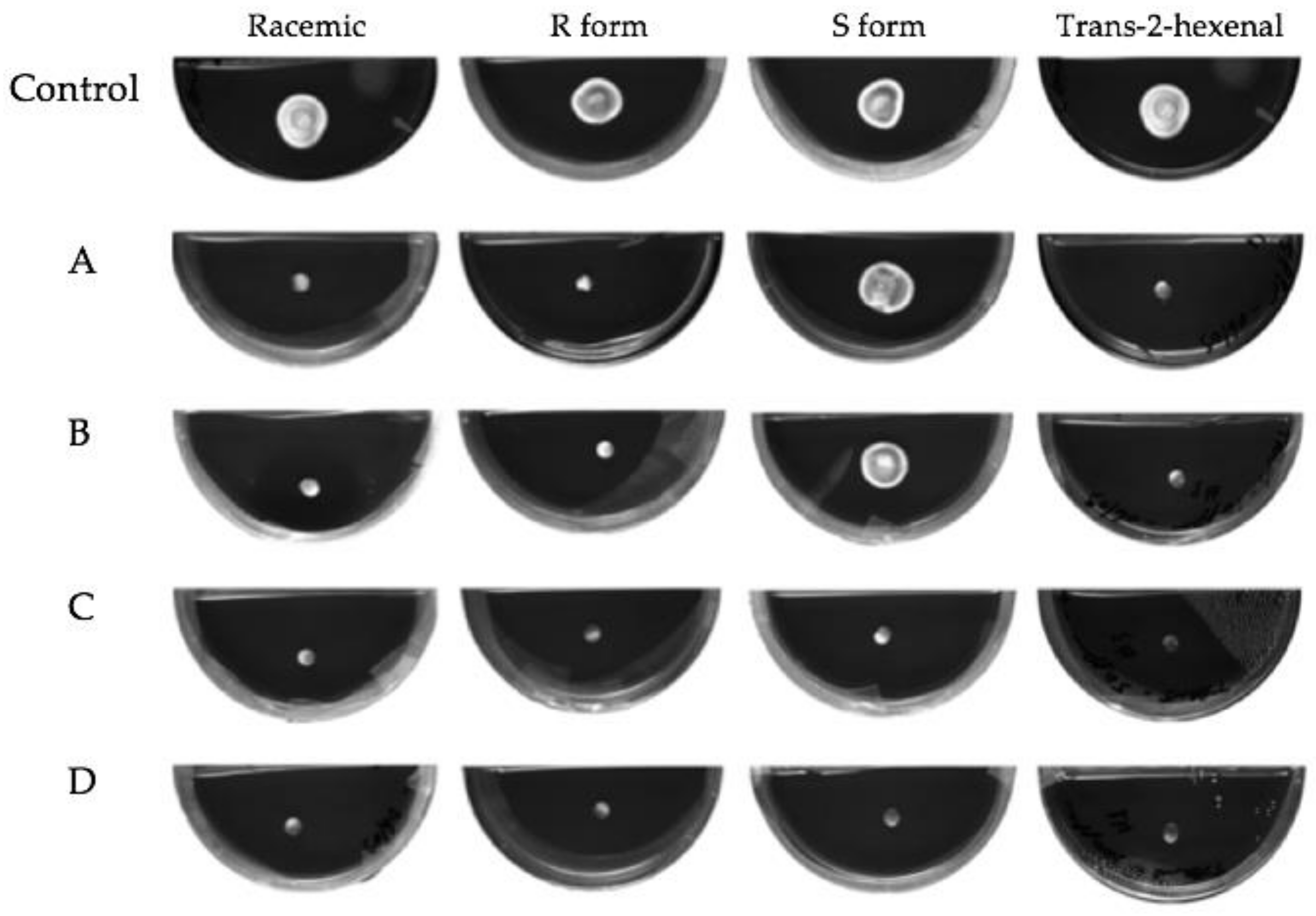
3.1. Growth of VOC Treated Mycelial Plugs
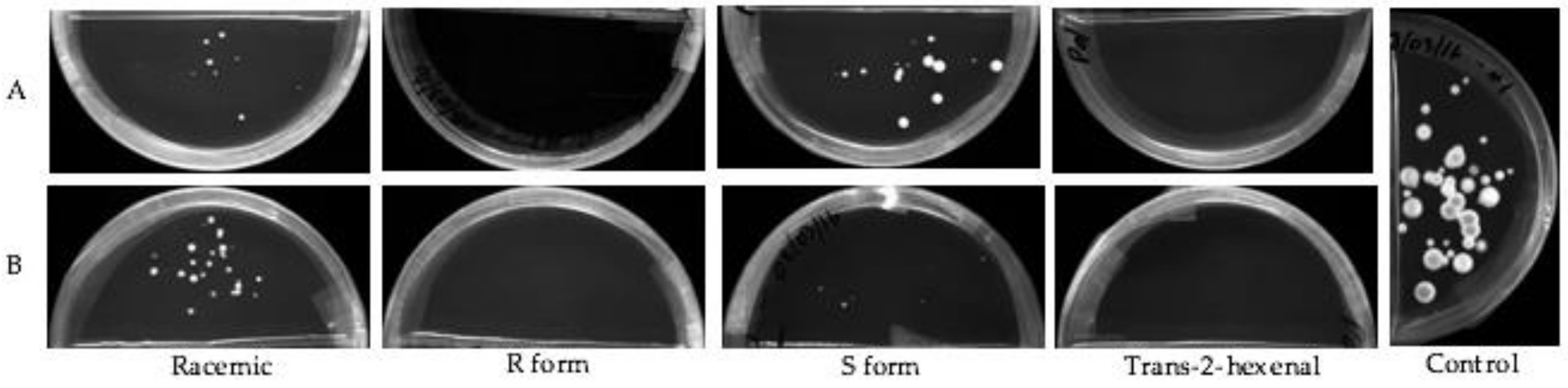
3.2. Subculture of Mycelial Plugs after VOC Treatment
3.3. Effect of VOCs on Growth from Conidiospores at 10 °C
3.4. Effect of 0.005, 0.01, 0.02 and 0.05 µmol/mL trans-2-hexenal on Conidiospore Growth at 10 °C
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blehart, D.S.; Hicks, A.C.; Behr, M.; Meteyer, C.U.; Berlowski-Zier, B.M.; Buckles, E.L.; Coleman, J.T.H.; Darling, S.R.; Gargas, A.; Niver, R.; et al. Bat white-nose syndrome: An emerging fungal pathogen? Science 2009, 323, 227. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.G.; Reeder, D.M.; Coleman, J.T. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Res. News 2011, 52, 13–27. [Google Scholar]
- Frick, W.F.; Pollock, J.F.; Hicks, A.C.; Langwig, K.E.; Reynolds, D.S.; Turner, G.G.; Butchkoski, C.M.; Kunz, T.H. An emerging disease causes regional population collapse of a common North American bat species. Science 2010, 329, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, S.; Blake, D.; Puechmaille, S.J. White-Nose Syndrome fungus introduced from Europe to North America. Curr. Biol. 2015, 25, R217–R219. [Google Scholar] [CrossRef] [PubMed]
- Field, K.A.; Johnson, J.S.; Lilley, T.M.; Reeder, S.M.; Rogers, E.J.; Behr, M.J.; Reeder, D.M. The White-Nose Syndrome transcriptome: Activation of anti-fungal host responses in wing tissue of hibernating little brown Myotis. PLoS Pathog. 2015, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Langwig, K.E.; Frick, W.F.; Bried, J.T.; Hicks, A.C.; Kunz, T.H.; Kilpatrick, A.M. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol. Lett. 2012, 15, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Thogmartin, W.E.; Sanders-Reed, C.A.; Szymanski, J.A.; McKann, P.C.; Pruitt, L.; King, R.A.; Runge, M.C.; Russell, R.E. White-nose syndrome is likely to extirpate the endangered Indiana bat over large parts of its range. Biol. Conserv. 2013, 160, 162–172. [Google Scholar] [CrossRef]
