Advances in Hydrogel-Based Drug Delivery Systems
Abstract
1. Introduction
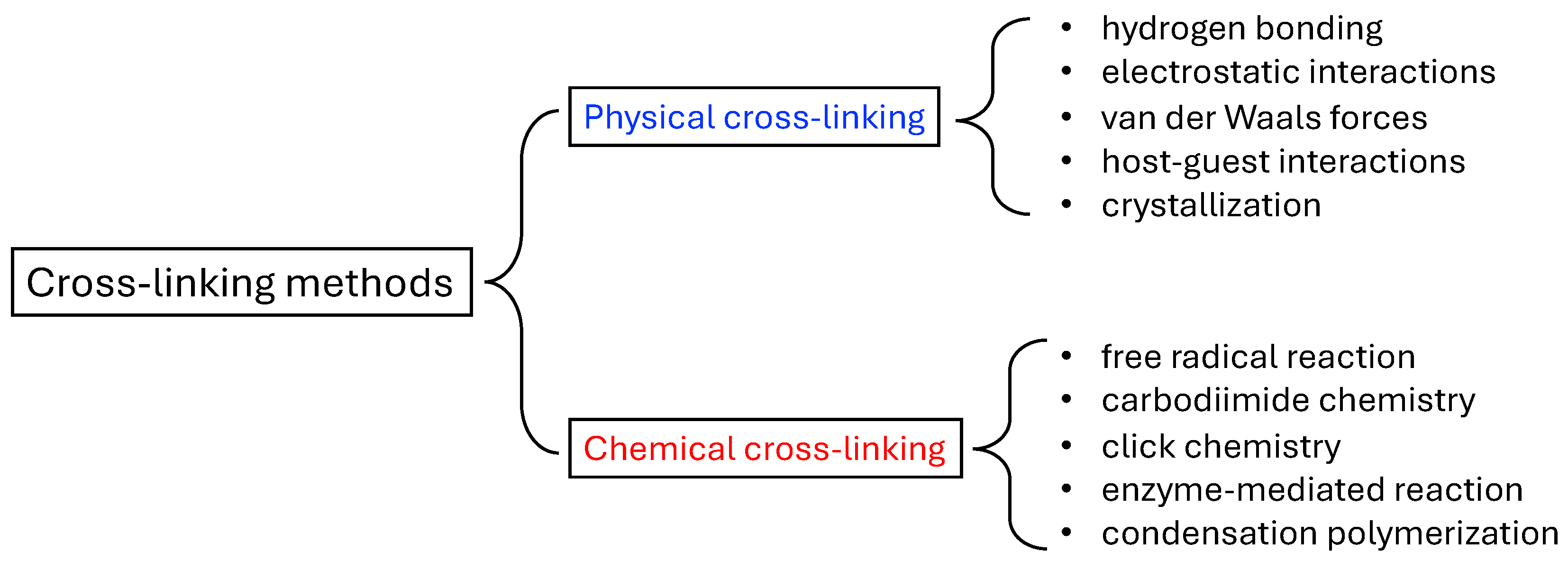
2. Classification of Hydrogels
2.1. Physcial Hydrogel
2.2. Chemical Hydrogel
3. Characterization of Hydrogels
3.1. Mesh Size and Swelling Behavior
3.2. Porosity and Microstructures
3.3. Mechanical Properties
3.4. Degradability
4. Hydrogel in Advanced Drug Delivery System
4.1. Oral Hydrogel-Based Drug Delivery
4.2. Injectable Hydrogel-Based Drug Delivery
4.3. Topical Hydrogel-Based Drug Delivery
4.4. Ocular Hydrogel-Based Drug Delivery
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Brown, T.E.; Anseth, K.S. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. [Google Scholar] [CrossRef] [PubMed]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Patel, D.K.; Jung, E.; Priya, S.; Won, S.Y.; Han, S.S. Recent advances in biopolymer-based hydrogels and their potential biomedical applications. Carbohydr. Polym. 2023, 323, 121408. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, J.; Guo, P.; Heng, L. Super water absorbency OMMT/PAA hydrogel materials with excellent mechanical properties. RSC Adv. 2017, 7, 14504–14510. [Google Scholar] [CrossRef]
- Tang, Y.; Heaysman, C.L.; Willis, S.; Lewis, A.L. Physical hydrogels with self-assembled nanostructures as drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Saldías, C.; Díaz, D.D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.; Tibbitt, M.W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.H.; Wu, F.G. Hydrogel-based growth factor delivery platforms: Strategies and recent advances. Adv. Mater. 2024, 36, 2210707. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, R.E.; Salama, A.; El-Fakharany, E.M.; Guarino, V. Mineralized polyvinyl alcohol/sodium alginate hydrogels incorporating cellulose nanofibrils for bone and wound healing. Molecules 2022, 27, 697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Sharma, A.; Panwar, V.; Chopra, V.; Ghosh, D. Polysaccharide-based hybrid self-healing hydrogel supports the paracrine response of mesenchymal stem cells. ACS Appl. Bio Mater. 2019, 2, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Zawrzykraj, M.; Sawicka, J.; Banach-Kopeć, A.; Tylingo, R.; Pikuła, M. Application of 3D-printed hydrogels in wound healing and regenerative medicine. Biomed. Pharmacother. 2023, 167, 115416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, C.; Xu, P.; Liu, K. Hydrogel-mediated extracellular vesicles for enhanced wound healing: The latest progress, and their prospects for 3D bioprinting. J. Nanobiotechnol. 