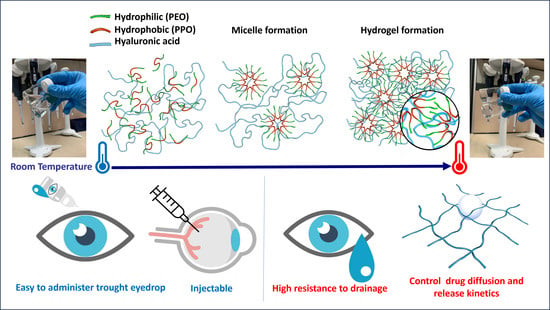
Thermosensitive In Situ Gelling Poloxamers/Hyaluronic Acid Gels for Hydrocortisone Ocular Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogel Selection and Characterization
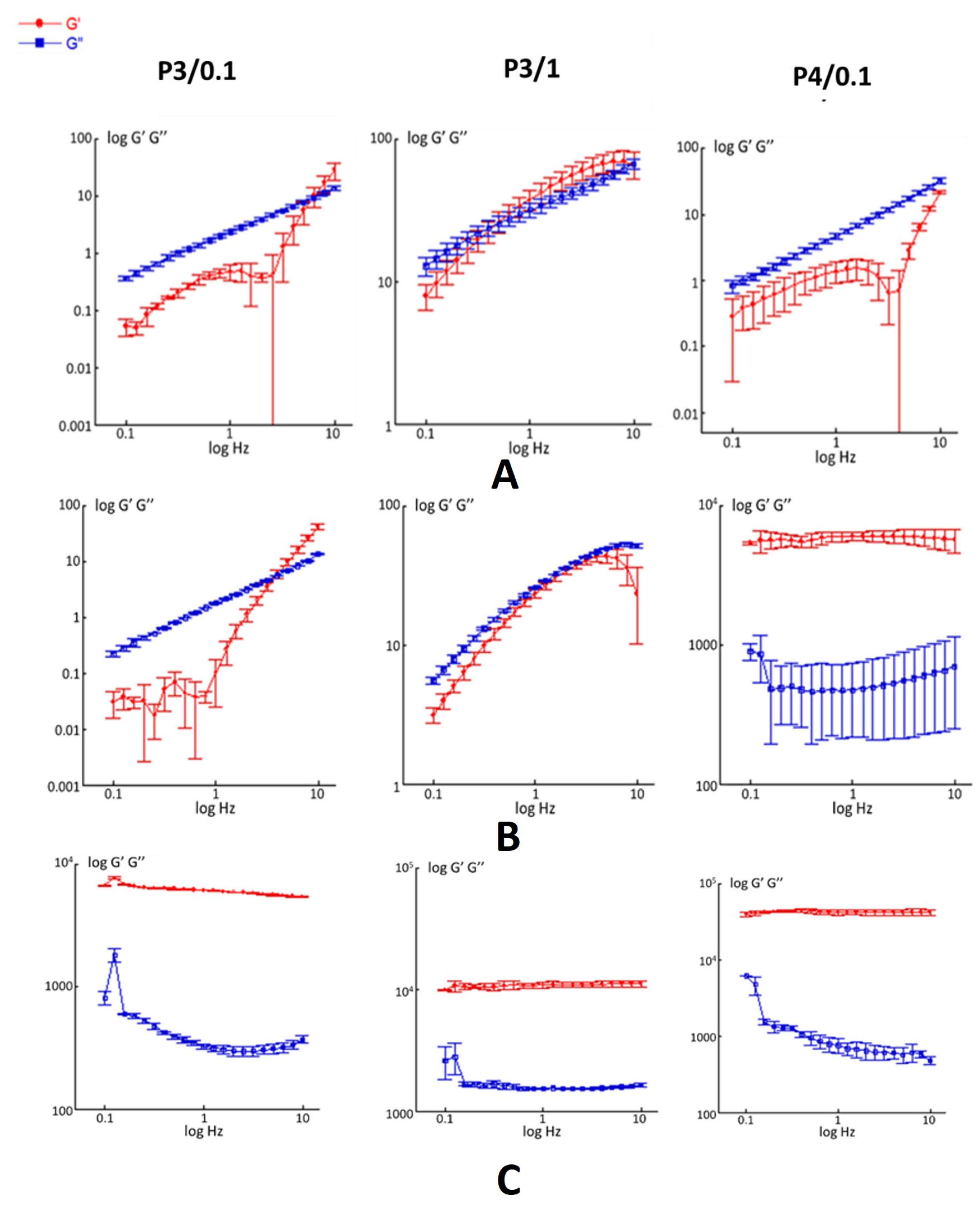
2.2. Rheological Characterization
2.3. Determination of the Gelation Temperature and Time
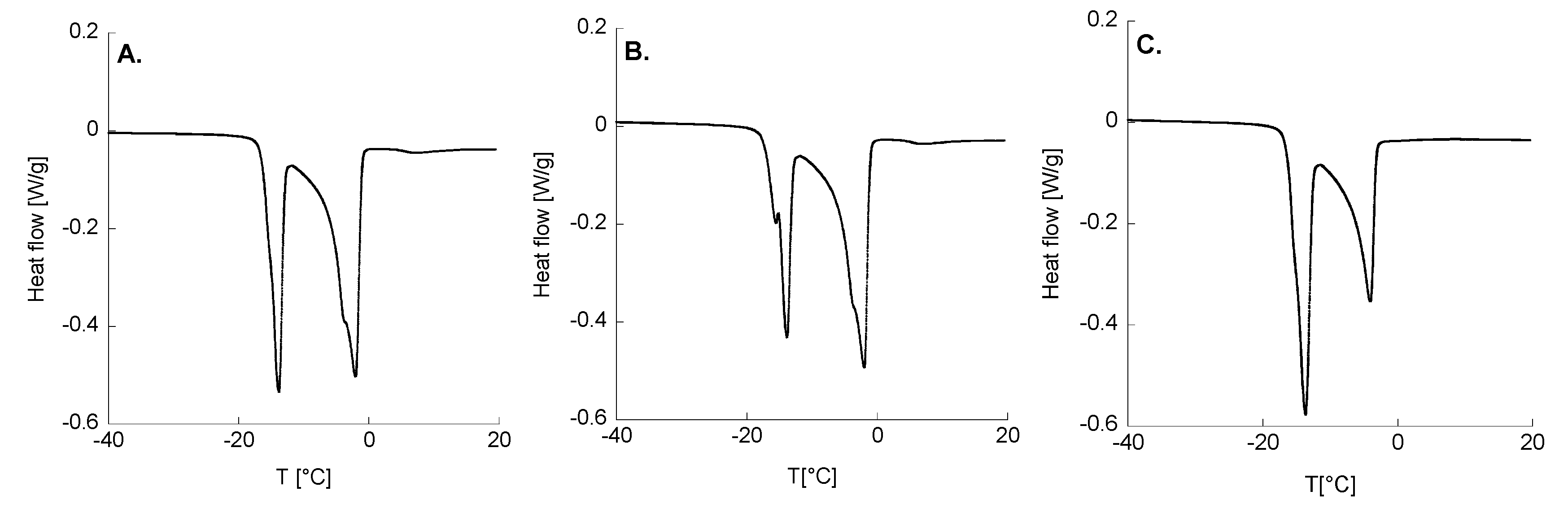
2.4. Thermal Analyses
2.5. Hydrogel Swelling and Release Kinetics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Polymeric Platforms
4.2.2. Measurements of pH and Osmolarity
4.2.3. Characterization of Hydrogels
4.2.4. Rheological Characterization
4.2.5. Determination of Temperature and Gelation Time
4.2.6. Thermodynamic Tests
4.2.7. Swelling Behavior
4.2.8. Hydrocortisone In Vitro Release Kinetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and Limitations of Drug Delivery Systems Formulated as Eye Drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to Prolong the Residence Time of Drug Delivery Systems on Ocular Surface. Adv. Colloid. Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug Delivery to the Anterior Segment of the Eye: A Review of Current and Future Treatment Strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-Targeting Drugs for the Treatment of Retinal Neovascularization in Diabetic Retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Maxwell, C.J.; Swindle-Reilly, K.E. Macro- and Microscale Properties of the Vitreous Humor to Inform Substitute Design and Intravitreal Biotransport. Curr. Eye Res. 2021, 46, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Vanrell, R.; Refojo, M.F. Biodegradable Microspheres for Vitreoretinal Drug Delivery. Adv. Drug Deliv. Rev. 2001, 52, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.L.; Lin, S.J.; Tsai, H.C.; Chan, W.H.; Tsai, C.H.; Cheng, C.H.D.; Hsiue, G.H. Mixed Micelle Systems Formed from Critical Micelle Concentration and Temperature-Sensitive Diblock Copolymers for Doxorubicin Delivery. Biomaterials 2009, 30, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.M.; Escamilla, A.P.; Ribeiro, A.C.F.; Esteso, M.A. Effect of 1,2-Propanediol on the Critical Micelle Concentration of Decyltrimethylammonium Bromide at Temperatures from 293.15 K to 308.15 K. Int. J. Mol. Sci. 2022, 23, 15884. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Biondi, M.; Quaglia, F.; Fusco, S.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Injectable Thermally Responsive Mucoadhesive Gel for Sustained Protein Delivery. Biomacromolecules 2011, 12, 28–33. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; Rotonda, M.I.L. A Novel Poloxamers/Hyaluronic Acid in Situ Forming Hydrogel for Drug Delivery: Rheological, Mucoadhesive and in Vitro Release Properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Silvestri, T.; Fusco, S.; Borzacchiello, A.; De Rosa, G.; Biondi, M. Drug Micro-Carriers with a Hyaluronic Acid Corona toward a Diffusion-Limited Aggregation within the Vitreous Body. Carbohydr. Polym. 2019, 220, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-Coated Nanoparticles for Active Tumor Targeting: Influence of Polysaccharide Molecular Weight on Cell Uptake. Colloids Surf. B Biointerfaces 2022, 210, 112240. [Google Scholar] [CrossRef] [PubMed]
- Kallab, M.; Szegedi, S.; Hommer, N.; Stegmann, H.; Kaya, S.; Werkmeister, R.M.; Schmidl, D.; Schmetterer, L.; Garhöfer, G. Topical Low Dose Preservative-Free Hydrocortisone Reduces Signs and Symptoms in Patients with Chronic Dry Eye: A Randomized Clinical Trial. Adv. Ther. 2020, 37, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; dell’Omo, R.; Gelso, A.; Rinaldi, M.; Bartollino, S.; Napolitano, P.; Russo, A.; Campagna, G.; Costagliola, C. Effects of Topical Low-Dose Preservative-Free Hydrocortisone on Intraocular Pressure in Patients Affected by Ocular Surface Disease with and without Glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Microfluidic Nanoprecipitation and Wet Milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Khawaja, A.P.; Pasquale, L.R.; Stein, J.D. A Review of Systemic Medications That May Modulate the Risk of Glaucoma. Eye 2020, 34, 12–28. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of Hydrophilic Polymeric Matrices on the Diffusion and Release of Water Soluble Drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-Based Natural Polymers as Smart Carriers for the Controlled Delivery of Timolol Maleate through the Cornea for Glaucoma. Int. J. Biol. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef]
