Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential
Abstract
:1. Introduction
2. Wound Healing Process
3. Hydrogel Wound Dressings
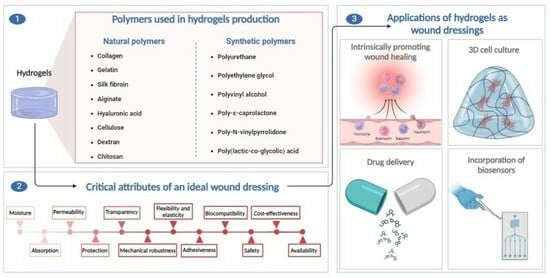
4. Polymers
4.1. Natural Polymers
4.1.1. Collagen
4.1.2. Gelatin
4.1.3. Silk Fibroin
4.1.4. Alginate
4.1.5. Hyaluronic Acid
4.1.6. Cellulose
4.1.7. Dextran
4.1.8. Chitosan
4.2. Synthetic Polymers
4.2.1. Polyurethane
4.2.2. Polyethylene Glycol
4.2.3. Polyvinyl Alcohol
4.2.4. Poly-ε-Caprolactone
4.2.5. Poly-N-Vinylpyrrolidone
4.2.6. Poly(lactic-co-glycolic) Acid
5. Overview of Critical Attributes of Polymers
6. Applications of Hydrogels as Wound Dressings
6.1. Hydrogels with the Intrinsic Ability to Promote Wound Healing
6.2. Hydrogels as Drug Delivery Systems and Other Substance Carriers
6.3. Hydrogels as 3D Scaffolds for Cell Adhesion and Proliferation
6.4. Hydrogels with Integrated Biosensors
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| BC | Bacterial cellulose |
| BCM | Bacterial cellulose membrane |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| FGF | Fibroblast growth factor |
| GA | Glycolic acid |
| HA | Hyaluronic acid |
| NF-kB | Nuclear Factor Kappa B |
| PCL | Poly-ε-caprolactone |
| PEG | Polyethylene glycol |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| PVP | Poly-N-vinylpyrrolidone |
| RGD | Arginine–glycine–aspartic acid peptide sequences |
| SF | Silk fibroin |
| TGF-β | Transforming growth factor |
| VEGF | Vascular endothelial growth factor |
References
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint. 2022, 8, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Stubbe, B.; Mignon, A.; Declercq, H.; Van Vlierberghe, S.; Dubruel, P. Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment. Macromol. Biosci. 2019, 19, 1900123. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- He, Y.; Cen, Y.; Tian, M. Immunomodulatory hydrogels for skin wound healing: Cellular targets and design strategy. J. Mater. Chem. B 2024, 12, 2435–2458. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.-Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Tefft, J.B.; Chen, C.S.; Eyckmans, J. Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure. APL Bioeng. 2021, 5, 016102. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, H.; Yang, K.; Wang, H.; Duan, C.; Ni, N.; An, L.; Luo, Y.; Zhao, P.; Gou, Y.; et al. Reversibly immortalized keratinocytes (iKera) facilitate re-epithelization and skin wound healing: Potential applications in cell-based skin tissue engineering. Bioact. Mater. 2022, 9, 523–540. [Google Scholar] [CrossRef]
- Yang, F.; Jin, S.; Tang, Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.L.; Cheng, B.; Mo, X.M.; Chen, C.; Pan, J.F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels To Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Etxabide, A.; Uranga, J.; Bayat, A.; Guerrero, P.; Igartua, M.; de la Caba, K.; Hernandez, R.M. Development of Bioinspired Gelatin and Gelatin/Chitosan Bilayer Hydrofilms for Wound Healing. Pharmaceutics 2019, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.R.; Shahzadi, L.; Tehseen, S.; Alvi, F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Amino acids loaded chitosan/collagen based new membranes stimulate angiogenesis in chorioallantoic membrane assay. Int. J. Biol. Macromol. 2019, 140, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A Gelatin-sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired Multifunctional Hybrid Hydrogel Promotes Wound Healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; He, A.; Liu, W.; Jiang, X.; Zheng, J.; Han, C.C.; Hsiao, B.S.; Chu, B.; Fang, D. Chemical crosslinking and biophysical properties of electrospun hyaluronic acid based ultra-thin fibrous membranes. Polymer 2009, 50, 3762–3769. [Google Scholar] [CrossRef]
- Kumar, V.B.; Tiwari, O.S.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gazit, E. Design of Functional RGD Peptide-Based Biomaterials for Tissue Engineering. Pharmaceutics 2023, 15, 345. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Ayello, E.A.; Loehne, H.; Chariker, M.; DiCosmo, F. Edge Effect: The Role of Collagen in Wound Healing. Adv. Skin Wound Care 2007, 22, 12–15. [Google Scholar] [CrossRef]
- Karlsson, M.; Olofsson, P.; Steinvall, I.; Sjöberg, F.; Thorfinn, J.; Elmasry, M. Three Years’ Experience of a Novel Biosynthetic Cellulose Dressing in Burns. Adv. Wound Care 2019, 8, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, Y.; Dai, J.; Yan, S.; Miao, W.; Ren, L. Calcium alginate/PNIPAAm hydrogel with body temperature response and great biocompatibility: Application as burn wound dressing. Int. J. Biol. Macromol. 2022, 216, 686–697. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef]
- Jafari, A.; Hassanajili, S.; Azarpira, N.; Bagher Karimi, M.; Geramizadeh, B. Development of thermal-crosslinkable chitosan/maleic terminated polyethylene glycol hydrogels for full thickness wound healing: In vitro and in vivo evaluation. Eur. Polym. J. 2019, 118, 113–127. [Google Scholar] [CrossRef]
- Almeida Cruz, M.; Araujo, T.A.; Avanzi, I.R.; Parisi, J.R.; Martins de Andrade, A.L.; Muniz Rennó, A.C. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: A Systematic Review. Mar. Biotechnol. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Arora, D.; Bhunia, B.K.; Janani, G.; Mandal, B.B. Bioactive three-dimensional silk composite in vitro tumoroid model for high throughput screening of anticancer drugs. J. Colloid Interface Sci. 2021, 589, 438–452. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, P.-X.; Huang, S.-X.; Hou, D.-Z.; Xu, X.-D.; Ci, L.-Y.; Chen, S. Quantitative analysis of straight-chain/branched-chain Ratio During Enzymatic Synthesis of Dextran Based on Periodate Oxidation. Biochem. Biophys. Res. Commun. 2020, 523, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Petrovici, A.R.; Anghel, N.; Dinu, M.V.; Spiridon, I. Dextran-Chitosan Composites: Antioxidant and Anti-Inflammatory Properties. Polymers 2023, 15, 1980. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Noor, A.; Afzal, A.; Masood, R.; Khaliq, Z.; Ahmad, S.; Ahmad, F.; Qadir, M.-B.; Irfan, M. Dressings for burn wound: A review. J. Mater. Sci. 2022, 57, 6536–6572. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Wang, P.; Hwang, H.H.; Zhu, W.; Warner, J.; Chen, S. Continuous Optical 3D Printing of Green Aliphatic Polyurethanes. ACS Appl. Mater. Interfaces 2017, 9, 836–844. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Ganguly, S. Polymer in hemostasis and follow-up wound healing. J. Appl. Polym. Sci. 2023, 140, e53559. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Jiang, Z.; Li, R.; Xue, T.; Lin, C.; Deng, Y.; Jin, Y.; Sun, B. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front. Chem. 2022, 10, 1038839. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Manna, S.; Roy, S.; Nandi, S.K.; Basak, P. Polymeric biomaterials-based tissue engineering for wound healing: A systemic review. Burn. Trauma 2023, 11, tkac058. [Google Scholar] [CrossRef]
