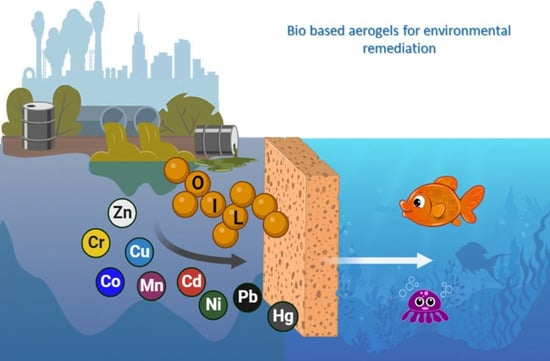
Bio-Based Aerogels for the Removal of Heavy Metal Ions and Oils from Water: Novel Solutions for Environmental Remediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aerogels for the Removal of Heavy Metal Ions
2.1.1. Cellulose-Based Aerogels
2.1.2. Chitosan-Based Aerogels
2.2. Aerogels for Oil Removal
2.2.1. Cellulose-Based Aerogels
2.2.2. Chitosan-Based Aerogels
2.3. Aerogels for the Recovery of Rare and Rare-Earth Elements
| Adsorbent | Modification | Heavy Metal | Adsorption Mechanism | Adsorption Performance | Specific Surface Area (m2/g) | Structure and Porosity | Average Pore Size (nm) | Z Potential a | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose filament fibers | Crosslinking reaction with bisacrylamide | Cu(II) | Physical adsorption | 51.3 mg/g | Continuously distributed large pores | From 20 mV to −10 mV (pH range: 2–10; pHzpc = 4.62) | [25] | ||
| 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) oxidized cellulose nanofibril (TO-CNF) | Crosslinking with trimethylolpropane-tris-(2-methyl-1-aziridine) propionate (TMPTAP) and polyethyleneimine (PEI) | Cu(II) | Amino-hydroxyl group complexation | 485.44 mg/g | 3D multi-wall perforated cellular structure; 99.02–99.51% porosity | From 45 mV to −15 mV (pH range: 2–11; pHzpc = 10.5) | [26] | ||
| Cellulose | Glutaraldehyde crosslinking reaction with polyethyleneimine (PEI) | Cr(VI) | Electrostatic interaction (–NH3+) | 229.1 mg/g | 36.8 | High porosity (volume = 0.12 cm3/g) | 13.5 | From 9 mV to 0.5 mV (pH range: 1–8; optimal absorption pH = 2) | [27] |
| Cellulose nanofibrils (CNFs)/carboxymethylcellulose | Crosslinked with polyethylene amine (BPEI) | Cu(II) | Electrostatic interaction, chelation, and ion exchange with the hydroxyl, amine, and carboxyl groups | 452.49 mg/g | 99.56–99.35% | [14] | |||
| Cellulose/lignin from natural corncob particles | Oxidation with citric acid (CA) | Cd(II) | Ion exchange; electrostatic and π-cation interaction | 1.62–42.9 mg/g | [34] | ||||
| Nanocellulose (from jute) | Reaction with maleic anhydride, followed by sodium exchange of protons | Pb(II) | Chemisorption/complexation | 20 mg/g, 40 mg/g, 115 mg/g, | Fused nano-whiskers; rough surface, porous and fluffy | [35] | |||
| Coconut shell biochar and tea factory waste | Introduction of carboxylic groups by adding methacrylic acid (MAA) | Cu(II), Cd(II), Pb(II), | Chelation by carboxylic and lactonic groups | [36] | |||||
| Cellulose nanofiber | Crosslinked with polyvinyl alcohol (PVA) and acrylic acid (AA) | Cu(II), Pb(II) | Coordination and ionic bond interactions | 30.0 mg/g (Cu); 131.5 mg/g (Pb) | Stable 3D structure, high porosity | [37] | |||
| Cellulose filament and chitosan | Crosslinked with citric acid | Cu(II) | Electrostatic interaction, hydrogen bonding | 206 mg/g | High porosity | [38] | |||
| Cellulose nanofiber and chitosan | Reaction with montmorillonite activated by acid | Cu(II), Cd(II), Pb(II) | Cu(II): 181.92 mg/g; Cd(II): 163.85 mg/g; Pb(II): 170.19 mg/g | Homogeneous 3D-oriented porous structure | [39] | ||||
| Cellulose nanofiber | Modification with tannic acid | Electrostatic interactions | 11.821 m2/g | Total pore volume 0.0641 cm3/g | Average pore diameter of 2.538 nm | [40] | |||
| Cellulose nanofiber grafted with cardanol-derived siloxane | Modification with tannic acid | Cu(II) | Chelation | 45.6 mg/L | 136.41–75.66 m2/g | Low density, 15.53 mg/cm3; 3D cellulosic porous structure | 5.510–3.822 nm | [41] | |
| Cellulose nanocrystals | Grafting with acrylic acid (AA) | Cu(II), Cd(II), Pb(II) | Chemisorption and coordination by sulfate Half-esters, carboxylic (AA), hydroxyl, and amine groups | Cu(II): 872.4 mg/g; Cd(II): 898.8 mg/g; Pb(II): 1026 mg/g | Highly porous and macroporous honeycomb structure | [42] | |||