- Gargas, A.; Trest, M.T.; Christensen, M.; Volk, T.J.; Blehert, D.S. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotoxon 2009, 108, 147–154. [Google Scholar] [CrossRef]
- Verant, M.L.; Boyles, J.G.; Waldrep, W., Jr.; Wibbelt, G.; Blehert, D.S. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blehert, D.S.; Lorch, J.M.; Ballmann, A.E.; Cryan, P.M.; Meteyer, C.U. Bat White-Nose Syndrome in North America. Microbe 2011, 6, 267–273. [Google Scholar] [CrossRef]
- Cryan, P.M.; Meteyer, C.U.; Boyles, J.G.; Blehert, D.S. Wing pathology of white-nose syndrome in bats suggests life threatening disruption of physiology. BMC Biol. 2010, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, L.; Turner, J.M.; Bollinger, T.K.; Lorch, J.M.; Misra, V.; Cryan, P.M.; Wibbelt, G.; Blehert, D.S.; Willis, C.K. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 6999–7003. [Google Scholar] [CrossRef] [PubMed]
- Reeder, D.M.; Frank, C.L.; Turner, G.G.; Meteyer, C.U.; Kurta, A.; Britzke, E.R.; Vodzak, M.E.; Darling, S.R.; Stihler, C.W.; Hicks, A.C.; et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE 2012, 7, e38920. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Reeder, D.M.; McMichael, J.W., III; Meierhofer, M.B.; Stern, D.W.; Lumadue, S.S.; Singler, L.E.; Winters, H.D.; Vodzak, M.E.; Kurta, A.; et al. Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown Myotis (Myotis lucifugus). PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, A.J.; Knudsen, G.M.; Beekman, C.; Perry, J.A.; Johnson, A.D.; DeRisi, J.L.; Craik, C.S.; Bennett, R.J. Destructin-1 is a collagen-degrading endopeptidase secreted by Pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 7478–7483. [Google Scholar] [CrossRef] [PubMed]