- Heczko, D.; Hachuła, B.; Maksym, P.; Kamiński, K.; Zięba, A.; Orszulak, L.; Paluch, M.; Kamińska, E. The Effect of Various Poly (N-vinylpyrrolidone) (PVP) Polymers on the Crystallization of Flutamide. Pharmaceuticals 2022, 15, 971. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Kim, S.-H. Lignocellulosic biomass as renewable feedstock for biodegradable and recyclable plastics production: A sustainable approach. Renew. Sustain. Energy Rev. 2022, 158, 112130. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen-Based Bioink for 3D Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chakravarty, T.; Roy, A.J.; Manna, S.; Nandi, S.K.; Basak, P. Sustainable development of Draksha- Beeja extract loaded gelatin and starch-based green and biodegradable mats for potential tissue engineering applications. Sustain. Chem. Pharm. 2023, 34, 101134. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Chi, B.; Zhang, X.; Wang, Y.; Wu, J.; Huang, Q. Construction of a dual-component hydrogel matrix for 3D biomimetic skin based on photo-crosslinked chondroitin sulfate/collagen. Int. J. Biol. Macromol. 2024, 254, 127940. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, D. Novel hyaluronic acid-tyrosine/collagen-based injectable hydrogels as soft filler for tissue engineering. Int. J. Biol. Macromol. 2019, 141, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, J.; Xiong, S.; Ding, Y.; Zhou, X.; Hu, Y.; Chen, W.; Lin, Y.; Dao, L. Fabrication and insights into the mechanisms of collagen-based hydrogels with the high cell affinity and antimicrobial activity. J. Appl. Polym. Sci. 2022, 139, 51623. [Google Scholar] [CrossRef]
- Li, J.; Zhai, Y.-N.; Xu, J.-P.; Zhu, X.-Y.; Yang, H.-R.; Che, H.-J.; Liu, C.-K.; Qu, J.-B. An injectable collagen peptide-based hydrogel with desirable antibacterial, self-healing and wound-healing properties based on multiple-dynamic crosslinking. Int. J. Biol. Macromol. 2024, 259, 129006. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Shahvandi, M.K.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D Printed Hydrogels for Ocular Wound Healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef]
- Rosenquist, J.; Folkesson, M.; Höglund, L.; Pupkaite, J.; Hilborn, J.; Samanta, A. An Injectable, Shape-Retaining Collagen Hydrogel Cross-linked Using Thiol-Maleimide Click Chemistry for Sealing Corneal Perforations. ACS Appl. Mater. Interfaces 2023, 15, 34407–34418. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen Peptides from Swim Bladders of Giant Croaker (Nibea japonica) and Their Protective Effects against H2O2-Induced Oxidative Damage toward Human Umbilical Vein Endothelial Cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef]
- Helary, C.; Zarka, M.; Giraud-Guille, M.M. Fibroblasts within concentrated collagen hydrogels favour chronic skin wound healing. J. Tissue Eng. Regen. Med. 2012, 6, 225–237. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2021, 608, 2278–2289. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, C.; Shao, J.; Chen, Y.; Yin, A.; Feng, Q.; Chen, S.; Peng, F.; Ma, X.; Xu, C.-Y.; et al. Integration of flexible, recyclable, and transient gelatin hydrogels toward multifunctional electronics. J. Mater. Sci. Technol. 2023, 145, 83–92. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Insect Cuticle-Mimetic Hydrogels with High Mechanical Properties Achieved via the Combination of Chitin Nanofiber and Gelatin. J. Agric. Food Chem. 2019, 67, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yao, Y.; Dai, L.; Jiao, M.; Ding, B.; Yu, Q.; Tang, J.; Liu, B. Sustainable and high-performance Zn dual-ion batteries with a hydrogel-based water-in-salt electrolyte. Energy Storage Mater. 2022, 47, 187–194. [Google Scholar] [CrossRef]
- Asadi, N.; Mehdipour, A.; Ghorbani, M.; Mesgari-Abbasi, M.; Akbarzadeh, A.; Davaran, S. A novel multifunctional bilayer scaffold based on chitosan nanofiber/alginate-gelatin methacrylate hydrogel for full-thickness wound healing. Int. J. Biol. Macromol. 2021, 193, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, X.; Ding, Z.; Tao, J.; Xu, G.; Wang, Y.; Huang, Y.; Liu, J. A polyacrylic acid/polyacrylamide-based hydrogel electrolyte containing gelatin for efficient electrochromic device with outstanding cycling stability and flexible compatibility. Eur. Polym. J. 2023, 190, 112024. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Mignon, A.; Minsart, M.; Van Hoorick, J.; Gardikiotis, I.; Caruntu, I.D.; Giusca, S.E.; Van Vlierberghe, S.; Profire, L. Gelatin-Based Versus Alginate-Based Hydrogels: Providing Insight in Wound Healing Potential. Macromol. Biosci. 2021, 21, 2100230. [Google Scholar] [CrossRef]
- Sharifi, S.; Islam, M.M.; Sharifi, H.; Islam, R.; Koza, D.; Reyes-Ortega, F.; Alba-Molina, D.; Nilsson, P.H.; Dohlman, C.H.; Mollnes, T.E.; et al. Tuning gelatin-based hydrogel towards bioadhesive ocular tissue engineering applications. Bioact. Mater. 2021, 6, 3947–3961. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Seetabhawang, S.; Sanchavanakit, N.; Phisalaphong, M. Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J. Biomater. Sci. Polym. Ed. 2019, 30, 961–982. [Google Scholar] [CrossRef]
- Dou, C.; Li, Z.; Luo, Y.; Gong, J.; Li, Q.; Zhang, J.; Zhang, Q.; Qiao, C. Bio-based poly (γ-glutamic acid)-gelatin double-network hydrogel with high strength for wound healing. Int. J. Biol. Macromol. 2022, 202, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zheng, L.; Wang, Y.; Fan, K.; Guo, S.; Kang, H.; Lin, J.; Xue, Y.; Liu, Z.; Li, C. Corneal stromal filler injection of gelatin-based photocurable hydrogels for maintaining the corneal thickness and reconstruction of corneal stroma. Compos. Part B Eng. 2023, 266, 111004. [Google Scholar] [CrossRef]
- Ahmed, A.; Nath, J.; Baruah, K.; Rather, M.A.; Mandal, M.; Dolui, S.K. Development of mussel mimetic gelatin based adhesive hydrogel for wet surfaces with self-healing and reversible properties. Int. J. Biol. Macromol. 2023, 228, 68–77. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D.; Quan, L.; Chai, H.; Sui, X.; Wang, B.; Xu, H.; Mao, Z. Mussel-inspired adhesive gelatin–polyacrylamide hydrogel wound dressing loaded with tetracycline hydrochloride to enhance complete skin regeneration. Soft Matter 2022, 18, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Yang, H.; Shu, Y.; Li, K.; Chen, K.; Xiao, W.; Liao, X. Preparation and antibacterial properties of an AgBr@SiO2/GelMA composite hydrogel. Biomed. Mater. 2022, 17, 025005. [Google Scholar] [CrossRef] [PubMed]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lu, C.; Yu, Z.; Lian, Y.; Ma, Y.; Chen, Z.; Jiang, X.; Zhang, Y. Transparent, High Stretchable, Environmental Tolerance, and Excellent Sensitivity Hydrogel for Flexible Sensors and Capacitive Pens. ACS Appl. Mater. Interfaces 2023, 15, 44280–44293. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.; Zhao, L.; Li, M.; Yang, W. Effective Removal of Dyes from Aqueous Solutions by a Gelatin Hydrogel. J. Polym. Environ. 2021, 29, 3497–3508. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Yang, J.; Du, H.; Chai, N.; Sha, Z.; Geng, M.; Zhou, X.; He, C. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef]