| Cellulose nanofiber | TEMPO oxidation and modification with 3-mercaptopropyltrimethoxysilane (MPTs) | Hg(II) | Coordination complex | 718.5 mg/g | 18.47–43.57 m2/g | Interconnected porous morphology, with high porosity: 99.05–99.53% | [44] | ||
| Cellulose and MOF (metal–organic framework) composites | In situ growth procedure | Cu(II), Pb(II) | Chelation | Pb(II): 81.30–89.40 mg/g; Cu(II): 31.23–39.33 mg/g | 1251.3 m2/g; 800.9 m2/g | Surface with many micron-sized macropores (porosity: 90.5%; 84.6%) | [46] | ||
| Cellulose and MOF (metal–organic framework) | Combination with zeolitic imidazolate framework | Cr(VI) | Metal ion reduction and capture by the amine (NH2) and carboxylic active groups (-COOH) | 41.8 mg/g | Homogeneous, highly porous structure with zeolitic imidazolate framework-8 (ZIF-8) | [47] | |||
| Lignocellulose/chitosan | Crosslinked with polyvinyl alcohol and glutaraldehyde | Hg(II), Ag(I), Al(III), Fe(III), Cu(II) | Rhodamine-6G as detection probe | Low density and high specific surface area | [53] | ||||
| Chitosan | Physicochemical double crosslinking with poly(acrylic acid-2-(dimethylamino)ethyl methacrylate) | Cu(II) | Chelation and complexation | 660% | 3.42–5.94 m2/g | Rough and porous structure, with super-low density (0.043 g/cm3), pore volumes of 0.005 and 0.009 cm3 g−1 | Mean pore sizes: 6.33 and 7.41 nm | (pHpzc = 9.83) | [58] |
| Chitosan | Composite with nanofibrillated cellulose (NFC) | Pb(II) | Interaction with amino groups | 252.6 mg/g | Highly oriented microchannel structure | [59] | |||
| Chitosan | Alginate/melamine composite | Pb(II) | Complexation by amino and aromatic nitrogen groups | 1331.6 mg/g | 3D network structures, with heterogeneous pores | [60] | |||
| Chitosan (shrimp waste) | Composite with cellulose (pineapple leaves) | Cr(VI) | Electrostatic interaction between Cr(VI) oxyanions and protonated amine groups | 210.6–211.4 mg/g | Ultra-lightweight (20–30 mg/cm3), highly porous (above 97.5%) | [61] | |||
| Modified chitosan | Combination with polydopamine and crosslinking with glutaraldehyde | Cr(VI), Pb(II) | Electrostatic attraction or chelation | Cr(VI): 374.4 mg/g; Pb(II): 441.2 mg/g | 77.3 m2/g | Perfect three-dimensional network structure, large pores | 24.9 nm | From 30/20 mV to −5/−10 mV (pH range: 2–8; pHpzc about 5.6 and 7.5) | [63] |
| Chitosan | Cellulose sulfate combination | Pb(II) | Ion interaction with functional groups | 197.1 mg/g | 0.786 m2/g | Total pore volume of 6.44 × 10−3 cm3/g) | Mean pore diameter of 32.77 nm | [64] | |
| Chitosan | Combination with citrus peel (CP) and bentonite (BT) | Cu(II) | Electrostatic interaction and chelation | 861.58 mg/g | 41.28, 48.36, and 46.57 m2/g | More ductile, dense, and compact three-dimensional porous structure (presence of abundant pores) | 5–25 μm | Between 20 mV and −25 mV (pH range: 1.5–5.5; pHpzc: 2.10, 3.22 and 3.71) | [65] |
| Chitosan/thiourea | Combination with formaldehyde | Pb(II), Ag(I) | Ion interaction with –NH and –S groups | 416.64–447.26 m2/g | High specific surface area and low density | [52] | |||
| Sodium alginate–streptomycin sulfate composite aerogel (Alg–Stre) | Chemical grafting | Pb(II), Cu(II) | Chelation process and ion exchange | 280 mg/g and 160.2 mg/g for Pb(II) or Cu(II) | High specific surface area and surface energy | [50] | |||
| Sodium alginate aerogel | Incorporation of modified L-cysteine/UiO-67 | Cd(II), Cu(II), Pb(II) | Chelation process and ion exchange | 661.2 mg/g for Pb(II); 296.2 mg/g for Cd(II); 326.4 mg/g for Cu(II) | 337.66–1037.14 m2/g | [51] | |||
