Study on Carbonation of Porcine Blood Hydrogel in the Composite Mortar of Ancient Chinese Architectural Painting
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Factors Related to the Formation of Porcine Blood Hydrogel
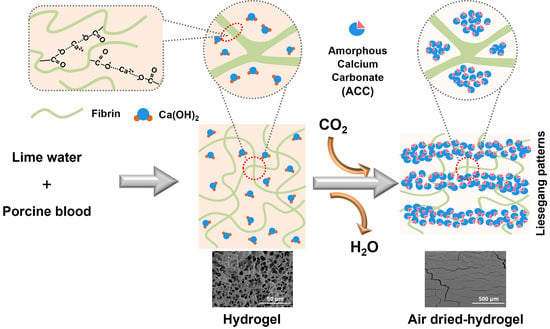
2.2. Surface Carbonation on the Porcine Blood Hydrogel during Air-Drying
2.3. Internal Carbonation in Porcine Blood Hydrogel during Air-Drying
2.4. Distribution Features of Carbonation in the Porcine Blood Hydrogel
3. Conclusions
- (1)
- Porcine blood mixed with lime water at the ratio found in the recipe can form a hydrogel, and the formed hydrogel has a hydrophobic surface. It can be predicted that these features may be potentially beneficial not only for easily dispersing porcine blood in hydrophobic tung oil in the preparation of composite mortar but also for enhancing the affinity between porcine blood and hydrophobic tung oil.
- (2)
- The lime water in porcine blood hydrogel can form calcium carbonate, nanocrystalline calcium carbonate, and amorphous calcium carbonate (ACC) in plain air. Calcite crystallites with highly terraced facets and distorted rhombohedral morphologies are formed when Ca2+ ions are present in a region with larger pores. In regions with networks of porcine blood hydrogel containing large amounts of Ca(OH)2, calcite aggregates with layered, ladder-like structures can form. Small, round calcite crystals are produced by Ca2+ ions in a region with a rich network of porcine blood hydrogel containing a lower amount of Ca(OH)2. ACC is primarily formed when Ca2+ ions are confined within hydrogel networks.
- (3)
- Lime water in porcine blood hydrogel can become calcium carbonate via standing in plain air, and the formed carbonate can be used as a filler in the composite mortar to enhance its mechanical performance.
- (4)
- The calcium carbonate formed in the porcine blood hydrogel exhibits a lamellar distribution. As a constituent material dispersed in the composite mortar, the layered distribution of calcium carbonate in the porcine blood hydrogel may be beneficial to reduce the internal stress of the composite mortar material.
4. Materials and Methods
4.1. Materials
4.2. Preparation of Carbonized Porcine Blood Hydrogel
4.3. Preparation of Samples Used in the Investigation
4.4. Analytical Method and Instrumentation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Ma, X.; Zhang, B. Analytical Investigations of Traditional Masonry Mortars from Ancient City Walls Built during Ming and Qing Dynasties in China. Int. J. Archit. Herit. 2016, 10, 663–673. [Google Scholar] [CrossRef]
- Weng, X.; Zhang, B.; Li, S. Analysis of Construction Materials of Xiongnu Tongwan City, Built 1600 Years Ago. Stud. Conserv. 2021, 66, 413–422. [Google Scholar] [CrossRef]
- Daodao, H.; Yuhu, L.; Juan, L.; Huiyun, L.; Xiaoqin, H.; Tao, M. Some scientific information in technical process of colored drawing on Chinese ancent building. Relics Museolgy 2009, 6, 435–450. [Google Scholar]
- Peifan, Q.; Deqi, Y.; Qi, M.; Aijun, S.; Jingqi, S.; Zengjun, Z.; Jianwei, H. Study and Restoration of the Yi Ma Wu Hui Layer of the Ancient Coating on the Putuo Zongcheng Temple. Int. J. Archit. Herit. 2021, 15, 1707–1721. [Google Scholar] [CrossRef]