- Indrakumar, S.; Joshi, A.; Dash, T.K.; Mishra, V.; Tandon, B.; Chatterjee, K. Photopolymerized silk fibroin gel for advanced burn wound care. Int. J. Biol. Macromol. 2023, 233, 123569. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, A.; Mao, Z.; Li, F.; Su, T.; Cao, L.; Wang, W.; Liu, Y.; Wang, C. Silk fibroin–gelatin photo-crosslinked 3D-bioprinted hydrogel with MOF-methylene blue nanoparticles for infected wound healing. Int. J. Bioprint. 2023, 9, 459–473. [Google Scholar] [CrossRef]
- Maity, B.; Alam, S.; Samanta, S.; Prakash, R.G.; Govindaraju, T. Antioxidant Silk Fibroin Composite Hydrogel for Rapid Healing of Diabetic Wound. Macromol. Biosci. 2022, 22, e2200097. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Pourjabbar, B.; Biazar, E.; Heidari Keshel, S.; Baradaran-Rafii, A. Improving the properties of fish skin collagen/silk fibroin dressing by chemical treatment for corneal wound healing. Int. Wound J. 2023, 20, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Shefa, A.A.; Amirian, J.; Kang, H.J.; Bae, S.H.; Jung, H.-I.; Choi, H.-J.; Lee, S.Y.; Lee, B.-T. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017, 177, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Cui, J.; Zhang, C.; Xing, C.; Bian, H.; Lv, J.; Chen, D.; Xiao, L.; Su, J.; et al. Advanced multilayer composite dressing with co-delivery of gelsevirine and silk fibroin for burn wound healing. Compos. Part B Eng. 2023, 253, 110549. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef]
- Zahra, D.; Shokat, Z.; Ahmad, A.; Javaid, A.; Khurshid, M.; Ashfaq, U.A.; Nashwan, A.J. Exploring the recent developments of alginate silk fibroin material for hydrogel wound dressing: A review. Int. J. Biol. Macromol. 2023, 248, 125989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, Y.; Chen, J.; He, Z.; Tan, P.; He, Y.; Pei, X.; Wang, J.; Tan, L.; Wan, Q. Structural-Functional Pluralistic Modification of Silk Fibroin via MOF Bridging for Advanced Wound Care. Adv. Sci. 2022, 9, e2204553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yin, C.; Qi, X.; Guo, C.; Wu, X. Silk Fibroin Crosslinked Glycyrrhizic Acid and Silver Hydrogels for Accelerated Bacteria-Infected Wound Healing. Macromol. Biosci. 2022, 22, 2100407. [Google Scholar] [CrossRef]
- Özen, N.; Özbaş, Z.; İzbudak, B.; Emik, S.; Özkahraman, B.; Bal-Öztürk, A. Boric acid-impregnated silk fibroin/gelatin/hyaluronic acid-based films for improving the wound healing process. J. Appl. Polym. Sci. 2022, 139, 51715. [Google Scholar] [CrossRef]
- Yin, C.; Han, X.; Lu, Q.; Qi, X.; Guo, C.; Wu, X. Rhein incorporated silk fibroin hydrogels with antibacterial and anti-inflammatory efficacy to promote healing of bacteria-infected burn wounds. Int. J. Biol. Macromol. 2022, 201, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xu, C.; Xiong, W.; Jiang, N.; Zheng, Y.; He, X.; Ding, F.; Lu, X.; Shen, J. Dual cross-linked organic-inorganic hybrid hydrogels accelerate diabetic skin wound healing. Chem. Eng. J. 2021, 417, 129335. [Google Scholar] [CrossRef]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2020, 417, 128278. [Google Scholar] [CrossRef]
- Li, Z.; Song, J.; Zhang, J.; Hao, K.; Liu, L.; Wu, B.; Zheng, X.; Xiao, B.; Tong, X.; Dai, F. Topical application of silk fibroin-based hydrogel in preventing hypertrophic scars. Colloids Surfaces B Biointerfaces 2020, 186, 110735. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Sun, F.; Zhang, X.; Peng, Z.; Jiang, B.; Liang, M.; Wang, Y. Silk fibroin hydrogel promote burn wound healing through regulating TLN1 expression and affecting cell adhesion and migration. J. Mater. Sci. Mater. Med. 2020, 31, 48. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Antimicrobial and wound healing activities of electrospun nanofibers based on functionalized carbohydrates and proteins. Cellulose 2022, 29, 1331–1347. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, C.; Fan, P.-S.; Zhang, X.-H.; Hou, D.-Y.; Wang, J.-Q.; Yang, H.; Wang, H.; Qiao, Z.-Y. Skin-like wound dressings with on-demand administration based on in situ peptide self-assembly for skin regeneration. J. Mater. Chem. B 2022, 10, 3624–3636. [Google Scholar] [CrossRef]
- Malinowski, C.; He, F.; Zhao, Y.; Chang, I.; Hatchett, D.W.; Zhai, S.; Zhao, H. Nanopatterned silk fibroin films with high transparency and high haze for optical applications. RSC Adv. 2019, 9, 40792–40799. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, J.; Wu, S.J. Highly Stretchable and Tough Physical Silk Fibroin–Based Double Network Hydrogels. Macromol. Rapid Commun. 2019, 40, e1900389. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Y.; Si, P.; Zhang, D. Tough silk fibroin hydrogel via polypropylene glycol (PPG) blending for wearable sensors. J. Appl. Polym. Sci. 2023, 140, e54689. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, L.; Zhou, B.; Zhao, P.; Wang, W.; Dong, S.; Cheng, B.; Yang, J.; Li, B.; Wang, X. In situ photo-crosslinking silk fibroin based hydrogel accelerates diabetic wound healing through antibacterial and antioxidant. Int. J. Biol. Macromol. 2023, 242, 125028. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xu, C.; Cao, Y.; Liu, S.; Reis, R.L.; Kundu, S.C.; Yang, X.; Xiao, B.; Duan, L. Transparent silk fibroin film-facilitated infected-wound healing through antibacterial, improved fibroblast adhesion and immune modulation. J. Mater. Chem. B 2023, 12, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.; Zhang, S.; Gu, X.; Wu, R.; Huang, T.; Zhou, Z.; Sun, C.; Ling, J.; Liu, M.; et al. Silk-Inspired In Situ Hydrogel with Anti-Tumor Immunity Enhanced Photodynamic Therapy for Melanoma and Infected Wound Healing. Adv. Funct. Mater. 2021, 31, 2101320. [Google Scholar] [CrossRef]
- Mitropoulos, A.N.; Marelli, B.; Ghezzi, C.E.; Applegate, M.B.; Partlow, B.P.; Kaplan, D.L.; Omenetto, F.G. Transparent, Nanostructured Silk Fibroin Hydrogels with Tunable Mechanical Properties. ACS Biomater. Sci. Eng. 2015, 1, 964–970. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- He, Y.; Zhao, W.; Dong, Z.; Ji, Y.; Li, M.; Hao, Y.; Zhang, D.; Yuan, C.; Deng, J.; Zhao, P.; et al. A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. Int. J. Biol. Macromol. 2021, 167, 182–192. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Wu, H.; Wang, Y.; Liu, Q.; Wang, Y.; Lincoln, S.F.; Guo, X.; Wang, J. Cyclodextrin Hydrogels: Rapid Removal of Aromatic Micropollutants and Adsorption Mechanisms. J. Chem. Eng. Data 2020, 65, 678–689. [Google Scholar] [CrossRef]
