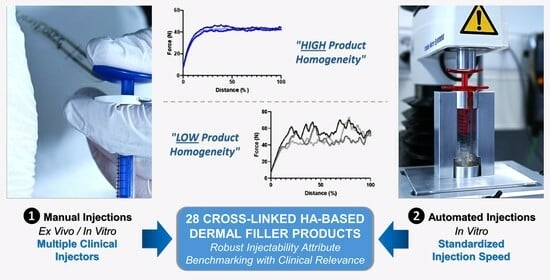
Clinical Perspectives on the Injectability of Cross-Linked Hyaluronic Acid Dermal Fillers: A Standardized Methodology for Commercial Product Benchmarking with Inter-Injector Assessments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Technical Benchmarking of Dermal Filler Product Parameters and Specificities
- Injectable dermal filler products based on BDDE-cross-linked HA;
- Products from well-established manufacturers with a brand presence on the market >10 years;
- Commercial dermal filler products with a CE mark and/or FDA approval (i.e., medical devices);
- Products indicated for injection in the face for managing fine to medium wrinkles and folds, and for volumizing purposes;
- Products well known and regularly injected by one or more of the practicing co-authors;
- Products available on the European and/or North American markets, with frequent clinical application in Switzerland (i.e., area of practice of the co-authors).
2.2. Comparative Manual Injectability Evaluation in Ex Vivo Human Skin and in SimSkin® Cutaneous Equivalents
2.3. Inter-Injector Variability Assessment and Influence of Lidocaine on Product Manual Injectability
2.4. Automated In Vitro Product Injectability Assessment: Comparative Injection Curves for Standardized Dermal Filler Product Benchmarking
2.5. Assessment of the Influence of Lidocaine Incorporation in Cross-Linked HA-Based Hydrogel Systems
2.6. Qualitative Clinical Perspectives for Cross-Linked HA-Based Dermal Filler Administration: Focus on the Point-by-Point Intradermal Injection Technique
2.7. Clinical Considerations, Performance Implications, and Perspectives on Product Injectability Attributes
2.8. Study Limitations
2.9. Future Research Perspectives Based on the Study
3. Conclusions
4. Materials and Methods
4.1. Materials Used in the Study
4.2. Comparative Manual Injectability Studies in Ex Vivo Human Skin and in SimSkin® Cutaneous Equivalents
4.3. Comparative Product Benchmarking: Multi-Injector Manual Injectability Study in Cutaneous Equivalents
4.4. Comparative Automated Injectability Study in Cutaneous Equivalents at Constant Injection Speed
4.5. Comparative Analysis of Different Manual Hydrogel Injection Techniques
4.6. Experimental Assessment of the Impact of Lidocaine on Hydrogel System Attributes during Product Sterilization
4.7. Statistical Analysis and Data Presentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDDE | 1,4-butanediol diglycidyl ether |
| CE | European mark of conformity |
| Da | Daltons |
| FDA | US Food and Drug Administration |
| G′ | storage modulus |
| G″ | loss modulus |
| HA | hyaluronic acid |
| ISO | International Standards Organization |
| MD | medical device |
| MDa | megaDaltons |
| min | minutes |
| MW | molecular weight |
| N | Newtons |
| NA | non-applicable |
| ns | non-significant |
| Pa | Pascals |
| Pa·s | Pascal seconds |
| ROS | reactive oxygen species |
| USA | United States of America |
References
- Vazirnia, A.; Braz, A.; Fabi, S.G. Nonsurgical jawline rejuvenation using injectable fillers. J. Cosmet. Dermatol. 2020, 19, 1940–1947. [Google Scholar] [CrossRef]
- Bacos, J.T.; Dayan, S.H. Superficial dermal fillers with hyaluronic acid. Facial Plast. Surg. 2019, 35, 219–223. [Google Scholar] [CrossRef]
- Fallacara, A.; Manfredini, S.; Durini, E.; Vertuani, S. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast. Surg. 2017, 33, 87–96. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 17 October 2023).