- Cryan, P.M.; Meteyer, C.U.; Blehert, D.S.; Lorch, J.M.; Reeder, D.M.; Turner, G.G.; Webb, J.; Behr, M.; Verant, M.; Russell, R.E.; Castle, K.T. Electrolyte depletion in white-nose syndrome bats. J. Wildl. Dis. 2013, 49, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Verant, M.L.; Meteyer, C.U.; Speakman, J.R.; Cryan, P.M.; Lorch, J.M.; Blehert, D.S. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; Kunz, T.H. White-Nose Syndrome: A fungal disease of North American hibernating bats. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Elsevier: Waltham, MA, USA, 2012; pp. 904–910. ISBN 978-0-12-383832-2. [Google Scholar]
- Moore, M.S.; Reichard, J.D.; Murtha, T.D.; Nabhan, M.L.; Pian, R.E.; Ferreira, J.S.; Kunz, T.H. Hibernating little brown Myotis (Myotis lucifugus) show variable immunological responses to white-nose syndrome. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Cornelison, C.T.; Gabriel, K.T.; Barlament, C.; Crow, S.A. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia 2014, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, C.T.; Keel, M.K.; Gabriel, K.T.; Barlament, C.K.; Tucker, T.A.; Pierce, G.E.; Crow, S.A. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodocrous strain DAP 96253. BMC Microbiol. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Raudabaugh, D.B.; Miller, A.N. Effect of trans, trans-farnesol on Pseudogymnoascus destructans and several closely related species. Mycopathologia 2015, 180, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Dias, I.; Bennett, J.W. Two volatile-phase alcohols inhibit growth of Pseudogymnoascus destructans, causative agent of white-nose syndrome in bats. Mycology 2016, 8, 1–6. [Google Scholar] [CrossRef]
- He, L.; Beesley, T.E. Applications of enantiomeric gas chromatography: A review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1075–1114. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 22, 70–81. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Subchapter B, Part 172—Food Additives Permitted for Direct Addition to Food for Human Consumption; Subpart F-Flavoring Agents and Related Substances; Sec. 172.515 Synthetic Flavoring Substances and Adjuvants. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=polysorbate%2020 (accessed on 3 April 2017).