- Benhalima, T.; Ferfera-Harrar, H. Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. Int. J. Biol. Macromol. 2019, 132, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the exemplary knowledge of microbial exopolysaccharides in a nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef] [PubMed]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-based wound dressing incorporated with hyaluronic acid and propolis: In vitro and in vivo studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Hyaluronic Acid-Based Scaffolds as Potential Bioactive Wound Dressings. Polymers 2021, 13, 2102. [Google Scholar] [CrossRef]
- Ganjoo, R.; Sharma, S.; Verma, C.; Quraishi, M.A.; Kumar, A. Heteropolysaccharides in sustainable corrosion inhibition: 4E (Energy, Economy, Ecology, and Effectivity) dimensions. Int. J. Biol. Macromol. 2023, 235, 123571. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; He, S.; Gao, Y.; Cui, X. Constructing of pH and reduction dual-responsive folic acid-modified hyaluronic acid-based microcapsules for dual-targeted drug delivery via sonochemical method. Colloid Interface Sci. Commun. 2021, 44, 100503. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Wang, Z.-Y.; Ren, Z.-W.; Zhang, X.-W.; Wei, D.-X. Advances in modified hyaluronic acid-based hydrogels for skin wound healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.-H.; Na, K.-S.; et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, J.; Jiang, Z.; Hu, H.; Yang, C.; Liu, W.; Han, B. A self-healing and injectable hydrogel based on water-soluble chitosan and hyaluronic acid for vitreous substitute. Carbohydr. Polym. 2021, 256, 117519. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Y.; Zhang, Z.; Gao, Y.; Liu, W.; Borjihan, Q.; Qu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.-J.; et al. Engineering a multifunctional N-halamine-based antibacterial hydrogel using a super-convenient strategy for infected skin defect therapy. Chem. Eng. J. 2019, 379, 122238. [Google Scholar] [CrossRef]
- Gwak, M.A.; Hong, B.M.; Seok, J.M.; Park, S.A.; Park, W.H. Effect of tannic acid on the mechanical and adhesive properties of catechol-modified hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2021, 191, 699–705. [Google Scholar] [CrossRef]
- Passi, A.; Vigetti, D. Hyaluronan as tunable drug delivery system. Adv. Drug Deliv. Rev. 2019, 146, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Caruso, M.R.; D’agostino, G.; Milioto, S.; Cavallaro, G.; Lazzara, G. A review on biopolymer-based treatments for consolidation and surface protection of cultural heritage materials. J. Mater. Sci. 2023, 58, 12954–12975. [Google Scholar] [CrossRef]
- Kwak, M.H.; Kim, J.E.; Go, J.; Koh, E.K.; Song, S.H.; Son, H.J.; Kim, H.S.; Yun, Y.H.; Jung, Y.J.; Hwang, D.Y. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr. Polym. 2015, 122, 387–398. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.-G.; Dinu-Pîrvu, C.-E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.d.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surfaces B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Jung, S.A.; Malyaran, H.; Demco, D.E.; Manukanc, A.; Häser, L.S.; Kučikas, V.; van Zandvoort, M.; Neuss, S.; Pich, A. Fibrin–Dextran Hydrogels with Tunable Porosity and Mechanical Properties. Biomacromolecules 2023, 24, 3972–3984. [Google Scholar] [CrossRef]
- Luo, H.-C.; Mai, K.-J.; Liu, E.; Chen, H.; Xie, Y.-J.; Zheng, Y.-X.; Lin, R.; Zhang, L.-M.; Zhang, Y. Efficiency and Safety of Dextran-PAMAM/siMMP-9 Complexes for Decreasing Matrix Metalloproteinase-9 Expression and Promoting Wound Healing in Diabetic Rats. Bioconjug. Chem. 2022, 33, 2398–2410. [Google Scholar] [CrossRef]
- Nonsuwan, P.; Matsugami, A.; Hayashi, F.; Hyon, S.-H.; Matsumura, K. Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties. Carbohydr. Polym. 2019, 204, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Rohiwal, S.S.; Ellederova, Z.; Tiwari, A.P.; Alqarni, M.; Elazab, S.T.; El-Saber Batiha, G.; Pawar, S.H.; Thorat, N.D. Self-assembly of bovine serum albumin (BSA)–dextran bio-nanoconjugate: Structural, antioxidant and in vitro wound healing studies. RSC Adv. 2021, 11, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, D.; Yu, Y.; Zheng, Y. Insights into the Role of Natural Polysaccharide-Based Hydrogel Wound Dressings in Biomedical Applications. Gels 2022, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2021, 253, 117213. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules 2023, 28, 4034. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int. J. Pharm. 2017, 532, 466–477. [Google Scholar] [CrossRef]
- Mostafavi Esfahani, M.; Koupaei, N.; Hassanzadeh-Tabrizi, S.A. Synthesis and characterization of polyvinyl alcohol/dextran/Zataria wound dressing with superior antibacterial and antioxidant properties. J. Vinyl Addit. Technol. 2023, 29, 380–394. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Lu, Q.; Wang, L.; Hu, X.; Zhang, H. Preparation, flocculation and application in sugar refining of eco-friendly dextran-polylysine complex flocculant. Sep. Purif. Technol. 2023, 306, 122673. [Google Scholar] [CrossRef]
- Maingret, V.; Courrégelongue, C.; Schmitt, V.; Héroguez, V. Dextran-Based Nanoparticles to Formulate pH-Responsive Pickering Emulsions: A Fully Degradable Vector at a Day Scale. Biomacromolecules 2020, 21, 5358–5368. [Google Scholar] [CrossRef]
- Trejo-Caballero, M.E.; Díaz-Patiño, L.; González-Reynac, M.; Molina, G.A.; López-Miranda, J.L.; Esparza, R.; España-Sánchez, B.L.; Arjona, N.; Estevez, M. Biopolymeric hydrogel electrolytes obtained by using natural polysaccharide-poly(itaconic acid-co-2-hydroxyethyl methacrylate) in deep eutectic solvents for rechargeable Zn-air batteries. Green Chem. 2023, 25, 6784–6796. [Google Scholar] [CrossRef]
- De Cicco, F.; Reverchon, E.; Adami, R.; Auriemma, G.; Russo, P.; Calabrese, E.C.; Porta, A.; Aquino, R.P.; Del Gaudio, P. In situ forming antibacterial dextran blend hydrogel for wound dressing: SAA technology vs. spray drying. Carbohydr. Polym. 2014, 101, 1216–1224. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Chen, H.; Wang, N.; Liu, X.; Sun, G.; Qiao, W. Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel. Int. J. Biol. Macromol. 2019, 132, 1098–1105. [Google Scholar] [CrossRef]
- Bochani, S.; Zarepour, A.; Kalantari-Hesari, A.; Haghi, F.; Shahbazi, M.-A.; Zarrabi, A.; Taheri, S.; Maleki, A. Injectable, antibacterial, and oxygen-releasing chitosan-based hydrogel for multimodal healing of bacteria-infected wounds. J. Mater. Chem. B 2023, 11, 8056–8068. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together is better: Poly(tannic acid) nanorods functionalized polysaccharide hydrogels for diabetic wound healing. Ind. Crop. Prod. 2022, 186, 115273. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Mehrban, S.F.; Aliabadi, H.A.M.; Karimi, M.; Mohammadi, A.; Maleki, A.; Mahdavi, M.; Larijani, B.; Shalan, A.E. Review: The latest advances in biomedical applications of chitosan hydrogel as a powerful natural structure with eye-catching biological properties. J. Mater. Sci. 2022, 57, 3855–3891. [Google Scholar] [CrossRef]