- Fagien, S.; Bertucci, V.; von Grote, E.; Mashburn, J.H. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plastic Reconstr. Surg. 2019, 143, 707e–720e. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The rheology and physicochemical characteristics of hyaluronic acid fillers: Their clinical implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef]
- Choi, M.S. Basic rheology of dermal filler. Arch. Plast. Surg. 2020, 47, 301–304. [Google Scholar] [CrossRef]
- Molliard, S.G.; Bétemps, J.B.; Hadjab, B.; Topchian, D.; Micheels, P.; Salomon, D. Key rheological properties of hyaluronic acid fillers: From tissue integration to product degradation. Plast. Aesthet. Res. 2018, 5, 17. [Google Scholar] [CrossRef]
- Rosamilia, G.; Hamade, H.; Freytag, D.L.; Frank, K.; Green, J.B.; Devineni, A.; Gavril, D.L.; Hernandez, C.A.; Pavicic, T.; Cotofana, S. Soft tissue distribution pattern of facial soft tissue fillers with different viscoelastic properties. J. Cosmet. Dermatol. 2020, 19, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.; Cassuto, D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast. Reconstruct. Surg. 2013, 132, 5S–21S. [Google Scholar] [CrossRef] [PubMed]
- Steenen, S.A.; Bauland, C.G.; van der Lei, B.; Su, N.; van Engelen, M.D.G.; Anandbahadoer-Sitaldin, R.D.R.R.A.L.; Koeiman, W.; Jawidan, T.; Hamraz, Y.; Lange, J. Head-to-head comparison of 4 hyaluronic acid dermal fillers for lip augmentation: A multicenter randomized, quadruple-blind, controlled clinical trial. J. Am. Acad. Dermatol. 2023, 88, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Safran, T.; Swift, A.; Cotofana, S.; Nikolis, A. Evaluating safety in hyaluronic acid lip injections. Expert Opin. Drug Safety 2021, 20, 1473–1486. [Google Scholar] [CrossRef]
- Trévidic, P.; Kaufman-Janette, J.; Weinkle, S.; Wu, R.; Dhillon, B.; Antunes, S.; Macé, E.; Maffert, P. Injection guidelines for treating midface volume deficiency with hyaluronic acid fillers: The ATP approach (Anatomy, Techniques, Products). Aesthet. Surg. J. 2022, 42, 920–934. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Ma, J.; Li, J. The efficacy and safety of hyaluronic acid injection in tear trough deformity: A systematic review and meta-analysis. Aesthet. Plast. Surg. 2023; in press. [Google Scholar] [CrossRef]
- Wongprasert, P.; Dreiss, C.A.; Murray, G. Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol. Ther. 2022, 35, e15453. [Google Scholar] [CrossRef]
- da Costa, A.; Biccigo, D.G.Z.; de Souza Weimann, E.T.; Mercadante, L.M.; Oliveira, P.R.G.; Prebianchi, S.B.; Abdalla, B.M.Z. Durability of three different types of hyaluronic acid fillers in skin: Are there differences among biphasic, monophasic monodensified, and monophasic polydensified products? Aesthet. Surg. J. 2017, 37, 573–581. [Google Scholar] [CrossRef]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The development of hyaluronic acids used for skin tissue regeneration. Current Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Carraux, P.; Micheels, P.; Kaya, G.; Salomon, D. In vivo bio-integration of three hyaluronic acid fillers in human skin: A histological study. Dermatology 2014, 228, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The degradation of hyaluronan in the skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Santer, V.; Molliard, S.G.; Micheels, P.; Río-Sancho, S.D.; Quinodoz, P.; Kalia, Y.N.; Salomon, D. Hyaluronic acid after subcutaneous injection-An objective assessment. Dermatol. Surg. 2019, 45, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Arlette, J.P.; Trotter, M.J. Anatomic location of hyaluronic acid filler material injected into nasolabial fold: A histologic study. Dermatol. Surg. 2008, 34, S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Micheels, P.; Goodman, L. Injection depth in intradermal therapy: Update and correction of published data. J. Drugs Dermatol. 2018, 17, 88–96. [Google Scholar]
- Olenius, M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthet. Plast. Surg. 1998, 22, 97–101. [Google Scholar] [CrossRef]
- Micheels, P.; Sarazin, D.; Besse, S.; Sundaram, H.; Flynn, T.C. A blanching technique for intradermal injection of the hyaluronic acid Belotero®. Plast. Reconstr. Surg. 2013, 132, 69S–76S. [Google Scholar] [CrossRef]
- Micheels, P.; Besse, S.; Flynn, T.C.; Sarazin, D.; Elbaz, Y. Superficial dermal injection of hyaluronic acid soft tissue fillers: Comparative ultrasound study. Dermatol. Surg. 2012, 38, 1162–1169. [Google Scholar] [CrossRef]
- Della Volpe, C.; Andrac, L.; Casanova, D.; Legré, R.; Magalon, G. Skin diversity: Histological study of 140 skin residues, adapted to plastic surgery. Ann. Chir. Plast. Esthet. 2012, 5, 423–449. [Google Scholar] [CrossRef]
- Kaya, G.; Saurat, J.-H. Dermatoporosis: A chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology 2007, 215, 284–294. [Google Scholar] [CrossRef]
- Tsukahara, K.; Tamatsu, Y.; Sugawara, Y.; Shimada, K. Relationship between the depth of facial wrinkles and the density of the retinacula cutis. Arch. Dermatol. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Kaya, G. Dermatoporose: Un syndrome emergent. Rev. Med. Suisse 2008, 155, 1078–1082. [Google Scholar]
- Kim, J. Effects of injection depth and volume of stabilized hyaluronic acid in human dermis on skin texture, hydration, and thickness. Arch. Aesthetic Plast. Surg. 2014, 20, 97–103. [Google Scholar] [CrossRef]
- Allergan Aesthetics, Allergan (Allergan, Annecy, France). Instructions for Use of the Juvéderm® Gel Range (Volbella®, Volift®, Voluma®, Juvéderm® Ultra 2, 3). 2022, unpublished work. Available online: https://media.allergan.com/actavis/actavis/media/general/Juvederm-voluma-IFU.pdf (accessed on 23 January 2024).
