Cellulose Diacetate Aerogels with Low Drying Shrinkage, High-Efficient Thermal Insulation, and Superior Mechanical Strength
Abstract
:1. Introduction
2. Results and Discussion
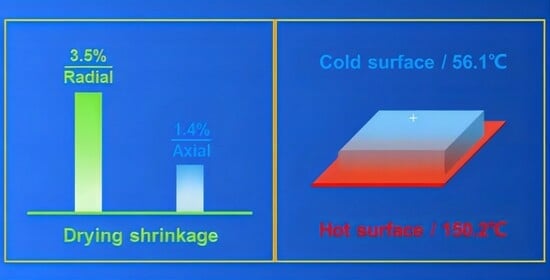
2.1. Macrostructure and Microstructural Evolution
2.2. Chemical Crosslinking Evidence
2.3. Thermal Insulation Performance
2.4. Mechanical Property
2.5. Thermal Stability Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CDAAs
4.3. Characterization Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; He, J.; Xiong, S.; Xiao, Q.; Xiao, Y.; Ding, F.; Ji, H.; Yang, Z.; Li, Z. Construction and nanostructure of chitosan/nanocellulose hybrid aerogels. Biomacromolecules 2021, 22, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yin, X.; Yu, Y.; Cai, Z.; Wang, X. Chemically functionalized natural cellulose materials for effective triboelectric nanogenerator development. Adv. Funct. Mater. 2017, 27, 1700794. [Google Scholar] [CrossRef]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Siddiqui, M.T.H.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent progress in high-strength and robust regenerated cellulose materials. Adv. Mater. 2021, 33, 2000682. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Z.; Chen, C.; Xia, Q.; Zhou, Y.; Ray, S.J.R.W.U.; Gan, W.; Li, C.; Chen, G.; Foster, B.; et al. All-natural, degradable, rolled-up straws based on cellulose micro-and nano-hybrid fibers. Adv. Funct. Mater. 2020, 30, 1910417. [Google Scholar] [CrossRef]
- Shchipunov, Y.; Postnova, I. Cellulose mineralization as a route for novel functional materials. Adv. Funct. Mater. 2018, 28, 1705042. [Google Scholar] [CrossRef]
- Xiong, S.; Hu, Y.; Zhang, S.; Xiao, Y.; Li, Z. Constructing Cellulose Diacetate Aerogels with Pearl-Necklace-like Skeleton Networking Structure. Gels 2021, 7, 210. [Google Scholar] [CrossRef]
- Zhou, S.; Nyholm, L.; Strømme, M.; Wang, Z. Cladophora cellulose: Unique biopolymer nanofibrils for emerging energy, environmental, and life science applications. Acc. Chem. Res. 2019, 52, 2232–2243. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Rudolph, T.; Walther, A.; Mülhaupt, R. Nanocellulose aerogels for supporting iron catalysts and in situ formation of polyethylene nanocomposites. Adv. Funct. Mater. 2017, 27, 1605586. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- Al Abdallah, H.; Abu-Jdayil, B.; Iqbal, M.Z. Improvement of mechanical properties and water resistance of bio-based thermal insulation material via silane treatment. J. Clean. Prod. 2022, 346, 131242. [Google Scholar] [CrossRef]
- Qian, H.; Liu, J.; Wang, X.; Pei, W.; Fu, C.; Ma, M.; Huang, C. The state-of-the-art application of functional bacterial cellulose-based materials in biomedical fields. Carbohydr. Polym. 2022, 300, 120252. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, D.; Lee, Y.-H.; Chen, W.; Lee, S.-Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef]
- AscanioVillabona, J.; Romero, B.E.T.; Duran, M.A.; Lengerke, O.; Betancur, L. Evaluation of the thermal performance of housing envelopes as passive cooling systems. Sustain. Eng. Innov. 2023, 5, 177–188. [Google Scholar] [CrossRef]
- Jain, R.; Bakare, Y.B.; Pattanaik, B.; Alaric, J.S.; Balam, S.K.; Ayele, T.B.; Nalagandla, R. Optimization of energy consumption in smart homes using firefly algorithm and deep neural networks. Sustain. Eng. Innov. 2023, 5, 161–176. [Google Scholar] [CrossRef]
- Adrian, M.; Purnomo, E.P.; Enrici, A.; Khairunnisa, T. Energy transition towards renewable energy in Indonesia. Herit. Sustain. Dev. 2023, 5, 107–118. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Rudolph, T.; Walther, A.; Mülhaupt, R. PLA coating improves the performance of renewable adsorbent pads based on cellulosic aerogels from aquatic waste biomass. Chem. Eng. J. 2020, 390, 124607. [Google Scholar]