- Song, K.; Hao, Y.; Liu, Y.; Cao, R.; Zhang, X.; He, S.; Wen, J.; Zheng, W.; Wang, L.; Zhang, Y. Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A self-adapting hydrogel based on chitosan/oxidized konjac glucomannan/AgNPs for repairing irregular wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-Based Hemostatic Hydrogels: The Concept, Mechanism, Application, and Prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, T.; Yan, W.; Zhang, F.; Xu, Y.; Du, Z. Structure and Properties of Oxidized Chitosan Grafted Cashmere Fiber by Amide Covalent Modification. Molecules 2020, 25, 3812. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Liu, F.; Han, R.; Naficy, S.; Casillas, G.; Sun, X.; Huang, Z. Few-Layered Boron Nitride Nanosheets for Strengthening Polyurethane Hydrogels. ACS Appl. Nano Mater. 2021, 4, 7988–7994. [Google Scholar] [CrossRef]
- He, M.; Hou, Y.; Zhu, C.; He, M.; Jiang, Y.; Feng, G.; Liu, L.; Li, Y.; Chen, C.; Zhang, L. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjug. Chem. 2021, 32, 1915–1925. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Lee, G.-H.; Chou, C.-W.; Chen, Y.-P.; Wu, T.-H.; Lin, H.-R. Stimulation of wound healing by PU/hydrogel composites containing fibroblast growth factor-2. J. Mater. Chem. B 2015, 3, 1931–1941. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, J.; Long, F.; Qiu, W.; Meng, G.; Zeng, Z.; Huang, H.; Wang, H.; Lin, N.; Liu, X.Y. Bioinspired Conductive Enhanced Polyurethane Ionic Skin as Reliable Multifunctional Sensors. Adv. Sci. 2023, 10, e2300857. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.I.; An, D.H.; Lim, J.W.; Oh, T.; Lee, H.; Park, S.-M.; Jeong, J.H.; Chung, J.W. Hydrogel Surface-Modified Polyurethane Copolymer Film with Water Permeation Resistance and Biocompatibility for Implantable Biomedical Devices. Micromachines 2021, 12, 447. [Google Scholar] [CrossRef]
- Iga, C.; Pawel, S.; Marcin, L.; Justyna, K.L. Polyurethane Composite Scaffolds Modified with the Mixture of Gelatin and Hydroxyapatite Characterized by Improved Calcium Deposition. Polymers 2020, 12, 410. [Google Scholar] [CrossRef]
- Xiang, S.L.; Su, Y.X.; Yin, H.; Li, C.; Zhu, M.Q. Visible-light-driven isotropic hydrogels as anisotropic underwater actuators. Nano Energy 2021, 85, 105965. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, J.; Gao, F.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Self-healable and recyclable polyurethane-polyaniline hydrogel toward flexible strain sensor. Compos. Part B Eng. 2021, 219, 108965. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Lewandowska, A.; Terebieniec, A.; Przybytek, A.; Cieśliński, H. Antibacterial polyurethanes, modified with cinnamaldehyde, as potential materials for fabrication of wound dressings. Polym. Bull. 2019, 76, 2725–2742. [Google Scholar] [CrossRef]
- Xiao, L.; Ni, W.; Zhao, X.; Guo, Y.; Li, X.; Wang, F.; Luo, G.; Zhan, R.; Xu, X. A moisture balanced antibacterial dressing loaded with lysozyme possesses antibacterial activity and promotes wound healing. Soft Matter 2021, 17, 3162–3173. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O. Synthesis and properties of water-dispersible polyurethanes based on various diisocyanates and PEG as the hard segment. J. Appl. Polym. Sci. 2023, 140, e53948. [Google Scholar] [CrossRef]
- Song, J.; Li, L.; Niu, Y.H.; Ke, R.Y.; Zhao, X. Preparation of humic acid water-retaining agent-modified polyurethane sponge as a soilless culture material. J. Appl. Polym. Sci. 2022, 139, 52182. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Ye, Z.; Zhang, Y.; Gao, S.; Rong, H.; Zhang, J.; Deng, L.; Dong, A. Superhydrophobic and superhydrophilic polyurethane sponge for wound healing. Chem. Eng. J. 2022, 446, 136985. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, X.; Pan, M.; Yuan, J.; Jia, Z.; Zhu, L. A Robust, Tough and Multifunctional Polyurethane/Tannic Acid Hydrogel Fabricated by Physical-Chemical Dual Crosslinking. Polymers 2020, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Giroto, A.S.; do Valle, S.F.; Ribeiro, T.; Ribeiro, C.; Mattoso, L.H.C. Towards urea and glycerol utilization as “building blocks” for polyurethane production: A detailed study about reactivity and structure for environmentally friendly polymer synthesis. React. Funct. Polym. 2020, 153, 104629. [Google Scholar] [CrossRef]
- Zanini, N.C.; de Souza, A.G.; Barbosa, R.F.C.; Rosa, D.S.; Mulinari, D.R. Eco-friendly composites of polyurethane and sheath palm residues. J. Cell. Plast. 2021, 58, 139–158. [Google Scholar] [CrossRef]
- Bourguignon, M.; Thomassin, J.M.; Grignard, B.; Vertruyen, B.; Detrembleur, C. Water-Borne Isocyanate-Free Polyurethane Hydrogels with Adaptable Functionality and Behavior. Macromol. Rapid Commun. 2021, 42, e2000482. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chou, P.-Y.; Chen, Z.-Y.; Lin, F.-H. Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion. Int. J. Mol. Sci. 2019, 20, 1625. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Sun, Y.; Wang, S.; Zhang, L.; Wu, A.; Zhang, Y. Superhydrophilic quaternized calcium alginate based aerogel membrane for oil-water separation and removal of bacteria and dyes. Int. J. Biol. Macromol. 2023, 227, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Huang, K.; Wang, S.; Quirino, R.L.; Zhang, Z.-X.; Zhang, C. Eco-Friendly Castor Oil-Based Delivery System with Sustained Pesticide Release and Enhanced Retention. ACS Appl. Mater. Interfaces 2020, 12, 37607–37618. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhou, X.; Fang, C.; Song, Y.; Li, Y. Eco-friendly waterborne polyurethane reinforced with cellulose nanocrystal from office waste paper by two different methods. Carbohydr. Polym. 2019, 209, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Feng, Y.; Hu, Y.; Yuan, T.; Yang, Z. A renewable and multifunctional eco-friendly coating from novel tung oil-based cationic waterborne polyurethane dispersions. J. Clean. Prod. 2019, 241, 118341. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Luo, L.; Ding, Q.; Tang, S. Bio-orthogonally crosslinked catechol–chitosan hydrogel for effective hemostasis and wound healing. Carbohydr. Polym. 2022, 281, 119039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhou, X.; Mo, Z.; Zeng, Z.; Wang, Z.; Cai, Z.; Luo, L.; Ding, Q.; Li, H.; Tang, S. A PEG-CMC-THB-PRTM hydrogel with antibacterial and hemostatic properties for promoting wound healing. Int. J. Biol. Macromol. 2023, 224, 370–379. [Google Scholar] [CrossRef]