- Amadori, M.L.; Mengacci, V.; Vagnini, M.; Casoli, A.; Holakooei, P.; Eftekhari, N.; Lin, K.; Maekawa, Y.; Germinario, G. Organic Matter and Pigments in the Wall Paintings of Me-Taw-Ya Temple in Bagan Valley, Myanmar. Appl. Sci. 2021, 11, 11441. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, P.; Zhu, H.; Zhang, B.; Hu, Y. A thorough detection of the mortar materials for Buddhist buildings in Bagan, Myanmar. Eur. Phys. J. Plus 2023, 138, 151. [Google Scholar] [CrossRef]
- Jia, M.; Wei, G.; Grifa, C.; Zhang, J.; Cui, B.; Li, M.; Xu, J.; Ma, X. Characterization of archaeological lime mortars in a Ming dynasty tomb: A multi-analytical approach. Archaeometry 2023, 65, 736–753. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, H.; Zhang, B.; Wei, G.; Li, G.; Zhou, Y. A study of the Chinese organic–inorganic hybrid sealing material used in “Huaguang No.1” ancient wooden ship. Thermochim. Acta 2013, 551, 20–26. [Google Scholar] [CrossRef]
- Weng, X.; Ma, M.; Zhang, B. Detection of early Chinese organic-inorganic composite lime surface from the Lushanmao site, 4300 years ago. J. Cult. Herit 2021, 52, 128–133. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, H.; Wang, H.; Fang, S.; Zhang, B.; Yang, F. An experimental study on application of sticky rice–lime mortar in conservation of the stone tower in the Xiangji Temple. Constr. Build. Mater. 2012, 28, 624–632. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, G.; Pant, R.; Shen, J.; Liu, M.; Luo, H. Nanoindentation Characterization of a Ternary Clay-Based Composite Used in Ancient Chinese Construction. Materials 2016, 9, 866. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhang, B.; Hu, Y.; Wan, J.; Zhang, J. Comprehensive test of tabia at the Humen Fort sites in Guangdong, China. J. Cult. Herit. 2021, 49, 298–304. [Google Scholar] [CrossRef]
- Zhang, K.; Sui, Y.; Wang, L.; Tie, F.; Yang, F.; Liu, Y.; Zhang, Y. Effects of sticky rice addition on the properties of lime-tile dust mortars. Herit. Sci. 2021, 9, 4. [Google Scholar] [CrossRef]
- Wei, G.F.; Fang, S.Q.; Li, Z.G.; Zhang, B.J. Physical properties and microscopic structure of tung oil-lime putty. J. Build. Eng. 2013, 16, 469–474. [Google Scholar]
- Fang, S.; Zhang, H.; Zhang, B.; Guoqing, L. A study of Tung-oil–lime putty—A traditional lime based mortar. Int. J. Adhes. Adhes. 2014, 48, 224–230. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.J.; Fang, S.Q. The application history and scientific nature of blood-based materials in traditional Chinese mortar. J. Archaeol. Sci. 2013, 25, 94–102. [Google Scholar]
- Zhao, P.; Zhang, Y.S.; Li, G.Y. Understanding and assessment of ancient Chinese pig blood–lime mortar. Adv. Mater. 2014, 3356, 446–449. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Zhang, H.; Zhang, B. A study of traditional blood lime mortar for restoration of ancient buildings. Cem. Concr. Res. 2015, 76, 232–241. [Google Scholar] [CrossRef]
- Zhang, K.; Grimoldi, A.; Rampazzi, L.; Sansonetti, A.; Corti, C. Contribution of thermal analysis in the characterization of lime-based mortars with oxblood addition. Thermochim. Acta 2019, 678, 178303. [Google Scholar] [CrossRef]
- Fang, S.Q.; Yang, T.; Zhang, B.J.; Wei, G.F. Impacts of sugar and egg-white as additives on the performance of traditional lime mortar and their mechanisms. Sci. Sin. Technol. 2015, 45, 865–873. [Google Scholar]
- Zhang, K.; Fang, S.Q.; Zhang, B.J. The application history and scientificity of traditional Chinese egg white mortar. Sci. Sin. Technol. 2015, 45, 635–642. [Google Scholar] [CrossRef]