2024, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Soon-Shiong, P.; Heintz, R.; Merideth, N.; Yao, Q.; Yao, Z.; Zheng, T.; Murphy, M.; Moloney, M.; Schmehl, M.; Harris, M. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994, 343, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Sengers, B.G.; Taylor, M.; Please, C.P.; Oreffo, R.O. Computational modelling of cell spreading and tissue regeneration in porous scaffolds. Biomaterials 2007, 28, 1926–1940. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A multiscale model for solute diffusion in hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Vernerey, F.J.; Lalitha Sridhar, S.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D cell–hydrogel interactions: Experiments, models, and mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Muthukumar, M. Theory of charged gels: Swelling, elasticity, and dynamics. Gels 2021, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Lee, C.S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Malta, R.; Marques, A.C.; Costa, P.C.d.; Amaral, M.H. Stimuli-Responsive Hydrogels for Protein Delivery. Gels 2023, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Sultana, F.; Imran-Ul-Haque, M.; Arafat, M.; Sharmin, S. An overview of nanogel drug delivery system. J. Appl. Pharm. Sci. 2013, 3, S95–S105. [Google Scholar] [CrossRef]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Kimura, M.; Fukumoto, K.; Watanabe, J.; Takai, M.; Ishihara, K. Spontaneously forming hydrogel from water-soluble random-and block-type phospholipid polymers. Biomaterials 2005, 26, 6853–6862. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Yoshimura, R.; Seki, C.; Fujioka, R. Synthesis and characterization of biodegradable hydrogels based on starch and succinic anhydride. Carbohydr. Polym. 2006, 64, 345–349. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-bonding reinforced injectable hydrogels: Application as a thermo-triggered drug controlled-release system. ACS Appl. Polym. Mater. 2020, 2, 1587–1596. [Google Scholar] [CrossRef]
- You, Y.; Yang, J.; Zheng, Q.; Wu, N.; Lv, Z.; Jiang, Z. Ultra-stretchable hydrogels with hierarchical hydrogen bonds. Sci. Rep. 2020, 10, 11727. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Hirokawa, Y.; Tanaka, T. Reentrant phase transition in acrylamide-derivative copolymer gels. Macromolecules 1984, 17, 2641–2643. [Google Scholar] [CrossRef]