- Peng, L.; Chang, L.; Si, M.; Lin, J.; Wei, Y.; Wang, S.; Liu, H.; Han, B.; Jiang, L. Hydrogel-Coated Dental Device with Adhesion-Inhibiting and Colony-Suppressing Properties. ACS Appl. Mater. Interfaces 2020, 12, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wu, G.; Zhou, H.; Qian, K.; Hu, J. Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Falqi, F.H.; Bin-Dahman, O.A.; Khair, A.; Al-Harthi, M.A. PVA/PEG/graphene shape memory composites responsive to multi-stimuli. Appl. Phys. A 2022, 128, 427. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Zhang, Y.; Zhang, S.; Wang, W.; Jin, X.; Tang, B. Novel bio-based phase change materials with high enthalpy for thermal energy storage. Appl. Energy 2020, 268, 114979. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Zhang, X.; Su, P.; Yue, F.; Zeng, S.; Du, S. Improved surface adhesion and wound healing effect of madecassoside liposomes modified by temperature-responsive PEG-PCL-PEG copolymers. Eur. J. Pharm. Sci. 2020, 151, 105373. [Google Scholar] [CrossRef]
- Qayyum, A.S.; Jain, E.; Kolar, G.; Kim, Y.; Sell, S.A.; Zustiak, S.P. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 2017, 9, 025019. [Google Scholar] [CrossRef]
- Chen, Q.; Passos, A.; Balabani, S.; Chivu, A.; Zhao, S.; Azevedo, H.S.; Butler, P.; Song, W. Semi-interpenetrating network hyaluronic acid microgel delivery systems in micro-flow. J. Colloid Interface Sci. 2018, 519, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Kang, Y.; Eom, Y.; Torati, S.R.; Kim, C. Functionalization of Biotinylated Polyethylene Glycol on Live Magnetotactic Bacteria Carriers for Improved Stealth Properties. Biology 2021, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Anada, T.; Kobayashi, S.; Ueda, T.; Tanaka, M. Effect of bound water content on cell adhesion strength to water-insoluble polymers. Acta Biomater. 2021, 134, 313–324. [Google Scholar] [CrossRef]
- Krishnadoss, V.; Melillo, A.; Kanjilal, B.; Hannah, T.; Ellis, E.; Kapetanakis, A.; Hazelton, J.; San Roman, J.; Masoumi, A.; Leijten, J.; et al. Bioionic Liquid Conjugation as Universal Approach To Engineer Hemostatic Bioadhesives. ACS Appl. Mater. Interfaces 2019, 11, 38373–38384. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Meng, Q. Adhesive polyethylene glycol-based hydrogel patch for tissue repair. Colloids Surfaces B Biointerfaces 2022, 218, 112751. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.Y.; Zou, M.L.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Yuan, Z.D.; Wu, J.J.; Ye, J.X.; Yu, S.; Li, X.; et al. Dual-Action Icariin-Containing Thermosensitive Hydrogel for Wound Macrophage Polarization and Hair-Follicle Neogenesis. Front. Bioeng. Biotechnol. 2022, 10, 902894. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, M.; Jang, H.; Kondaveeti, S.; Sun, K.; Kim, S.; Park, H.H.; Jeong, H.E. Bifunctional Amphiphilic Nanospikes with Antifogging and Antibiofouling Properties. ACS Appl. Mater. Interfaces 2022, 14, 39478–39488. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Wu, C.; Yu, D.; Ding, Q.; Li, R. Novel TEMPO-oxidized cellulose nanofiber/polyvinyl alcohol/polyethyleneimine nanoparticles for Cu2+ removal in water. Cellulose 2021, 28, 10999–11011. [Google Scholar] [CrossRef]
- Rahmani, S.; Olad, A.; Rahmani, Z. Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: Study of drug delivery behavior. Polym. Bull. 2023, 80, 4117–4138. [Google Scholar] [CrossRef]
- Takács, T.; Abdelghafour, M.M.; Lamch, Ł.; Szenti, I.; Sebők, D.; Janovák, L.; Kukovecz, Á. Facile modification of hydroxyl group containing macromolecules provides autonomously self-healing polymers through the formation of dynamic Schiff base linkages. Eur. Polym. J. 2022, 168, 111086. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Montaser, A.S.; Rehan, M.; El-Naggar, M.E. pH-Thermosensitive hydrogel based on polyvinyl alcohol/sodium alginate/N-isopropyl acrylamide composite for treating re-infected wounds. Int. J. Biol. Macromol. 2019, 124, 1016–1024. [Google Scholar] [CrossRef]
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; He, J.; Wei, X.; Li, H.; Liu, X.; Cheng, F. A polyphenol and ε-polylysine functionalized bacterial cellulose/PVA multifunctional hydrogel for wound healing. Int. J. Biol. Macromol. 2023, 247, 125663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, M.; Wang, T.; Chen, X.; Li, Q.; Zhao, X. Preparation of aloe polysaccharide/honey/PVA composite hydrogel: Antibacterial activity and promoting wound healing. Int. J. Biol. Macromol. 2022, 211, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Yang, Y.; Fan, A.; Chi, R.; Shi, J.; Zhang, X. Poly (vinyl alcohol) (PVA) hydrogel incorporated with Ag/TiO2 for rapid sterilization by photoinspired radical oxygen species and promotion of wound healing. Appl. Surf. Sci. 2019, 494, 708–720. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Fan, D.; Fu, R.; Ma, P.; Duan, Z.; Li, X.; Lei, H.; Chi, L. Construction of porous sponge-like PVA-CMC-PEG hydrogels with pH-sensitivity via phase separation for wound dressing. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 505–515. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Synthesis, Physical, Mechanical and Antibacterial Properties of Nanocomposites Based on Poly(vinyl alcohol)/Graphene Oxide–Silver Nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef]
- Jackson, J.; Burt, H.; Lange, D.; Whang, I.; Evans, R.; Plackett, D. The Design, Characterization and Antibacterial Activity of Heat and Silver Crosslinked Poly(Vinyl Alcohol) Hydrogel Forming Dressings Containing Silver Nanoparticles. Nanomaterials 2021, 11, 96. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Gradinaru, L.-M.; Morariu, S.; Plugariu, I.-A.; Gradinaru, R.V. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Funct. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Y.; Zeng, Y.; Yuan, R.; Lu, X.; Yang, X.; Wei, E.; Cui, C. Phytic acid/tannic acid reinforced hydrogels with ultra-high strength for human motion monitoring and arrays. Soft Matter 2024, 20, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Z.; Pan, W.; Zhang, J.; Qi, Y.; Qin, Y.; Zhang, Y. Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation. e-Polymers 2023, 23, 20230056. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 2020, 134, 109853. [Google Scholar] [CrossRef]
- Souza, S.O.L.; Cotrim, M.A.P.; Oréfice, R.L.; Carvalho, S.G.; Dutra, J.A.P.; de Paula Careta, F.; Resende, J.A.; Villanova, J.C.O. Electrospun poly(ε-caprolactone) matrices containing silver sulfadiazine complexed with β-cyclodextrin as a new pharmaceutical dosage form to wound healing: Preliminary physicochemical and biological evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 67. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; James, J.; Grohens, Y.; Kalarikkal, N.; Thomas, S. Additive Manufacturing of Poly (ε-Caprolactone) for Tissue Engineering. JOM 2020, 72, 4127–4138. [Google Scholar] [CrossRef]
- Thangunpai, K.; Hu, D.; Kajiyama, M.; Neves, M.A.; Enomae, T. Effects of Grafting Maleic Anhydride onto Poly-ɛ-caprolactone on Facilitative Enzymatic Hydrolysis. Macromol. Mater. Eng. 2023, 308, 2300067. [Google Scholar] [CrossRef]
- Salehi, M.; Niyakan, M.; Ehterami, A.; Haghi-Daredeh, S.; Nazarnezhad, S.; Abbaszadeh-Goudarzi, G.; Vaez, A.; Hashemi, S.F.; Rezaei, N.; Mousavi, S.R. Porous electrospun poly(ε-caprolactone)/gelatin nanofibrous mat containing cinnamon for wound healing application: In vitro and in vivo study. Biomed. Eng. Lett. 2019, 10, 149–161. [Google Scholar] [CrossRef]