- Silvestri, T.; D’Aria, F.; Villapiano, F.; Scala, F.; Mayol, L.; Aleo, D.; Cardullo, N.; Fraziano, S.F.; Biondi, M.; Giancola, C. Physico-chemical studies of inclusion complex between hydrocortisone and cyclodextrins. J. Mol. Liq 2023, 390, 123031. [Google Scholar] [CrossRef]




| Acronyms | F127 (% w/v) | F68 (% w/v) | HA 830 kDa (% w/v) |
|---|---|---|---|
| P1 | 15 | 10 | - |
| P2 | 15 | 15 | - |
| P3 | 21.43 | 21.43 | - |
| P4 | 30 | 30 | - |
| P1/0.1 | 15 | 10 | 0.1 |
| P1/1 | 15 | 10 | 1 |
| P1/2 | 15 | 10 | 2 |
| P2/0.1 | 15 | 15 | 0.1 |
| P2/1 | 15 | 15 | 1 |
| P2/2 | 15 | 15 | 2 |
| P3/0.1 * | 21.43 | 21.43 | 0.1 |
| P3/1 * | 21.43 | 21.43 | 1 |
| P3/2 | 21.43 | 21.43 | 2 |
| P4/0.1 * | 30 | 30 | 0.1 |
| Formulation | Ti (°C) | Tgel (°C) | Tgel (min) |
|---|---|---|---|
| P3/0.1 | 22.0 ± 2.0 | 38.3 ± 0.3 | 8.21 ± 0.30 |
| P3/1 | 22.5 ± 4.1 | 38.2 ± 0.4 | 6.33 ± 0.19 |
| P4/0.1 | 20.5 ± 2.4 | 28.7 ± 0.5 | 0.15 ± 0.05 |
| Formulation | T (°C) | G’ (Pa) ± SD | G’’ (Pa) ± SD |
|---|---|---|---|
| P3/0.1 | 4 | 0.15 ± 0.05 | 0.13 ± 0.08 |
| 25 | 0.14 ± 0.04 | 1.80 ± 0.04 | |
| 37 | (7.72 ± 1.37) × 103 | (4.82 ± 2.11) × 102 | |
| P3/1 | 4 | 38.0 ± 6.03 | 32.0 ± 3.56 |
| 25 | 23.5 ± 2.40 | 27.6 ± 0.58 | |
| 37 | (7.88 ± 3.73) × 103 | (2.74 ± 1.32) × 102 | |
| P4/0.1 | 4 | 1.38 ± 0.49 | 4.70 ± 0.41 |
| 25 | (7.87 ± 6.59) × 103 | (1.59 ± 0.57) × 103 | |
| 37 | (4.26 ± 0.02) × 104 | (5.07 ± 1.12) × 102 |
| Formulation | T (°C) | G’ (Pa) ± SD | G’’ (Pa) ± SD |
|---|---|---|---|
| P3/0.1 + HC | 4 | 0.56 ± 0.05 | 2.42 ± 0.08 |
| 25 | 1.41 ± 2.25 | 3.43 ± 2.22 | |
| 37 | (5.30 ± 0.52) × 103 | (1.71 ± 0.07) × 102 | |
| P3/1 + HC | 4 | 36.3 ± 4.78 | 30.8 ± 3.28 |
| 25 | 26.4 ± 1.83 | 30.2 ± 2.15 | |
| 37 | (1.49 ± 0.08) × 104 | (2.18 ± 0.13) × 103 | |
| P4/0.1 + HC | 4 | 1.41 ± 0.10 | 5.33 ± 0.11 |