- Centauro, I.; Cantisani, E.; Grandin, C.; Salvini, A.; Vettori, S. The Influence of Natural Organic Materials on the Properties of Traditional Lime-Based Mortars. Int. J. Archit. Herit. 2017, 11, 670–684. [Google Scholar] [CrossRef]
- Shivakumar, M.; Selvaraj, T. A scientific study on the role of organic lime mortars of Padmanabhapuram Palace, Thuckalay, Tamilnadu, India. Eur. Phys. J. Plus 2020, 135, 923. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, B.; Liang, X. A case study and mechanism investigation of typical mortars used on ancient architecture in China. Thermochim. Acta 2008, 473, 1–6. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Pan, C.; Zeng, Y. Traditional mortar represented by sticky rice lime mortar—One of the great inventions in ancient China. Sci. China Technol. Sci. 2009, 52, 1641–1647. [Google Scholar] [CrossRef]
- Peng, H.T.; Zhang, Q.; Li, N.S.; Wng, D.F. Effect of Sticky Rice Paste on Properties of Tabia Used to Repair Ancient Earthen Ruins. J. Build. Mater. 2011, 14, 718–722. [Google Scholar]
- Zhao, P.; Jackson, M.D.; Zhang, Y.; Li, G.; Monteiro, P.J.M.; Yang, L. Material characteristics of ancient Chinese lime binder and experimental reproductions with organic admixtures. Constr. Build Mater. 2015, 84, 477–488. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B. Why Ancient Chinese People Like to Use Organic–Inorganic Composite Mortars?—Application History and Reasons of Organic–Inorganic Mortars in Ancient Chinese Buildings. J. Archaeol. Method Theory 2019, 26, 502–536. [Google Scholar] [CrossRef]
- Fei, M.; Tang, X.; Yu, Y.; Li, K.; Fan, Z.; Xiang, Q. Synergistic Improvement Earthen Mortars with Tung Oil, Sticky Rice Pulp, and Aerial Lime: Application for the Conservation of Rammed Earth Structures Damaged by Exfoliation. Int. J. Archit. Herit. 2023, 1–16. [Google Scholar] [CrossRef]
- Lin, H.; Gunasekaran, S. Cow blood adhesive: Characterization of physicochemical and adhesion properties. Int. J. Adhes. Adhes. 2010, 30, 139–144. [Google Scholar] [CrossRef]
- Lee, Y.; Martin, S.; Oc, L.; Sánchez, I.; Bolívar-Galiano, F.; Doménech-Carbó, M. Evaluation of a gelatin-based adhesive for historic paintings that incorporates citronella oil as an eco-friendly biocide. J. Adhes. Sci. Technol. 2018, 32, 2320–2349. [Google Scholar] [CrossRef]
- Coradin, T.; Wang, K.; Law, T.; Trichet, L. Type I Collagen-Fibrin Mixed Hydrogels: Preparation, Properties and Biomedical Applications. Gels 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Isobe, K.; Tsujino, T.; Koyata, Y.; Ohyagi, F.; Watanabe, T.; Nakamura, M.; Kitamura, Y.; Okudera, H.; Nakata, K.; et al. Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int. J. Implant Dent. 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.; Li, J.; Xu, M.; Shao, Y.; Tu, Y. Formation mechanism of ovalbumin gel induced by alkali. Food Hydrocoll. 2016, 61, 390–398. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, B.A.; Strong, D.D.; Watt, K.W.; Doolittle, R.F. Amino acid sequence studies on the alpha chain of human fibrinogen. Exact location of cross-linking acceptor sites. Biochemistry 1979, 18, 5405–5410. [Google Scholar] [CrossRef] [PubMed]
- Côté, H.C.; Lord, S.T.; Pratt, K.P. γ-Chain Dysfibrinogenemias: Molecular Structure-Function Relationships of Naturally Occurring Mutations in the γ Chain of Human Fibrinogen. Blood 1998, 92, 2195–2212. [Google Scholar] [CrossRef]