| Mesoporous silica/gelatin-based aerogel | Hg(II) | Metal complex coordination by gelatin | 209 mg/g | Hybrid backbones built from spherical blocks (globules) | From 32 to 17 nm | From −22 mV to −14 mV at pH = 4; from −27 mV to −23 mV at pH = 6 | [55] |
| Adsorbent | Modification Method | Absorbed Oil | Performance | Specific Surface Area (m2/g) | Structure and Porosity | Average Pore Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| Cellulose nanofibers (CNFs) | Methyltrimethoxysilane and 3-glycidoxypropyltrimethoxysilane modification | Toluene | 56 g/g | 3.9 m2/g | 3D open-cell geometry interconnected with minor cellular pores, thin sheets, nanoparticles, and nanofilaments; high pore volume (0.06 m3/g) | Macropores (>50 nm), mesopores (2–50 nm), and micropores (<2 nm) | [78] |
| Recycled cellulose fibers | Kymene crosslinking, methyltrimethoxysilane (MTMS) coating | 5w40 motor oil | 95 g/g | Highly porous 3D structures (porosity: 97.2–99.4%) | Macropores (>50 nm) | [79] | |
| Modified cellulose/polyurethane foam (PUF) | Fe3O4 NP enrichment | n-Hexane | >97.68% | Highly porous interconnected 3D structure | [80] | ||
| Cellulose from bamboo powder | Composite formation with graphene oxide; crosslinking agent: epichlorohydrin (ECH) | Methylene blue (MB); tetracycline (TC) | MB: 421.9 mg/g TC: 163.4 mg/g | 173 m2/g | 3D network structure, fluffy and porous architectures | 3.6 nm | [87] |
| Cellulose nanofiber (CNF) | Modification with deep eutectic solvents; carbonization | Acetone, n-hexane, methanol, sesame oil, paraffin oil, decane, gasoline, diesel, phenoxin, chloroform, toluene, ether, pump oil, acetic ether, oleic acid | From 74% to 95% | Ordered lamellar structure, rough surface; many macropores | Micro- and mesoporous features: broad pore size distributions (from 0 to 50 nm) | [84] | |
| Cellulose from waste biomass | Post-pyrolysis | Series of oils and organics (ethanol, methanol, acetone, chloroform, ethyl acetate, benzene and methylbenzene, gasoline, cooking oil, olive oil, and pump oil) | pump oil 63.9%, ethanol 97.0%, gasoline 100% | Porous and interconnected 3D networks | [85] | ||
| Chitosan | Graphene oxide modification; glutaraldehyde crosslinking | Diesel oil | 12.56–12.03 g/g; | 641.6 m2/g; 530.3 m2/g; and 606.4 m2/g | Interconnected, mesoporous, three-dimensional network | [90] | |
| Chitosan | Glutaraldehyde crosslinking | Crude oil, diesel | 1.07 g/g, 31.07 g/g | Porous structure, low density (0.0283 g/cm3), high porosity (97.98%) | [56] | ||
| Chitosan | Sodium stearate and cellulose | Surfactant in water–oil emulsions | Non-porous surface and porous internal layer (density and porosity of 0.065 g/cm3 and 95%, respectively) | [91] | |||
| Chitosan/cellulose nanofibrillated fiber (CNF) | Crosslinking and CVD | Several organic solvents | more than 100 g/g; chloroform up to 232 g/g | Highly oriented wavy structures | [92] | ||
| Chitosan | Microbubble template technique/ultrasonic technology/crosslinking | Hexane, cyclohexane, vacuum pump oil, silicone oil, and soybean oil | separation efficiency > 91% | 3D interconnected porous architectures, porosity of 97.6% | [93] | ||
| Chitosan | Bio-origin genipin as crosslinker | Oil spill, crude vegetable oil | Efficiency of 99% pure water recovered | Highly porous structure, large pore size | Macroporous range of 40–50 μm | [94] | |
| Chitosan | Oxidized cellulose; crosslinking and cold plasma modification | Selectivity for various oils | 13.77–28.20 g/g | Macroporous structures in 3D network structures with high porosity (96.44%, 96.79%, and 96.81%) | [95] |