- Puttipipatkhachorn, S.; Nunthanid, J.; Yamamoto, K.; Peck, G. Drug physical state and drug–polymer interaction on drug release from chitosan matrix films. J. Control. Release 2001, 75, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Du, Y.; Sun, L.; Zhang, B.; Dou, A. Polyelectrolyte complex beads composed of water-soluble chitosan/alginate: Characterization and their protein release behavior. J. Appl. Polym. Sci. 2006, 100, 4614–4622. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Lin, Y.C.; Lu, H.T.; Ho, Y.C.; Weng, S.C.; Tsai, M.L.; Mi, F.L. A novel injectable in situ forming gel based on carboxymethyl hexanoyl chitosan/hyaluronic acid polymer blending for sustained release of berberine. Carbohydr. Polym. 2019, 206, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Nguyen, M.T.; Luong, T.H.; Nguyen, D.T.; Truong, M.D.; Le Thi, P.; Le, H.K.; Dang, T.T.; Tran, N.Q. Syringeable hydrogel based β-cyclodextrin and mixed micelles for methotrexate delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105299. [Google Scholar]
- Sun, R.; Xia, Q.; Sun, Y. A Novel Strategy for Topical Administration by Combining Chitosan Hydrogel Beads with Nanostructured Lipid Carriers: Preparation, Characterization, and Evaluation. Gels 2024, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Lu, P.L.; Lai, J.Y.; Ma, D.H.K.; Hsiue, G.H. Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: Characterization and interaction with corneal endothelial cells. J. Biomater. Sci. Polym. Ed. 2008, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, C.; Shi, J.; Yu, W.; Zhu, C.; Zhang, Q.; Mizuno, M. A Carbodiimide Cross-Linked Silk Fibroin/Sodium Alginate Composite Hydrogel with Tunable Properties for Sustained Drug Delivery. Macromol. Mater. Eng. 2021, 306, 2100470. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Beckman, E.J.; Russell, A.J. Light-induced tailoring of PEG-hydrogel properties. Biomaterials 1998, 19, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Anseth, K. Characterization of hydrogels formed from acrylate modified poly (vinyl alcohol) macromers. Polymer 2000, 41, 7715–7722. [Google Scholar] [CrossRef]
- Ward, J.H.; Peppas, N.A. Preparation of controlled release systems by free-radical UV polymerizations in the presence of a drug. J. Control. Release 2001, 71, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Elvira, C.; Mano, J.; San Roman, J.; Reis, R. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials 2002, 23, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Leta, S.; Picci, N. pH-Sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int. J. Pharm. 2006, 312, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Satish, C.; Satish, K.; Shivakumar, H. Hydrogels as controlled drug delivery systems: Synthesis, crosslinking, water and drug transport mechanism. Indian J. Pharm. Sci. 2006, 68, 2. [Google Scholar]