- Shen, F.H.; Feng, Q.L.; Wang, C.M. The modulation of collagen on crystal morphology of calcium carbonate. J. Cryst. Growth 2002, 242, 239–244. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, F.; Zhang, J.; Yang, L.; Shi, X.; Fang, Q.; Ma, X. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids. Res. Chem. Intermed. 2013, 39, 2407–2415. [Google Scholar] [CrossRef]
- Su, L.; Liang, X.; Zhou, Q.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Structural characterization of amorphous calcium carbonate-binding protein: An insight into the mechanism of amorphous calcium carbonate formation. Biochem. J. 2013, 453, 179–186. [Google Scholar] [CrossRef]
- Qingling, F. Principles of calcium-based biomineralization. Prog. Mol. Subcell. Biol. 2011, 52, 141–197. [Google Scholar]
- Parvinzadeh Gashti, M.; Stir, M.; Bourquin, M.; Hulliger, J. Mineralization of Calcium Phosphate Crystals in Starch Template Inducing a Brushite Kidney Stone Biomimetic Composite. Cryst. Growth Des. 2013, 13, 2166–2173. [Google Scholar] [CrossRef]
- Polowczyk, I.; Fiedot, M.; Bastrzyk, A. Protein-Mediated Precipitation of Calcium Carbonate. Materials 2016, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ma, W.; Wang, L.; Wan, P.; Hu, J.; Cao, L. Control over the crystal phase, shape, size and aggregation of calcium carbonate via a l-aspartic acid inducing process. Biomaterials 2004, 25, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Liu, C.; Yang, D.; Yan, Y.; Chen, Y.; Huang, J.; Liu, Y.; Zheng, G.; Xie, L.; Zhang, R. Alv Protein Plays Opposite Roles in the Transition of Amorphous Calcium Carbonate to Calcite and Aragonite during Shell Formation. Cryst. Growth Des. 2018, 18, 3794–3804. [Google Scholar] [CrossRef]
- Lopez-Berganza, J.A.; Chen, S.; Espinosa-Marzal, R.M. Tailoring Calcite Growth through an Amorphous Precursor in a Hydrogel Environment. Cryst. Growth Des. 2019, 19, 3192–3205. [Google Scholar] [CrossRef]
- Greer, H.F.; Zhou, W.; Guo, L. Reversed Crystal Growth of Calcite in Naturally Occurring Travertine Crust. Crystals 2017, 7, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, Y.; Li, B.; Wang, C.; Han, Z.; Weng, L. Reaction-Diffusion Process for Hydrogels with a Tailored Layer Structure. Processes 2023, 11, 1975. [Google Scholar] [CrossRef]
- Yu, J.; Lei, M.; Cheng, B.; Zhao, X. Effects of PAA additive and temperature on morphology of calcium carbonate particles. J. Solid State Chem. 2004, 177, 681–689. [Google Scholar] [CrossRef]
- Meldrum, F.C. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 2003, 48, 187–224. [Google Scholar] [CrossRef]
- Kosanović, C.; Falini, G.; Kralj, D. Mineralization of Calcium Carbonates in Gelling Media. Cryst. Growth Des. 2011, 11, 269–277. [Google Scholar] [CrossRef]
- Smeets, P.J.M.; Cho, K.R.; Kempen, R.G.E.; Sommerdijk, N.A.J.M.; De Yoreo, J.J. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat. Mater. 2015, 14, 394–399. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Li, H.; Keene, E.C.; Seh, Z.W.; Estroff, L.A. Crystal Growth of Calcium Carbonate in Hydrogels as a Model of Biomineralization. Adv. Funct. Mater. 2012, 22, 2891–2914. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium Carbonate Polyamorphism and Its Role in Biomineralization: How Many Amorphous Calcium Carbonates Are There? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, Via Vaterite. Nanoscale 2010, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; With, G.D.; Sommerdijk, N.A.J.M. In situ techniques in biomimetic mineralization studies of calcium carbonate. Chem. Soc. Rev. 2010, 39, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Różycka, M.; Coronado, I.; Brach, K.; Olesiak-Bańska, J.; Samoć, M.; Zarębski, M.; Dobrucki, J.; Ptak, M.; Weber, E.; Polishchuk, I.; et al. Lattice Shrinkage by Incorporation of Recombinant Starmaker-Like Protein within Bioinspired Calcium Carbonate Crystals. Chem. Eur. J. 2019, 25, 12740–12750. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Zhou, Y.; Nie, F.; Duan, X.; Pei, C. Hen eggwhite-mediated stack crystallization of calcium carbonate. J. Cryst. Growth 2010, 312, 831–836. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015, 190, 291–303. [Google Scholar] [CrossRef]
- Song, K.; Bang, J.H.; Chae, S.C.; Kim, J.; Lee, S.W. Phase and morphology of calcium carbonate precipitated by rapid mixing in the absence of additives. RSC Adv. 2022, 12, 19340–19349. [Google Scholar] [CrossRef]
- Nabika, H.; Itatani, M.; Lagzi, I. Pattern Formation in Precipitation Reactions: The Liesegang Phenomenon. Langmuir 2020, 36, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Rácz, Z. Formation of Liesegang patterns. Phys. A 1999, 274, 50–59. [Google Scholar] [CrossRef]
- Tóth, R.; Walliser, R.M.; Lagzi, I.; Boudoire, F.; Düggelin, M.; Braun, A.; Housecroft, C.E.; Constable, E.C. Probing the mystery of Liesegang band formation: Revealing the origin of self-organized dual-frequency micro and nanoparticle arrays. Soft Matter. 2016, 12, 8367–8374. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-González, O.; Campos-Escamilla, C.; Flores-Ibarra, A.; Esturau-Escofet, N.; Arreguin-Espinosa, R.; Stojanoff, V.; Cuéllar-Cruz, M.; Moreno, A. Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals 2019, 9, 443. [Google Scholar] [CrossRef]
- Cheng, C.; Zhu, Y.; Zhang, J.; Li, W.; Teri, G.; Zheng, L.; Hu, D. Mechanism for formation of porcine blood hydrogels used as additives in the mortar of traditional Chinese architectural painting. Herit. Sci. 2024, 12, 74. [Google Scholar] [CrossRef]












| Samples | Blood (g) | 1M CaCl2 (mL) | Saturated NaOH (mL) | Lime Water (g) | Form | pH |
|---|---|---|---|---|---|---|
| A | 100 | 6 | / | / | sol | 7.56 ± 0.04 |
| B | 100 | / | 2 | / | hydrogel | 11.86 ± 0.63 |
| C | 100 | 6 | 3 | / | hydrogel | 12.95 ± 0.03 |
| D | 100 | / | / | 5 | hydrogel | 12.73 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Ma, W.; Chen, R.; Zhu, Y.; Zheng, L.; Li, W.; Hu, D. Study on Carbonation of Porcine Blood Hydrogel in the Composite Mortar of Ancient Chinese Architectural Painting. Gels 2024, 10, 191. https://doi.org/10.3390/gels10030191
Cheng C, Ma W, Chen R, Zhu Y, Zheng L, Li W, Hu D. Study on Carbonation of Porcine Blood Hydrogel in the Composite Mortar of Ancient Chinese Architectural Painting. Gels. 2024; 10(3):191. https://doi.org/10.3390/gels10030191
Chicago/Turabian StyleCheng, Cong, Wenhua Ma, Rui Chen, Yeting Zhu, Lizhen Zheng, Wei Li, and Daodao Hu. 2024. "Study on Carbonation of Porcine Blood Hydrogel in the Composite Mortar of Ancient Chinese Architectural Painting" Gels 10, no. 3: 191. https://doi.org/10.3390/gels10030191