3. Conclusions and Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdel Maksoud, M.I.A.; Kumar, R.; Maegawa, K.; Kawamura, G.; Tan, W.K.; Matsuda, A. Nanocomposite matrix conjugated with carbon nanomaterials for photocatalytic wastewater treatment. J. Hazard. Mater. 2021, 410, 124657. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2020, 19, 1457–1475. [Google Scholar] [CrossRef]
- Kumar, V.; Younis, S.A.; Vikrant, K.; Kim, K.-H. Trends in advanced materials for sustainable environmental remediation. In Advanced Materials for Sustainable Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–29. [Google Scholar]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2017, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorptive removal of dye crystal violet onto low-cost carbon produced from Eichhornia plant: Kinetic, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2013, 53, 543–556. [Google Scholar] [CrossRef]
- Janani, R.; Gurunathan, B.; Sivakumar, K.; Varjani, S.; Ngo, H.H.; Gnansounou, E. Advancements in heavy metals removal from effluents employing nano-adsorbents: Way towards cleaner production. Environ. Res. 2022, 203, 111815. [Google Scholar]
- Pandey, A.K.; Gaur, V.K.; Udayan, A.; Varjani, S.; Kim, S.-H.; Wong, J.W.C. Biocatalytic remediation of industrial pollutants for environmental sustainability: Research needs and opportunities. Chemosphere 2021, 272, 129936. [Google Scholar] [CrossRef]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-based material derived from biomass waste for wastewater treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2008, 84, 13–28. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process—A Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Tan, Y.; Shen, Y.; Zhang, S. Highly compressible nanocellulose aerogels with a cellular structure for high-performance adsorption of Cu(II). Chemosphere 2022, 291, 132887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-H.; Zhang, Q.; Sun, G.-T.; Chen, C.-Z.; Zhu, M.-Q.; Huang, X.-H. The synthesis of tannin-based graphene aerogel by hydrothermal treatment for removal of heavy metal ions. Ind. Crops Prod. 2022, 176, 114304. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef]
- Boccia, A.C.; Pulvirenti, A.; García-González, C.A.; Grisi, F.; Neagu, M. Compendium of Safety Regulatory for Safe Applications of Aerogels. Gels 2023, 9, 842. [Google Scholar] [CrossRef]
- Syeda, H.I.; Yap, P.-S. A review on three-dimensional cellulose-based aerogels for the removal of heavy metals from water. Sci. Total Environ. 2022, 807, 150606. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Sajid, M.; Khan, S.; Bilal, M. Aerogel-based adsorbents as emerging materials for the removal of heavy metals from water: Progress, challenges, and prospects. Sep. Purif. Technol. 2022, 291, 120923. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Liu, K.; Chen, L.; Huang, L.; Lai, Y. Evaluation of ethylenediamine-modified nanofibrillated cellulose/chitosan composites on adsorption of cationic and anionic dyes from aqueous solution. Carbohydr. Polym. 2016, 151, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, T.-W.; Yang, Y.-L.; Bai, Z.-C.; Xia, L.-R.; Wang, M.; Lv, X.-L.; Li, L. Removal of heavy metal ions and anionic dyes from aqueous solutions using amide-functionalized cellulose-based adsorbents. Carbohydr. Polym. 2020, 230, 115619. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Pang, H.; Tan, Y.; Zhang, S.; Li, J. 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu(II) ions from water. Chem. Eng. J. 2019, 378, 122157. [Google Scholar] [CrossRef]