- Van Rie, J.; Declercq, H.; Van Hoorick, J.; Dierick, M.; Van Hoorebeke, L.; Cornelissen, R.; Thienpont, H.; Dubruel, P.; Van Vlierberghe, S. Cryogel-PCL combination scaffolds for bone tissue repair. J. Mater. Sci. Mater. Med. 2015, 26, 123. [Google Scholar] [CrossRef]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Alvarez, V.A. Eco-friendly films prepared from plantain flour/PCL blends under reactive extrusion conditions using zirconium octanoate as a catalyst. Carbohydr. Polym. 2017, 178, 260–269. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Schlachet, I.; Rouxel, D.; Menu, P.; Sosnik, A. Chitosan ascorbate hydrogel improves water uptake capacity and cell adhesion of electrospun poly(epsilon-caprolactone) membranes. Int. J. Pharm. 2019, 559, 420–426. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yuan, X.; Ma, L.; Bi, B.; Zhu, H.; Lei, Z.; Liu, W.; Pu, H.X.; Jiang, J.; Jiang, X.; et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone) /nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics 2020, 10, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Ratwatte, R.; Booth, M.A.; Tran, H.M.; Tran, P.A. High Nanodiamond Content-PCL Composite for Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Parmar, V.S.; Malhotra, S.; Chhillar, A.K. Biodegradable diblock copolymeric PEG-PCL nanoparticles: Synthesis, characterization and applications as anticancer drug delivery agents. Polymer 2020, 207, 122901. [Google Scholar] [CrossRef]
- Rangel, A.; Nguyen, T.N.; Egles, C.; Migonney, V. Different real-time degradation scenarios of functionalized poly(ε-caprolactone) for biomedical applications. J. Appl. Polym. Sci. 2021, 138, 50479. [Google Scholar] [CrossRef]
- Arbade, G.K.; Srivastava, J.; Tripathi, V.; Lenka, N.; Patro, T.U. Enhancement of hydrophilicity, biocompatibility and biodegradability of poly(ε-caprolactone) electrospun nanofiber scaffolds using poly(ethylene glycol) and poly(L-lactide-co-ε-caprolactone-co-glycolide) as additives for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 1648–1670. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, D.; Ahmed, N.; Zafar, A.; Shah, K.U.; ur Rehman, A. Lipase-sensitive fusidic acid polymeric nanoparticles based hydrogel for on-demand delivery against MRSA-infected burn wounds. J. Drug Deliv. Sci. Technol. 2023, 80, 104110. [Google Scholar] [CrossRef]
- Gupta, A.; Upadhyay, N.K.; Parthasarathy, S.; Rajagopal, C.; Roy, P.K. Nitrofurazone-loaded PVA–PEG semi-IPN for application as hydrogel dressing for normal and burn wounds. J. Appl. Polym. Sci. 2013, 128, 4031–4039. [Google Scholar] [CrossRef]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and physico-chemical properties of poly(N-vinyl pyrrolidone)-based hydrogels with titania nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, L.; Liang, F.C.; Zhang, Z.X.; Cho, C.J.; Ercan, E.; Chueh, C.C.; Chen, W.C.; Borsali, R.; Kuo, C.C. Improving the Performance and Stability of Perovskite Light-Emitting Diodes by a Polymeric Nanothick Interlayer-Assisted Grain Control Process. ACS Omega 2020, 5, 8972–8981. [Google Scholar] [CrossRef]
- Nam, H.G.; Nam, M.G.; Yoo, P.J.; Kim, J.-H. Hydrogen bonding-based strongly adhesive coacervate hydrogels synthesized using poly(N-vinylpyrrolidone) and tannic acid. Soft Matter 2019, 15, 785–791. [Google Scholar] [CrossRef]
- Kouser, S.; Prabhu, A.; Prashantha, K.; Nagaraja, G.K.; D’souza, J.N.; Meghana Navada, K.; Qurashi, A.; Manasa, D.J. Modified halloysite nanotubes with Chitosan incorporated PVA/PVP bionanocomposite films: Thermal, mechanical properties and biocompatibility for tissue engineering. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 634, 127941. [Google Scholar] [CrossRef]
- Sun, S.; Hao, M.; Ding, C.; Zhang, J.; Ding, Q.; Zhang, Y.; Zhao, Y.; Liu, W. SF/PVP nanofiber wound dressings loaded with phlorizin: Preparation, characterization, in vivo and in vitro evaluation. Colloids Surfaces B Biointerfaces 2022, 217, 112692. [Google Scholar] [CrossRef]
- Ajaz, N.; Khan, I.U.; Khalid, I.; Khan, R.U.; Khan, H.A.; Asghar, S.; Khalid, S.H.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; et al. In vitro and toxicological assessment of dexamethasone sodium phosphate loaded pH sensitive Pectin-g-poly(AA)/PVP semi interpenetrating network. Mater. Today Commun. 2020, 25, 101325. [Google Scholar] [CrossRef]
- Pushp, P.; Bhaskar, R.; Kelkar, S.; Sharma, N.; Pathak, D.; Gupta, M.K. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol. Bioeng. 2021, 118, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-W.; Liu, H.; Ying, J.; Liu, C.-T.; Shen, C.-Y.; Wang, Y.-M. Effect of Physical Aging on Heterogeneity of Poly(ε-caprolactone) Toughening Poly(lactic acid) Probed by Nanomechanical Mapping. Chin. J. Polym. Sci. 2023, 41, 143–152. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Wang, X.Y.; Wu, Z.Y.; Weng, Y.X. Kinetic analysis of PGA/PBAT plastic films for strawberry fruit preservation quality and enzyme activity. J. Food Compos. Anal. 2022, 108, 104439. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Ren, Y.; Wang, R.; Sun, M.; Liang, W. Enhancing melt strength of polyglycolic acid by reactive extrusion with chain extenders. J. Appl. Polym. Sci. 2022, 139, 51796. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; de Lucas, A.; Rodríguez, J.; García, M. Measurement, correlation and modelling of high-pressure phase equilibrium of PLGA solutions in CO2. J. Supercrit. Fluids 2020, 155, 104637. [Google Scholar] [CrossRef]
- Zuhour, M.; Güneş, C.; Fındık, S.; Dündar, M.A.; Gök, O.; Altuntaş, Z. Effect of methylprednisolone loaded poly lactic-co-glycolic acid (PLGA) bioabsorbable nanofibers on tendon healing and adhesion formation. J. Drug Deliv. Sci. Technol. 2023, 89, 104988. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three Dimensional Printing Bilayer Membrane Scaffold Promotes Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, e1703509. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, e1800069. [Google Scholar] [CrossRef]
- Dong, M.; Mao, Y.; Zhao, Z.; Zhang, J.; Zhu, L.; Chen, L.; Cao, L. Novel fabrication of antibiotic containing multifunctional silk fibroin injectable hydrogel dressing to enhance bactericidal action and wound healing efficiency on burn wound: In vitro and in vivo evaluations. Int. Wound J. 2022, 19, 679–691. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Z.; Cai, K. Recent advances in 3D hydrogel culture systems for mesenchymal stem cell-based therapy and cell behavior regulation. J. Mater. Chem. B 2022, 10, 1486–1507. [Google Scholar] [CrossRef]
- Morales, X.; Cortés-Domínguez, I.; Ortiz-De-Solorzano, C. Modeling the Mechanobiology of Cancer Cell Migration Using 3D Biomimetic Hydrogels. Gels 2021, 7, 17. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, X.; Lei, X.; Tan, J.; Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burn. Trauma 2021, 9, tkab002. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall’ Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]