- Saboktakin, M.R.; Maharramov, A.; Ramazanov, M.A. pH-sensitive starch hydrogels via free radical graft copolymerization, synthesis and properties. Carbohydr. Polym. 2009, 77, 634–638. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Fernandez-Trillo, F.; Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. Click chemistry with polymers, dendrimers, and hydrogels for drug delivery. Pharm. Res. 2012, 29, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Suzuki, Y.; Suhara, T.; Omichi, K.; Shimizu, A.; Hasegawa, K.; Kokudo, N.; Ohta, S.; Ito, T. In situ cross-linkable hydrogel of hyaluronan produced via copper-free click chemistry. Biomacromolecules 2013, 14, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol–ene click hydrogels for therapeutic delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, D.; Lim, D.K.; Koo, H.; Kim, K. Copper-Free Click Chemistry: Applications in Drug Delivery, Cell Tracking, and Tissue Engineering. Adv. Mater. 2022, 34, 2107192. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q. Enzyme-laden bioactive hydrogel for biocatalytic monitoring and regulation. Accounts Chem. Res. 2021, 54, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, J.M. Radiation formation of hydrogels for drug delivery. J. Control. Release 1994, 31, 9–19. [Google Scholar] [CrossRef]
- Raza, M.A.; Jeong, J.O.; Park, S.H. State-of-the-art irradiation technology for polymeric hydrogel fabrication and application in drug release system. Front. Mater. 2021, 8, 769436. [Google Scholar] [CrossRef]
- Sperinde, J.J.; Griffith, L.G. Synthesis and characterization of enzymatically-cross-linked poly (ethylene glycol) hydrogels. Macromolecules 1997, 30, 5255–5264. [Google Scholar] [CrossRef]
- Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced methods for the characterization of supramolecular hydrogels. Gels 2021, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.i. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.i.; Shibayama, M. Structure characterization of tetra-PEG gel by small-angle neutron scattering. Macromolecules 2009, 42, 1344–1351. [Google Scholar] [CrossRef]
- Chen, K.; Muthukumar, M. Substantial slowing of electrophoretic translocation of DNA through a nanopore using coherent multiple entropic traps. ACS Nano 2023, 17, 9197–9208. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Muthukumar, M. Topologically frustrated dynamics of crowded charged macromolecules in charged hydrogels. Nat. Commun. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Chen, K.; Muthukumar, M. Entropic barrier of topologically immobilized DNA in hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e2106380118. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, S.F.; Muthukumar, M. Boundaries of the topologically frustrated dynamical state in polymer dynamics. ACS Macro Lett. 2022, 11, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, N.; Yagi, T.; Kume, T.; Yoshii, F. Radiation crosslinking of carboxymethyl starch. Carbohydr. Polym. 2004, 58, 109–113. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, H.h.; Jing, Z.x.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.i.; Nakajima, T.; Matsuda, T.; Sakai, T.; Gong, J.P. Network elasticity of a model hydrogel as a function of swelling ratio: From shrinking to extreme swelling states. Soft Matter 2018, 14, 9693–9701. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Tanaka, T.; Han, C.C. Small-angle neutron scattering study on weakly charged temperature sensitive polymer gels. J. Chem. Phys. 1992, 97, 6842–6854. [Google Scholar] [CrossRef]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly (vinyl alcohol) membranes prepared by freezing/thawing techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Bastide, J.; Leibler, L. Large-scale heterogeneities in randomly cross-linked networks. Macromolecules 1988, 21, 2647–2649. [Google Scholar] [CrossRef]
- Bode, F.; Da Silva, M.A.; Smith, P.; Lorenz, C.D.; McCullen, S.; Stevens, M.M.; Dreiss, C.A. Hybrid gelation processes in enzymatically gelled gelatin: Impact on nanostructure, macroscopic properties and cellular response. Soft Matter 2013, 9, 6986–6999. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.E.B.; Barbouche, M.; Hamzaoui, A.H. Historical view of hydrogel characterization. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–479. [Google Scholar]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, Y.; Wang, J.; Song, W.; Cong, Y.; Wei, X.; Mei, Y.; Miyatake, H.; Ito, Y.; Chen, Y.M. Biomimetic Glucose Trigger-Insulin Release System Based on Hydrogel Loading Bidentate β-Cyclodextrin. Adv. Funct. Mater. 2021, 31, 2104488. [Google Scholar] [CrossRef]
- Yanez, F.; Gomez-Amoza, J.L.; Magarinos, B.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrogels porosity and bacteria penetration: Where is the pore size threshold? J. Membr. Sci. 2010, 365, 248–255. [Google Scholar] [CrossRef]
- Salerno, A.; Borzacchiello, R.; Netti, P.A. Pore structure and swelling behavior of porous hydrogels prepared via a thermal reverse-casting technique. J. Appl. Polym. Sci. 2011, 122, 3651–3660. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; Nomizu, M.; Nishi, N. Preparation of DNA-loaded polysulfone microspheres by liquid–liquid phase separation and its functional utilization. J. Colloid Interface Sci. 2004, 275, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef]