- Guo, D.-M.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Polyethylenimine-functionalized cellulose aerogel beads for efficient dynamic removal of chromium(vi) from aqueous solution. RSC Adv. 2017, 7, 54039–54052. [Google Scholar] [CrossRef]
- Tang, X.Z.; Yu, B.; Hansen, R.V.; Chen, X.; Hu, X.; Yang, J. Grafting Low Contents of Branched Polyethylenimine onto Carbon Fibers to Effectively Improve Their Interfacial Shear Strength with an Epoxy Matrix. Adv. Mater. Interfaces 2015, 2, 1500122. [Google Scholar] [CrossRef]
- Weidt, D.; Figiel, Ł. Effect of CNT waviness and van der Waals interaction on the nonlinear compressive behaviour of epoxy/CNT nanocomposites. Compos. Sci. Technol. 2015, 115, 52–59. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nanocomposites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Li, J.; Miao, X.; Hao, Y.; Zhao, J.; Sun, X.; Wang, L. Synthesis, amino-functionalization of mesoporous silica and its adsorption of Cr(VI). J. Colloid Interface Sci. 2008, 318, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiang, Z.; Song, T.; Qi, H. Wet-strength agent improves recyclability of dip-catalyst fabricated from gold nanoparticle-embedded bacterial cellulose and plant fibers. Cellulose 2019, 26, 3375–3386. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Landin-Rodriguez, L.E.; Leyva-Ramos, S.; Medellin-Castillo, N.A. Modification of corncob with citric acid to enhance its capacity for adsorbing cadmium(II) from water solution. Chem. Eng. J. 2012, 180, 113–120. [Google Scholar] [CrossRef]
- Vadakkekara, G.J.; Thomas, S.; Nair, C.P.R. Maleic acid modified cellulose for scavenging lead from water. Int. J. Biol. Macromol. 2019, 129, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C.; Ziyath, A.M.; Jinadasa, K.B.S.N.; Egodawatta, P.; Sarina, S.; Goonetilleke, A. Quantifying the influence of surface physico-chemical properties of biosorbents on heavy metal adsorption. Chemosphere 2019, 234, 488–495. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Tian, C.; Wu, Y.; Li, X.; Luo, S.; Qing, Y.; Jiang, Z. Cellulose Nanofibrils Aerogel Cross-Linked by Poly(vinyl alcohol) and Acrylic Acid for Efficient and Recycled Adsorption with Heavy Metal Ions. J. Nanosci. Nanotechnol. 2018, 18, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Varamesh, A.; Abraham, B.D.; Wang, H.; Berton, P.; Zhao, H.; Gourlay, K.; Minhas, G.; Lu, Q.; Bryant, S.L.; Hu, J. Multifunctional fully biobased aerogels for water remediation: Applications for dye and heavy metal adsorption and oil/water separation. J. Hazard. Mater. 2023, 457, 131824. [Google Scholar] [CrossRef]
- Rong, N.; Chen, C.; Ouyang, K.; Zhang, K.; Wang, X.; Xu, Z. Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep. Purif. Technol. 2021, 274, 119120. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.; Ma, C.; Li, H.; Qiu, Z. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2020, 138, 49880. [Google Scholar] [CrossRef]
- Ji, Y.; Wen, Y.; Wang, Z.; Zhang, S.; Guo, M. Eco-friendly fabrication of a cost-effective cellulose nanofiber-based aerogel for multifunctional applications in Cu(II) and organic pollutants removal. J. Clean. Prod. 2020, 255, 120276. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; An, B.; Wu, Z.; Yang, R.; Ma, C.; Huang, Q.; Li, W.; Li, J.; Liu, S. A facile hydrothermal method–fabricated robust and ultralight weight cellulose nanocrystal-based hydro/aerogels for metal ion removal. Environ. Sci. Pollut. Res. 2019, 26, 25583–25595. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, X.; Yang, W.; Hu, J.; Zhang, C.; Liu, K.; Jiang, S. Nanocellulose-based composite aerogels toward the environmental protection: Preparation, modification and applications. Environ. Res. 2023, 236, 116736. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Wang, H.; Wu, S.; Ru, J.; Tong, C.; Chen, Y.; Liu, H.; Wu, S.; Liu, X. Surface-Tailored Nanocellulose Aerogels with Thiol-Functional Moieties for Highly Efficient and Selective Removal of Hg(II) Ions from Water. ACS Sustain. Chem. Eng. 2017, 5, 11715–11726. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon Aerogels for Environmental Clean-Up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141. [Google Scholar] [CrossRef]