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406. [Google Scholar] [CrossRef]
- Zhang, Y.C.L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar]
- Gong, C.; Ni, J.-P.; Tian, C.; Su, Z.-H. Research in porous structure of cellulose aerogel made from cellulose nanofibrils. Int. J. Biol. Macromol. 2021, 172, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Lu, Y.; Li, Z.; Zhang, J.; Zong, L. Biomimetic, hierarchical-ordered cellulose nanoclaw hybrid aerogel with high strength and thermal insulation. Carbohydr. Polym. 2022, 297, 119990. [Google Scholar] [CrossRef]
- Chen, G.; Hong, F.F.; Yuan, J.; Li, L.; Fang, M.; Wei, W.; Wang, X.; Wei, Y. Super solvent of cellulose with extra high solubility for tunable cellulose structure with versatile application. Carbohydr. Polym. 2022, 296, 119917. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, X.; Hu, G.; Huang, T.; Yu, H.; Yu, B.; Zhu, M. In situ crosslinking of mechanically robust waterproof and moisture permeable cellulose diacetate nanofiber aerogels for warm clothing. Chem. Eng. J. 2022, 444, 136528. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Z.; Lyu, S.; Fu, F.; Wang, S. Highly flexible cross-linked cellulose nanofibril sponge-like aerogels with improved mechanical property and enhanced flame retardancy. Carbohydr. Polym. 2018, 179, 333–340. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanofibril aerogels: Synergistic improvement of hydrophobicity, strength, and thermal stability via cross-linking with diisocyanate. ACS Appl. Mater. Interfaces 2017, 9, 2825–2834. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Crosslinking polydopamine/cellulose nanofibril composite aerogels by metal coordination bonds for significantly improved thermal stability, flame resistance, and thermal insulation properties. Cellulose 2021, 28, 10987–10997. [Google Scholar]
- Zhou, T.; Cheng, X.; Pan, Y.; Li, C.; Gong, L. Mechanical performance and thermal stability of polyvinyl alcohol-cellulose aerogels by freeze drying. Cellulose 2019, 26, 1747–1755. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, X.; Feng, J.; Qi, F.; Dianyu, E.; Jiang, Y.; Li, L.; Xiong, S.; Fen, J. Structure, compression and thermally insulating properties of cellulose diacetate-based aerogels. Mater. Des. 2020, 189, 108502. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Hu, Y.; Ji, H.; Xiao, Y.; Wang, J.; Ding, G.X.F. Ambient Pressure Drying to Construct Cellulose Acetate/Benzoxazine Hybrid Aerogels with Flame Retardancy, Excellent Thermal Stability, and Superior Mechanical Strength Resistance to Cryogenic Temperature. Biomacromolecules 2022, 23, 5056–5064. [Google Scholar] [CrossRef]
- Martínez-Lázaro, A.; Ramírez-Montoya, L.A.; Ledesma-García, J.; Montes-Morán, M.A.; Gurrola, M.P.; Menéndez, J.A.; Arenillas, A.; Arriaga, L.G. Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell. Materials 2022, 15, 1422. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Lu, K.; Hu, Y.; Xu, G.; Wang, J.; Liao, Y.; Yu, S. Cellulose Diacetate Aerogels with Low Drying Shrinkage, High-Efficient Thermal Insulation, and Superior Mechanical Strength. Gels 2024, 10, 210. https://doi.org/10.3390/gels10030210
Zhang S, Lu K, Hu Y, Xu G, Wang J, Liao Y, Yu S. Cellulose Diacetate Aerogels with Low Drying Shrinkage, High-Efficient Thermal Insulation, and Superior Mechanical Strength. Gels. 2024; 10(3):210. https://doi.org/10.3390/gels10030210
Chicago/Turabian StyleZhang, Sizhao, Kunming Lu, Yangbiao Hu, Guangyu Xu, Jing Wang, Yanrong Liao, and Shuai Yu. 2024. "Cellulose Diacetate Aerogels with Low Drying Shrinkage, High-Efficient Thermal Insulation, and Superior Mechanical Strength" Gels 10, no. 3: 210. https://doi.org/10.3390/gels10030210