- Stratigaki, M.; Baumann, C.; van Breemen, L.C.; Heuts, J.P.; Sijbesma, R.P.; Göstl, R. Fractography of poly (N-isopropylacrylamide) hydrogel networks crosslinked with mechanofluorophores using confocal laser scanning microscopy. Polym. Chem. 2020, 11, 358–366. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Peng, X.; Han, L.; Wang, X.; Wang, J.; Zeng, H. Recent advances in mechano-responsive hydrogels for biomedical applications. ACS Appl. Polym. Mater. 2020, 2, 1092–1107. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Poly (ethylene glycol)–poly (lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J. Control. Release 2013, 172, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Young, C.; Bozzetto, K.; Poole-Warren, L.; Martens, P. Degradable, click poly (vinyl alcohol) hydrogels: Characterization of degradation and cellular compatibility. Biomed. Mater. 2012, 7, 024106. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jacobsen, M.T.; Pan, H.; Kopeček, J. Synthesis and characterization of enzymatically degradable PEG-based peptide-containing hydrogels. Macromol. Biosci. 2010, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y.; Li, Y.; Zhou, Z.; Cheng, Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials 2017, 112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Shmidov, Y.; Zhu, Y.; Matson, J.B.; Bitton, R. Effect of crosslinker topology on enzymatic degradation of hydrogels. Biomacromolecules 2020, 21, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The future of drug delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Hosseini-Nami, S.; Abed, Z.; Beik, J.; Aranda-Lara, L.; Samadian, H.; Morales-Avila, E.; Jaymand, M.; Shakeri-Zadeh, A. Recent advances in ultrasound-triggered drug delivery through lipid-based nanomaterials. Drug Discov. Today 2020, 25, 2182–2200. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in oral drug delivery systems: Challenges and opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, P.; Zhou, Y.; Zhou, X.; Ma, X.; Tan, T.; Cao, H. Hydrogel-encapsulated medium chain lipid-modified zeolite imidazole framework-90 as a promising platform for oral delivery of proteins. J. Control. Release 2024, 367, 93–106. [Google Scholar] [CrossRef] [PubMed]
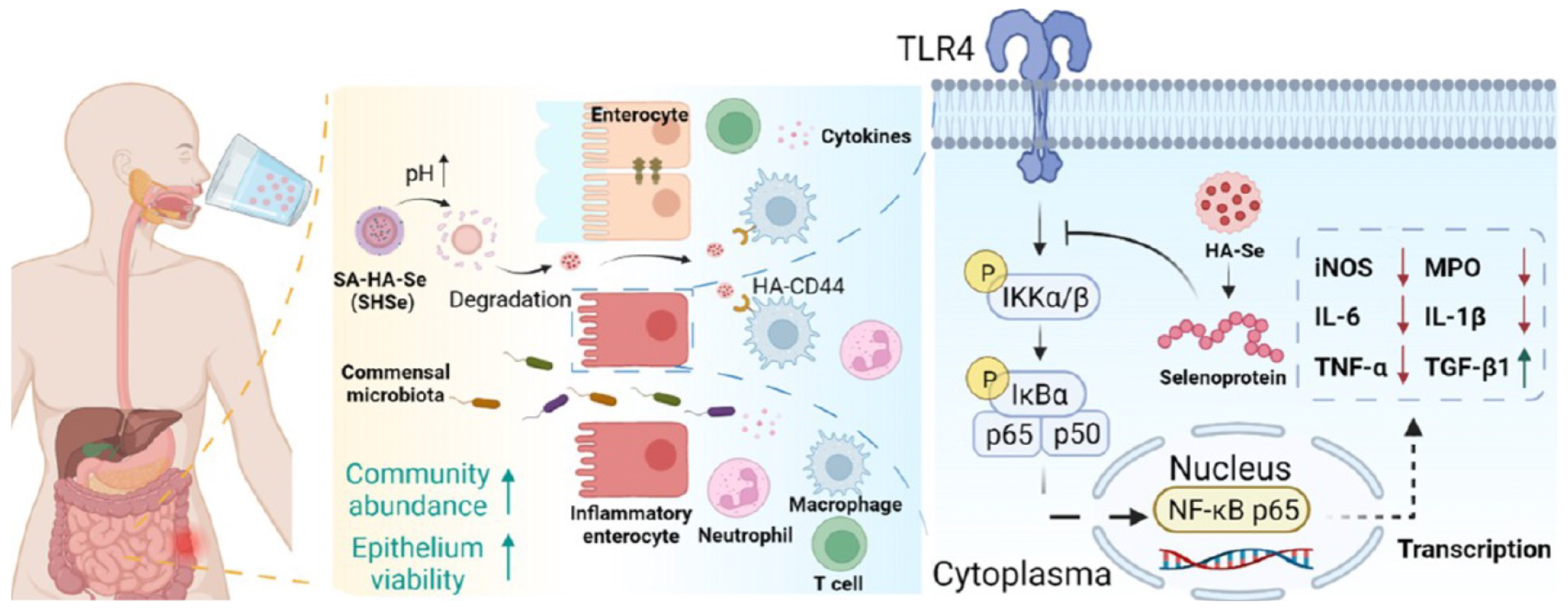