- Thamara, K.J.; Stevenson, P.C.; Hall, D.R.; Belmain, S.R. Effect of volatile constituents from Securidaca longepedunculata on insect pests of stored grain. J. Chem. Ecol. 2005, 31, 303–313. [Google Scholar]
- Cao, H.; Baldini, R.L.; Rahme, L.G. Common mechanisms for pathogens of plants and animals. Ann. Rev. Phytopathol. 2001, 39, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.; Hamel, C.; Yang, C.; Matsubara, T.; Gan, Y.; Singh, A.K.; Kuwada, K.; Ishii, T. Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. Phytochemistry 2012, 78, 72–80. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J.; Carter-Wientjes, C.H.; Boue, S.; Bhatnagar, D. Volatile trans-2-hexenal, a soybean aldehyde, inhibits Aspergillus flavus growth and aflatoxin production in corn. J. Food Sci. 2011, 76, M381–M386. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Lanciotti, R.; Guerzoni, M.E. Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett. Appl. Microbiol. 2001, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.M.; Lankton, J.; Werner, K.; Falendysz, E.A.; McCurley, K.; Blehert, D.S. Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. mBio 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A. The Chemistry and Biology of Volatiles, 1st ed.; Wiley: Chichester, UK, 2011; pp. 1–534. ISBN 9780470777787. [Google Scholar]
- Wright, M.S.; Greene-McDowelle, D.M.; Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. Effects of volatile aldehydes from Aspergillus-resistant varieties of corn on Aspergillus parasiticus growth and aflatoxin biosynthesis. Toxicon 2000, 38, 1215–1223. [Google Scholar] [CrossRef]
- Yin, G.; Padhi, S.; Lee, S.; Hung, R.; Zhao, G.; Bennett, J.W. Effects of three volatile oxylipins on colony development in two species of fungi and on Drosophila larval metamorphosis. Curr. Microbiol. 2015, 71, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Gardner, H.W. Lipoxygenase-derived aldehydes inhibit fungi pathogenic on soybean. J. Chem. Ecol. 1993, 19, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.M.; Franco, P.; Lindner, W. Separation of enantiomers: Needs, challenges, perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.; Dobrovin-Pennington, A.; Hobbs, P.J.; Pederby, J.; Rodger, A. Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 2009, 101, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Garcia, E.; Garzia, A.; Cordobés, S.; Espeso, E.A.; Ugalde, U. 8-Carbon oxylipins inhibit germination and growth, and stimulate aerial conidiation in Aspergillus nidulans. Fungal Biol. 2011, 115, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Gardner, H.W. Recent investigations into the lipoxygenase pathway in plants. Biochim. Biophys. Acta 1991, 1084, 221–239. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Flavor components of olive oil—A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Morales, M.T.; Alonso, M.V.; Rios, J.J.; Aparicio, R. Virgin olive oil aroma: Relationship between volatile compounds and sensory attributes by chemometrics. J. Agric. Food Chem. 1995, 43, 2925–2931. [Google Scholar] [CrossRef]
- Kubo, A.; Lunde, C.S.; Kub, I. Antimicrobial activity of the olive oil flavor compounds. J. Agric. Food Chem. 1995, 43, 1629–1633. [Google Scholar] [CrossRef]
- Battinelli, L.; Daniele, C.; Cristiani, M.; Bisignano, G.; Saija, A.; Mazzanti, G. In vitro antifungal and anti-elastase activity of some aliphatic aldehydes from Olea europaea L. fruit. Phytomedicine 2006, 13, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, H.; Kikusui, T.; Takeuchi, Y.; Sano, K.; Hatanaka, A.; Mori, Y. Alleviating effects of plant-derived fragrances on stress-induced hyperthermia in rats. Physiol. Behav. 2002, 75, 355–360. [Google Scholar] [CrossRef]
- Ito, A.; Miyoshi, M.; Ueki, S.; Fukada, M.; Komaki, R.; Watanabe, T. “Green odor” inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. Neurosci. Res. 2009, 65, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.J.; Merkel, C.; Auras, R.; Almenar, E. Development and characterization of antimicrobial poly(1-lactic acid) containing trans-2-hexenal trapped in cyclodextrins. Int. J. Food Microbiol. 2012, 153, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Gianotti, A.; Petrignani, F.; Belletti, N.; Guerzoi, M.E.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2003, 15, 201–208. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhi, S.; Dias, I.; Korn, V.L.; Bennett, J.W. Pseudogymnoascus destructans: Causative Agent of White-Nose Syndrome in Bats Is Inhibited by Safe Volatile Organic Compounds. J. Fungi 2018, 4, 48. https://doi.org/10.3390/jof4020048
Padhi S, Dias I, Korn VL, Bennett JW. Pseudogymnoascus destructans: Causative Agent of White-Nose Syndrome in Bats Is Inhibited by Safe Volatile Organic Compounds. Journal of Fungi. 2018; 4(2):48. https://doi.org/10.3390/jof4020048
Chicago/Turabian StylePadhi, Sally, Itamar Dias, Victoria L. Korn, and Joan W. Bennett. 2018. "Pseudogymnoascus destructans: Causative Agent of White-Nose Syndrome in Bats Is Inhibited by Safe Volatile Organic Compounds" Journal of Fungi 4, no. 2: 48. https://doi.org/10.3390/jof4020048