- Q-Med AB, Galderma SA (Galderma, Zug, Switzerland). Instructions for Use of the Restylane® Gel Range (Restylane®, Restylane® Lyft). 2017, unpublished work. Available online: https://www.restylane.com/ca/sites/default/files/2018-03/Restylane%20LYFT%20Lidocaine.pdf (accessed on 23 January 2024).
- Merz Aesthetics, Anteis SA (Anteis, Plan-les-Ouates, Switzerland). Instructions for Use of the Belotero® Gel Range (Belotero® Soft, Balance, Intense, Volume). 2015, unpublished work. Available online: https://www.merz.ch/wp-content/uploads/2016/03/BELOTERO_Volume_Lidocaine.pdf (accessed on 23 January 2024).
- Teoxane SA (Teoxane, Geneva, Switzerland). Instructions for Use of the RHA® Gel Range (RHA® 1, 2, 3, 4). 2015, unpublished work. Available online: https://irp-cdn.multiscreensite.com/e193bd60/files/uploaded/Teosyal-Rha-Dr.-brochure-en-1.pdf (accessed on 25 January 2024).
- Laboratoires VIVACY (Vivacy, Paris, France). Instructions for Use of the Stylage® Gel Range (Stylage® S, M, L, XL, XXL). 2024, unpublished work. Available online: https://vivacy.com/fr/produits/medecine-esthetique/ (accessed on 23 January 2024).
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Exp. Rev. Med. Dev. 2021, 18, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Pluda, S.; Salvagnini, C.; Fontana, A.; Marchetti, A.; Di Lucia, A.; Galesso, D.; Guarise, C. Investigation of crosslinking parameters and characterization of hyaluronic acid dermal fillers: From design to product performances. Gels 2023, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, J.; Deglesne, P.A.; Arroyo, R.; Sepúlveda, L.; Ranneva, E.; Deprez, P. Detection of a new reaction by-product in BDDE cross-linked autoclaved hyaluronic acid hydrogels by LC-MS analysis. Med. Dev. 2018, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.T.; Kam, J.; Bloom, J.D. Hyaluronic acid basics and rheology. Facial Plast. Surg. Clin. N. Am. 2022, 30, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Micheels, P.; Obamba, M. Rheological properties of several hyaluronic acid-based gels: A comparative study. J. Drugs Dermatol. 2018, 17, 602–608. [Google Scholar]
- Micheels, P.; Besse, S.; Sarazin, D.; Obamba, M. Hyaluronic acid gel based on CPM® technology with and without lidocaine: Is there a difference? J. Cosmet. Dermatol. 2018, 18, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Russo, L.; Argenzio, V.; Borzacchiello, A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J. Appl. Biomater. Biomech. 2011, 9, 127–136. [Google Scholar] [CrossRef]
- De la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and physicochemical characteristics of hyaluronic acid fillers: Overview and relationship to product performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef]
- Conrozier, T.; Mathieu, P.; Rinaudo, M. Mannitol preserves the viscoelastic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol. Ther. 2014, 1, 45–54. [Google Scholar] [CrossRef]
- Rinaudo, M.; Lardy, B.; Grange, L.; Conrozier, T. Effect of mannitol on hyaluronic acid stability in two in vitro models of oxidative stress. Polymers 2014, 6, 1948–1957. [Google Scholar] [CrossRef]
- Available online: https://www.postersessiononline.eu/173580348_eu/congresos/WBC2020/aula/-WBC2020-LATE_4410_WBC2020.pdf (accessed on 13 November 2023).