- Aylaz, G.; Okan, M.; Duman, M.; Aydin, H.M. Study on Cost-Efficient Carbon Aerogel to Remove Antibiotics from Water Resources. ACS Omega 2020, 5, 16635–16644. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, Z.; Meng, Y.; Wang, M.; Wang, Z. Effective removal of lead and copper ions from water using a novel sodium alginate–streptomycin sulfate composite aerogel. New J. Chem. 2023, 47, 6739–6748. [Google Scholar] [CrossRef]
- Du, M.; Cao, Y.; Luo, X.; Yang, W.; Lin, W.; Wang, Y.; Tang, W.; Li, Z. Shapeable sodium alginate aerogel beads incorporated with L-cysteine-modified defective UiO-67 for heavy metal ions removal. Chem. Eng. J. 2023, 475, 146289. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Lazar, M.M.; Platon, I.-V.; Humelnicu, D.; Doroftei, F.; Dinu, M.V. Feather-weight cryostructured thiourea-chitosan aerogels for highly efficient removal of heavy metal ions and bacterial pathogens. Int. J. Biol. Macromol. 2023, 235, 123910. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Zhang, M.; Xiao, Z.; Yang, Q.; Feng, L.; Li, S.; Shi, M. Lignocellulose/chitosan hybrid aerogel composited with fluorescence molecular probe for simultaneous adsorption and detection of heavy metal pollutants. J. Environ. Chem. Eng. 2023, 11, 111205. [Google Scholar] [CrossRef]
- Silva, C.C.B.d.; Terashima, F.J.H.; Barbieri, N.; Lima, K.F.d. Sound absorption coefficient assessment of sisal, coconut husk and sugar cane fibers for low frequencies based on three different methods. Appl. Acoust. 2019, 156, 92–100. [Google Scholar] [CrossRef]
- Herman, P.; Fábián, I.; Kalmár, J. Mesoporous Silica–Gelatin Aerogels for the Selective Adsorption of Aqueous Hg(II). ACS Appl. Nano Mater. 2019, 3, 195–206. [Google Scholar] [CrossRef]
- Li, A.; Lin, R.; Lin, C.; He, B.; Zheng, T.; Lu, L.; Cao, Y. An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion. Carbohydr. Polym. 2016, 148, 272–280. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Fan, S.; Chen, J.; Fan, C.; Chen, G.; Liu, S.; Zhou, H.; Liu, R.; Zhang, Y.; Hu, H.; Huang, Z.; et al. Fabrication of a CO2-responsive chitosan aerogel as an effective adsorbent for the adsorption and desorption of heavy metal ions. J. Hazard. Mater. 2021, 416, 126225. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Shi, R.; Zhang, H.; Gong, L.; Dai, L. Chitosan/nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb (II) ions from aqueous solution. Carbohydr. Polym. 2019, 223, 115048. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Tan, L.N.; Nguyen, N.C.T.; Trinh, A.M.H.; Do, N.H.N.; Le, K.A.; Le, P.K. Eco-friendly synthesis of durable aerogel composites from chitosan and pineapple leaf-based cellulose for Cr(VI) removal. Sep. Purif. Technol. 2023, 304, 122415. [Google Scholar] [CrossRef]
- Do, N.H.N.; Truong, B.Y.; Nguyen, P.T.X.; Le, K.A.; Duong, H.M.; Le, P.K. Composite aerogels of TEMPO-oxidized pineapple leaf pulp and chitosan for dyes removal. Sep. Purif. Technol. 2022, 283, 120200. [Google Scholar] [CrossRef]