| 25 | (6.63 ± 0.98) × 103 | (1.59 ± 0.22) × 103 | |
| 37 | (4.21 ± 0.12) × 104 | (7.20 ± 0.29) × 102 |
| Formulation | F68 (% p/V) | F127 (% p/V) | HA (% p/V) | Free H2O (% ± SD) | Bound H2O (% ± SD) | Amorphous H2O (% ± SD) |
|---|---|---|---|---|---|---|
| P3/0.1 | 21.43 | 21.43 | 0.1 | 34.8 ± 0.7 | 65.2 ± 0.9 | 32.3 ± 1.1 |
| P3/1 | 21.43 | 21.43 | 1 | 37.9 ± 6.1 | 62.1 ± 6.1 | 40.0 ± 9.8 |
| P4/0.1 | 30 | 30 | 0.1 | 54.2 ± 1.1 | 45.8 ± 1.1 | 36.3 ± 0.1 |
| Formulation | 1° Peak ENDO | 2° Peak ENDO | |||||
|---|---|---|---|---|---|---|---|
| T1 [°C] | ΔH1 [J/g] | T1 onset [°C] | T2 [°C] | ΔH2 [J/g] | T2 onset [°C] | ΔH tot [J/g] | |
| P3/0.1 | −13.7 ± 0.2 | 78.1 ± 1.1 | −15.6 ± 0.1 | −1.94 ± 0.11 | 146.6 ± 2.3 | −6.32 ± 0.00 | 224.6 ± 3.5 |
| P3/1 | −13.8 ± 0.1 | 74.6 ± 0.2 | −15.5 ± 0.1 | −2.04 ± 0.13 | 124.8 ± 32.5 | −6.32 ± 0.00 | 199.3 ± 32.7 |
| P4/0.1 | −13.8 ± 0.3 | 114.7 ± 2.1 | −16.0 ± 0.1 | −4.06 ± 0.06 | 96.7 ± 2.5 | −6.96 ± 0.40 | 211.4 ± 0.4 |
| P3/0.1 | P3/1 | P4/0.1 | |
|---|---|---|---|
| k, h−n | 0.0389 | 0.120 | 0.0533 |
| n | 0.779 | 0.665 | 0.898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villapiano, F.; Silvestri, T.; Lo Gatto, C.; Aleo, D.; Campani, V.; Graziano, S.F.; Giancola, C.; D’Aria, F.; De Rosa, G.; Biondi, M.; et al. Thermosensitive In Situ Gelling Poloxamers/Hyaluronic Acid Gels for Hydrocortisone Ocular Delivery. Gels 2024, 10, 193. https://doi.org/10.3390/gels10030193
Villapiano F, Silvestri T, Lo Gatto C, Aleo D, Campani V, Graziano SF, Giancola C, D’Aria F, De Rosa G, Biondi M, et al. Thermosensitive In Situ Gelling Poloxamers/Hyaluronic Acid Gels for Hydrocortisone Ocular Delivery. Gels. 2024; 10(3):193. https://doi.org/10.3390/gels10030193
Chicago/Turabian StyleVillapiano, Fabrizio, Teresa Silvestri, Camilla Lo Gatto, Danilo Aleo, Virginia Campani, Sossio Fabio Graziano, Concetta Giancola, Federica D’Aria, Giuseppe De Rosa, Marco Biondi, and et al. 2024. "Thermosensitive In Situ Gelling Poloxamers/Hyaluronic Acid Gels for Hydrocortisone Ocular Delivery" Gels 10, no. 3: 193. https://doi.org/10.3390/gels10030193