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced concepts in rheology for the evaluation of hyaluronic acid-based soft tissue fillers. Dermatol. Surg. 2021, 47, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- De Maio, M. MD Codes™: A methodological approach to facial aesthetic treatment with injectable hyaluronic acid fillers. Aesthet. Plast. Surg. 2021, 45, 690–709. [Google Scholar] [CrossRef]
- Sundaram, H.; Rohrich, R.J.; Liew, S.; Sattler, G.; Talarico, S.; Trévidic, P.; Gavard Molliard, S. Cohesivity of hyaluronic acid fillers: Development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast. Reconstr. Surg. 2015, 136, 678–686. [Google Scholar] [CrossRef]
- Mrestani, Y.; Hammitzch, M.; Neubert, R.H.H. Investigation of the interaction between lidocaine and the components of hyaluronic acid using frontal analysis continuous capillary electrophoresis. Chromatographia 2009, 69, 1321–1324. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Haridas, N.; Rosemary, M.J. Effect of steam sterilization and biocompatibility studies of hyaluronic acid hydrogel for viscosupplementation. Polymer Degrad. Stab. 2019, 163, 220–227. [Google Scholar] [CrossRef]
- Chen, J.; Peng, C.; Nie, J.; Kennedy, J.F.; Ma, G. Lyophilization as a novel approach for preparation of water resistant HA fiber membranes by crosslinked with EDC. Carbohydrate Polym. 2014, 102, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ángeles, G.; Nešporová, K.; Ambrožová, G.; Kubala, L.; Velebný, V. An effective translation: The development of hyaluronan-based medical products from the physicochemical, and preclinical aspects. Front. Bioeng. Biotechnol. 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- King, M. Management of Tyndall effect. J. Clin. Aesthet. Dermatol. 2016, 9, E6–E8. [Google Scholar]
- Urdiales-Gálvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Moreno, A.; Ortíz-Martí, F.; Del Rio-Reyes, R.; Romero-Álvarez, N.; Del Cueto, S.R.; et al. Treatment of soft tissue filler complications: Expert consensus recommendations. Aesthetic Plast. Surg. 2018, 42, 498–510. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Micheels, P.; (Private Medical Practice, Chêne-Bougeries, Switzerland); Porcello, A.; (University of Geneva, Geneva, Switzerland). Characterization of Commercial Dermal Fillers for Expert Review by Dr. Patrick Micheels. 2023; unpublished work. [Google Scholar]





| Parameters | Cross-Linked HA-Based Dermal Filler Product Manufacturers 1 | ||||
|---|---|---|---|---|---|
| Company Name | Allergan Aesthetics (Subsidiary of AbbVie Inc.) | Galderma SA | Merz Aesthetics (Subsidiary of Merz Group) | Teoxane SA | Laboratoires VIVACY |
| Company Headquarters | Irvine, CA, USA | Zug, Switzerland | Frankfurt am Main, Germany | Geneva, Switzerland | Paris, France |
| Product Brand of Interest | JUVÉDERM® | Restylane® | BELOTERO® | TEOSYAL RHA® | STYLAGE® |
| Product Brand Launch Year | 2000 | 1996 | 2005 | 2004 | 2008 |
| Product Types | Class III medical device | Class III medical device | Class III medical device | Class III medical device | Class III medical device |
| Product Approvals | CE-marked; FDA-approved | CE-marked; FDA-approved | CE-marked; FDA-approved | CE-marked; FDA-approved | CE-marked |
| Product Brand and Name 1 | Specified Product Clinical Uses | Needle Gauge (G) 2 | HA Concentration 3 | Cross-Linked HA | Contains Lidocaine | Cross-Linking Technology 4 |
|---|---|---|---|---|---|---|
| JUVÉDERM® VOLBELLA® | Fine lines; tear through | 30 G | 15 mg/mL | Yes | Yes | VYCROSS® |
| JUVÉDERM® VOLIFT® | Medium fold; lips | 30 G | 17.5 mg/mL | Yes | Yes | VYCROSS® |