- Guo, D.-M.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Yang, D.-J. Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels. Carbohydr. Polym. 2018, 202, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Najaflou, S.; Rad, M.F.; Baghdadi, M.; Nabi Bidhendi, G.R. Removal of Pb(II) from contaminated waters using cellulose sulfate/chitosan aerogel: Equilibrium, kinetics, and thermodynamic studies. J. Environ. Manag. 2021, 286, 112167. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Feng, D.; Shang, J.; Nasen, B.; Jiang, T.; Liu, Y.; Hou, S. Green composite aerogel based on citrus peel/chitosan/bentonite for sustainable removal Cu(II) from water matrices. Sci. Rep. 2023, 13, 15443. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Kitanaka, A.; Nishijima, W.; Baes, A.U.; Okada, M. Removal of oil pollutants in seawater as pretreatment of reverse osmosis desalination process. Water Res. 1999, 33, 1857–1863. [Google Scholar] [CrossRef]
- Ayotamuno, M.J.; Kogbara, R.B.; Ogaji, S.O.T.; Probert, S.D. Petroleum contaminated ground-water: Remediation using activated carbon. Appl. Energy 2006, 83, 1258–1264. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Pasila, A. A biological oil adsorption filter. Mar. Pollut. Bull. 2004, 49, 1006–1012. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Ouyang, D.; Lei, X.; Zheng, H. Recent Advances in Biomass-Based Materials for Oil Spill Cleanup. Nanomaterials 2023, 13, 620. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.N.; Thomas, L.H.; Altaner, C.M.; Callow, P.; Forsyth, V.T.; Apperley, D.C.; Kennedy, C.J.; Jarvis, M.C. Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195-203. [Google Scholar] [CrossRef] [PubMed]
- Marchessault, R.H.; Sundararajan, P.R. Cellulose. In The Polysaccharides; Academic Press: Cambridge, MA, USA, 1983; pp. 11–95. [Google Scholar]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Wang, M.; Tsai, H.-S.; Zhang, C.; Wang, C.; Ho, S.-H. Effective purification of oily wastewater using lignocellulosic biomass: A review. Chin. Chem. Lett. 2022, 33, 2807–2816. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Tao, F.; An, Y.; Zhong, Y.; Liu, Z.; Hu, Z.; Zhang, X.; Wang, X. An overview of biomass-based Oil/Water separation materials. Sep. Purif. Technol. 2023, 316, 123767. [Google Scholar] [CrossRef]
- Agaba, A.; Marriam, I.; Tebyetekerwa, M.; Yuanhao, W. Janus hybrid sustainable all-cellulose nanofiber sponge for oil-water separation. Int. J. Biol. Macromol. 2021, 185, 997–1004. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Alazab, A.A.; Saleh, T.A. Magnetic hydrophobic cellulose-modified polyurethane filter for efficient oil-water separation in a complex water environment. J. Water Process Eng. 2022, 50, 103125. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon Fiber Aerogel Made from Raw Cotton: A Novel, Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Wu, X.-L.; Wen, T.; Guo, H.-L.; Yang, S.; Wang, X.; Xu, A.-W. Biomass-Derived Sponge-like Carbonaceous Hydrogels and Aerogels for Supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Feng, Y.; Liu, Y.; Zheng, L.; Gan, L.; Liu, J.; Zeng, X.; Long, M. Renewable and robust biomass carbon aerogel derived from deep eutectic solvents modified cellulose nanofiber under a low carbonization temperature for oil-water separation. Sep. Purif. Technol. 2021, 254, 117577. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and facile fabrication of carbon aerogels from cellulose-based waste newspaper for solving organic pollution. Carbohydr. Polym. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Silbaugh, T.; Pfeffer, R.; Lin, Y.S. Removal of emulsified oil from water by inverse fluidization of hydrophobic aerogels. Powder Technol. 2010, 203, 298–309. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Zheng, P. Sulfo-functional 3D porous cellulose/graphene oxide composites for highly efficient removal of methylene blue and tetracycline from water. Int. J. Biol. Macromol. 2019, 140, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.-C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Guo, X.; Qu, L.; Zhu, S.; Tian, M.; Zhang, X.; Sun, K.; Tang, X. Preparation of Three-Dimensional Chitosan–Graphene Oxide Aerogel for Residue Oil Removal. Water Environ. Res. 2016, 88, 768–778. [Google Scholar] [CrossRef]