| Critical Attributes | Justification |
|---|---|
| Moisture | Ensuring a balanced and moist environment to promote cell migration and proliferation. It is considered as key factor for wound healing. (supplying moisture to the dry wounds and absorbing moisture and exudates from wet wounds) |
| Absorption | Controlling the level of wound exudate and preventing tissue maceration by effectively absorbing excess fluid |
| Permeability | Facilitating gaseous exchange (water vapor, O2, CO2) to the wound bed to accelerate cellular activity |
| Protection | Preventing microbial infections that could impede the wound healing process and prolong its duration |
| Transparency | Enabling clinicians to visualize the wound and monitor the healing process |
| Mechanical robustness | Mimicking the structure of native skin while being rigid enough to allow fixation on the wound |
| Flexibility and elasticity | Adapting to body movements and minimizing patient discomfort and pain during application and dressing replacement |
| Adhesiveness | Providing good adhesion to healthy skin without sticking to the wound itself, facilitating easy removal after re-epithelialization, and preventing secondary injuries in the newly formed tissue |
| Biocompatibility | Minimizing the risk of immune reactions or side effects |
| Safety | Ensuring the absence of toxic substances that could cause damage or result in dire consequences; ensuring that products resulting from degradation are safe and follow the normal metabolic pathway |
| Cost-effectiveness | Supplying a cost-effective wound dressing solution |
| Availability | Widely available to all patients and healthcare centers |
| Polymers | Main Natural Sources/Chemical Synthesis Pathways | References | |
|---|---|---|---|
| Natural polymers | Collagen | Bovine, porcine, red algae, fish, from species such as Prionace glauca, Oreochromis niloticus, and Lophius litulon, octopuses, starfish, jellyfish such as Rhopilema esculentum, polar bears, whales, seals, and marine sponges | [4,42] |
| Gelatin | Bovine, porcine, caprine, mammalian tissues, squid, sponges, jellyfish, and snails | [17] | |
| Silk Fibroin | Cocoons of Mulberry silkworm Bombyx mori and the non-mulberry silkworm Antheraea assama | [43] | |
| Alginate | Brown marine algae such as Laminaria, Ascophyllum, and Macrocystis, red and green marine macroalgae, or bacteria like Pseudomonas and Azotobacter | [1,34,38,44] | |
| Hyaluronic acid | Human vitreous humor, joints in the umbilical cord, connective tissue | [45] | |
| Bacterial cellulose | Bacteria strains such as Gluconacetobacter, Rhizobium, Agrobacterium, Sarcina, and Rhodobacter | [1,16,46,47] | |
| Dextran | Bacteria, such as Leuconostoc, Weissella, Lactobacillus, and Streptococcus | [48,49] | |
| Chitosan | Insects, marine invertebrates, and crustaceans such as crabs, shrimp, and lobster, as well as in the cell walls of fungi | [9,16,50,51,52,53] | |
| Synthetic polymers | Polyurethane | Result from the polyaddition polymerization between polyols (molecules containing hydroxyl groups) and isocyanates, in the presence of a chain extender and a catalyst | [54,55,56] |
| Polyethylene glycol | Result from the condensation of ethylene oxide and water | [57,58] | |
| Polyvinyl alcohol | Results from the hydrolysis, aminolysis, or alcoholysis of vinyl acetate | [59] | |
| Poly-ε-caprolactone | Results from the ring-opening polymerization of ε-caprolactone | [60] | |
| Poly-N-vinylpyrrolidone | Results from high-pressure free-radical polymerizations | [61] | |
| Poly(lactic-co-glycolic) acid | Results from the blend of polylactic acid (derives from lactic acid and results from renewable sources such as corn, starch, or sugarcane) and polyglycolic acid (results from the hydrating carbonylation of formaldehyde, with H2SO4 as a catalyst, or from the pyrolysis of renewable sources such as sugar beets, sugarcane, cantaloupe, and grapes) | [9,54,60,62] | |
| Polymers | Critical Attributes | Eco-Friendly | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Absorption | Permeability | Protection | Transparency | Mechanical Robustness | Flexibility and Elasticity | Adhesiveness | Biocompatibility | Safety | Cost-Effectiveness | Availability | ||||
| Natural polymers | Collagen | ++ | ++ | NA | + | +++ | + | ++ | + | +++ | ++ | + | ++ | √ | [4,16,33,37,38,42,64,67,68,69,70,73,75,76,78,79] |
| Gelatin | ++ | +++ | NA | + | +++ | + | ++ | + | +++ | +++ | ++ | ++ | √ | [17,27,31,67,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,99] | |
| Silk Fibroin | ++ | ++ | ++ | + | CV | +++ | +++ | ++ | +++ | +++ | ++ | ++ | √ | [16,17,54,57,60,102,103,104,105,106,107,108,109,110,112,113,114,115,116,117,119,120,121,122,123,124,125,126,127] | |
| Alginate | ++ | +++ | ++ | + | +++ | + | ++ | + | +++ | +++ | ++ | ++ | √ | [1,16,21,34,38,39,44,53,128,129,130,131,132,133,134] | |
| Hyaluronic acid | ++ | +++ | ++ | + | +++ | + | ++ | + | +++ | +++ | + | ++ | √ | [7,9,19,45,137,138,140,141,142,143,144,145,146,147] | |
| Bacterial cellulose | ++ | +++ | ++ | + | NA | +++ | ++ | NA | +++ | +++ | ++ | +++ | √ | [8,16,36,46,47,51,52,130,131,149,150,151,152,153,154,155] | |
| Dextran | ++ | +++ | NA | + | +++ | + | ++ | + | +++ | +++ | ++ | ++ | √ | [130,133,158,159,160,161,162,167,168,169,170,171,172,173,174] | |
| Chitosan | ++ | ++ | ++ | ++ | NA | + | ++ | +++ | +++ | +++ | ++ | +++ | √ | [9,11,16,38,46,50,51,52,53,67,78,98,130,131,132,133,134,137,154,176,177,178,179,180,181] | |
| Synthetic polymers | Polyurethane | NA | NA * | ++ | + | +++ | +++ | +++ | + | +++ | + | +++ | NA | X | [9,39,54,55,60,186,187,188,190,191,192,193,194,195,199,202] |
| Polyethylene glycol | ++ | +++ | ++ | + | +++ | CV | ++ | + | +++ | +++ | ++ | NA | √ | [2,17,19,30,41,54,57,58,60,209,210,211,212,213,214,215,216,223,224,226] | |
| Polyvinyl alcohol | ++ | +++ | ++ | + | ++ | CV | + | CV | +++ | +++ | ++ | NA | √ | [11,17,39,40,59,60,86,119,215,227,228,229,230,231,232,233,234,235,236,237,243,244] | |
| Poly-ε-caprolactone | NA | + | + | + | + | +++ | ++ | + | ++ | +++ | ++ | NA | √ | [39,40,54,59,60,237,247,248,249,251,252,253,254] | |
| Poly-N-vinylpyrrolidone | NA | CV | +++ | + | +++ | CV | NA | ++ | ++ | +++ | ++ | NA | √ | [17,39,40,54,262,263,264] | |
| Poly(lactic-co-glycolic) acid | - | - | + | NA ** | NA | ++ | NA | + | ++ | +++ | NA | NA | √ | [40,54,57,60,237,272,273,274] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. https://doi.org/10.3390/gels10030188
Ribeiro M, Simões M, Vitorino C, Mascarenhas-Melo F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels. 2024; 10(3):188. https://doi.org/10.3390/gels10030188
Chicago/Turabian StyleRibeiro, Mariana, Marco Simões, Carla Vitorino, and Filipa Mascarenhas-Melo. 2024. "Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential" Gels 10, no. 3: 188. https://doi.org/10.3390/gels10030188