| JUVÉDERM® VOLUMA® | Volumizer | 27 G | 20 mg/mL | Yes | Yes | VYCROSS® |
| JUVÉDERM® VOLUX® | Cheeks; temples; jaw line; chin volumizer | 27 G | 25 mg/mL | Yes | Yes | VYCROSS® |
| JUVÉDERM® Ultra 2 | Medium lines; lip border | 30 G | 24 mg/mL | Yes | Yes | HYLACROSS® |
| JUVÉDERM® Ultra 3 | Deep folds; lip volume | 27 G | 24 mg/mL | Yes | Yes | HYLACROSS® |
| Restylane® | Medium lines | 30 G | 20 mg/mL | Yes | No | NASHA® |
| Restylane® | Medium lines | 29 G | 20 mg/mL | Yes | No | NASHA® |
| Restylane® Lido | Medium lines | 30 G | 20 mg/mL | Yes | Yes | NASHA® |
| Restylane® Lido | Medium lines | 29 G | 20 mg/mL | Yes | Yes | NASHA® |
| Restylane® Lyft | Deep folds; volumizer | 27 G | 20 mg/mL | Yes | No | NASHA® |
| Restylane® Lyft Lido | Deep folds; volumizer | 27 G | 20 mg/mL | Yes | Yes | NASHA® |
| BELOTERO® Soft | Fine lines | 30 G | 20 mg/mL | Yes | No | CPM® |
| BELOTERO® Soft + | Fine lines | 30 G | 20 mg/mL | Yes | Yes | CPM® |
| BELOTERO® Balance | Medium lines; lip border | 30 G | 22.5 mg/mL | Yes | No | CPM® |
| BELOTERO® Balance + | Medium lines; lip border | 30 G | 22.5 mg/mL | Yes | Yes | CPM® |
| BELOTERO® Intense | Deep folds; lip volumizer | 27 G | 25.5 mg/mL | Yes | No | CPM® |
| BELOTERO® Intense + | Deep folds; lip volumizer | 27 G | 25.5 mg/mL | Yes | Yes | CPM® |
| BELOTERO® Volume | Volumizer | 30 G | 26 mg/mL | Yes | No | CPM® |
| BELOTERO® Volume + | Volumizer | 30 G | 26 mg/mL | Yes | Yes | CPM® |
| TEOSYAL RHA® 1 | Fine lines | 30 G | 15 mg/mL | Yes (mix) | Yes | Preserved Network® |
| TEOSYAL RHA® 2 | Medium folds; lip contour | 30 G | 23 mg/mL | Yes (mix) | Yes | Preserved Network® |
| TEOSYAL RHA® 3 | Deep folds; lip volumizer | 27 G | 23 mg/mL | Yes (mix) | Yes | Preserved Network® |
| TEOSYAL RHA® 4 | Volumizer | 27 G | 23 mg/mL | Yes (mix) | Yes | Preserved Network® |
| TEOSYAL Ultra Deep | Strong volumizer | 25 G | 25 mg/g | Yes | Yes | Teosyal PureSense |
| STYLAGE® S | Fine lines | 30 G | 16 mg/g | Yes | Yes | IPN-Like® + mannitol |
| STYLAGE® M | Medium folds; lip contour | 30 G | 20 mg/g | Yes | Yes | IPN-Like® + mannitol |
| STYLAGE® L | Deep folds; lip volumizer | 27 G | 24 mg/g | Yes | Yes | IPN-Like® + mannitol |
| STYLAGE® XL | Volumizer | 27 G | 26 mg/g | Yes | Yes | IPN-Like® + mannitol |
| STYLAGE® XXL | Cheeks; temples; jawline; chin volumizer | 27 G | 21 mg/g | Yes | No | IPN-Like® + mannitol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheels, P.; Porcello, A.; Bezzola, T.; Perrenoud, D.; Quinodoz, P.; Kalia, Y.; Allémann, E.; Laurent, A.; Jordan, O. Clinical Perspectives on the Injectability of Cross-Linked Hyaluronic Acid Dermal Fillers: A Standardized Methodology for Commercial Product Benchmarking with Inter-Injector Assessments. Gels 2024, 10, 101. https://doi.org/10.3390/gels10020101
Micheels P, Porcello A, Bezzola T, Perrenoud D, Quinodoz P, Kalia Y, Allémann E, Laurent A, Jordan O. Clinical Perspectives on the Injectability of Cross-Linked Hyaluronic Acid Dermal Fillers: A Standardized Methodology for Commercial Product Benchmarking with Inter-Injector Assessments. Gels. 2024; 10(2):101. https://doi.org/10.3390/gels10020101
Chicago/Turabian StyleMicheels, Patrick, Alexandre Porcello, Thierry Bezzola, Daniel Perrenoud, Pierre Quinodoz, Yogeshvar Kalia, Eric Allémann, Alexis Laurent, and Olivier Jordan. 2024. "Clinical Perspectives on the Injectability of Cross-Linked Hyaluronic Acid Dermal Fillers: A Standardized Methodology for Commercial Product Benchmarking with Inter-Injector Assessments" Gels 10, no. 2: 101. https://doi.org/10.3390/gels10020101