- Meng, G.; Peng, H.; Wu, J.; Wang, Y.; Wang, H.; Liu, Z.; Guo, X. Fabrication of superhydrophobic cellulose/chitosan composite aerogel for oil/water separation. Fibers Polym. 2017, 18, 706–712. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, W.; Xi, J.; Chu, Y.; Xiao, H.; Wu, W. Ultralight and shape recovery bio-based aerogel for oil-water separation. J. Environ. Chem. Eng. 2022, 10, 108822. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y. Facile and green fabrication of porous chitosan aerogels for highly efficient oil/water separation and metal ions removal from water. J. Environ. Chem. Eng. 2023, 11, 109689. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, L.; Hu, W.; Zheng, T.; Lu, L.; Cao, Y.; Chen, Y. Excellent reusable chitosan/cellulose aerogel as an oil and organic solvent absorbent. Carbohydr. Polym. 2018, 191, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, R.; Cook, D.R. Rare earths: A review of the landscape. MRS Energy Sustain. 2018, 5, 6. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.-W.; Park, I.-S.; Hong, H.J. Adsorptive recovery of rare earth elements from aqueous solution by citric acid crosslinked carboxymethylated cellulose nanofibril aerogel. J. Clean. Prod. 2023, 418, 138189. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.-M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhao, T.; Wang, X.; Isaeva, V.I.; Kustov, L.M.; Yao, J.; Gao, J. Bimetal-organic framework-derived nanotube@cellulose aerogels for peroxymonosulfate (PMS) activation. Carbohydr. Polym. 2022, 296, 119969. [Google Scholar] [CrossRef]
- Georgiou, E.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Efficient Removal of Polyvalent Metal Ions (Eu(III) and Th(IV)) from Aqueous Solutions by Polyurea-Crosslinked Alginate Aerogels. Gels 2022, 8, 478. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, Y.; Zhang, Z.; Li, Y.; Li, W. Nanocellulose aerogel for highly efficient adsorption of uranium (VI) from aqueous solution. Carbohydr. Polym. 2021, 267, 118233. [Google Scholar] [CrossRef]
- Pu, Y.; Qiang, T.; Li, G.; Ruan, X.; Ren, L. Efficient adsorption of low-concentration uranium from aqueous solutions by biomass composite aerogel. Ecotoxicol. Environ. Saf. 2023, 259, 115053. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, B.; Qian, Y.; Mei, P.; Wang, N. A simple and universal strategy to construct robust and anti-biofouling amidoxime aerogels for enhanced uranium extraction from seawater. Chem. Eng. J. 2020, 397, 125337. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccia, A.C.; Neagu, M.; Pulvirenti, A. Bio-Based Aerogels for the Removal of Heavy Metal Ions and Oils from Water: Novel Solutions for Environmental Remediation. Gels 2024, 10, 32. https://doi.org/10.3390/gels10010032
Boccia AC, Neagu M, Pulvirenti A. Bio-Based Aerogels for the Removal of Heavy Metal Ions and Oils from Water: Novel Solutions for Environmental Remediation. Gels. 2024; 10(1):32. https://doi.org/10.3390/gels10010032
Chicago/Turabian StyleBoccia, Antonella Caterina, Monica Neagu, and Alfio Pulvirenti. 2024. "Bio-Based Aerogels for the Removal of Heavy Metal Ions and Oils from Water: Novel Solutions for Environmental Remediation" Gels 10, no. 1: 32. https://doi.org/10.3390/gels10010032