- Ouyang, J.; Deng, B.; Zou, B.; Li, Y.; Bu, Q.; Tian, Y.; Chen, M.; Chen, W.; Kong, N.; Chen, T.; et al. Oral hydrogel microbeads-mediated in situ synthesis of selenoproteins for regulating intestinal immunity and microbiota. J. Am. Chem. Soc. 2023, 145, 12193–12205. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.J.; Bourre, L.; Melgar, S.; O’Driscoll, C.M. Intestinal delivery of non-viral gene therapeutics: Physiological barriers and preclinical models. Drug Discov. Today 2011, 16, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin. Mater. Sci. Eng. C 2016, 69, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Xia, G.; Zhang, X.; Deng, S.; Zhao, X.; Xu, Y.; Chang, G.; Tao, Y.; Li, M.; et al. Oral Delivery of Bioactive Glass-Loaded Core–Shell Hydrogel Microspheres for Effective Treatment of Inflammatory Bowel Disease. Adv. Sci. 2023, 10, 2207418. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.K.; Chapa-Villarreal, F.A.; Oldenkamp, H.F.; Elder, M.G.; Venkataraman, A.K.; Peppas, N.A. Stimuli-responsive self-assembled polymer nanoparticles for the oral delivery of antibodies. J. Control. Release 2023, 361, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Touzout, Z.; Abdellaoui, N.; Hadj-Hamou, A.S. Conception of pH-sensitive calcium alginate/poly vinyl alcohol hydrogel beads for controlled oral curcumin delivery systems. Antibacterial and antioxidant properties. Int. J. Biol. Macromol. 2024, 263, 130389. [Google Scholar] [CrossRef] [PubMed]
- Andretto, V.; Rosso, A.; Zilio, S.; Sidi-Boumedine, J.; Boschetti, G.; Sankar, S.; Buffier, M.; Miele, A.E.; Denis, M.; Choffour, P.A.; et al. Peptide-Based Hydrogel for Nanosystems Encapsulation: The Next Generation of Localized Delivery Systems for the Treatment of Intestinal Inflammations. Adv. Healthc. Mater. 2024, 1, 2303280. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable hydrogels: From laboratory to industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Kaneko, J.; Nagasaki, Y. Development of a long-acting, protein-loaded, redox-active, injectable gel formed by a polyion complex for local protein therapeutics. Biomaterials 2016, 84, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Kang, T.; Cha, G.D.; Park, O.K.; Cho, H.R.; Kim, M.; Lee, J.; Kim, D.; Lee, B.; Chu, J.; Koo, S.; et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano 2023, 17, 5435–5447. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Huang, A.P.H.; Hsu, S.h. Injectable, Micellar Chitosan Self-Healing Hydrogel for Asynchronous Dual-Drug Delivery to Treat Stroke Rats. Adv. Funct. Mater. 2023, 33, 2303853. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Wu, Y.; Zhang, B.; Hu, C.; Guo, C.; Kong, Q.; Wang, Y. An injectable and self-strengthening nanogel encapsuled hydrogel gene delivery system promotes degenerative nucleus pulposus repair. Compos. Part B Eng. 2023, 250, 110469. [Google Scholar] [CrossRef]
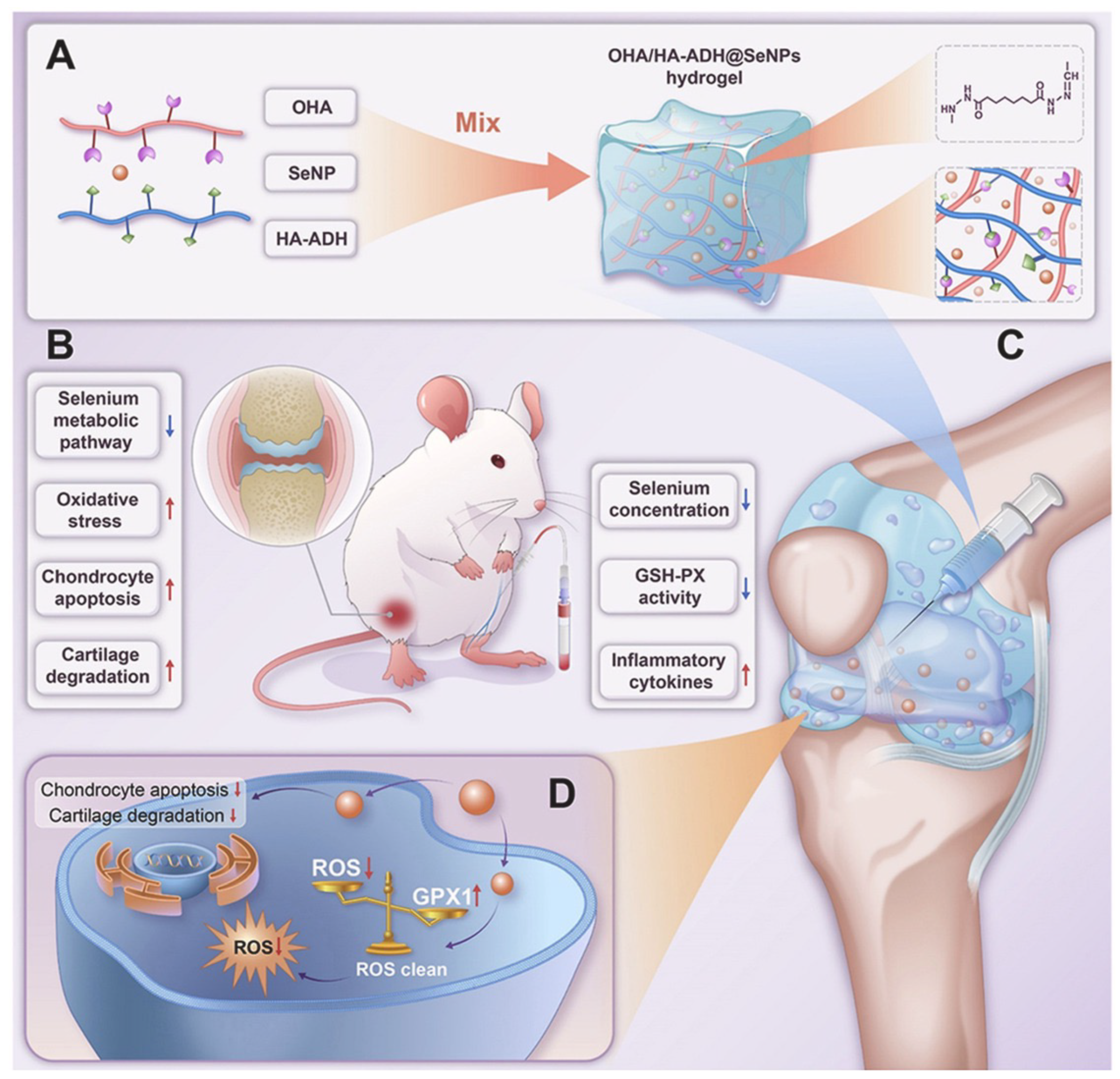
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Chu, R.; Ge, H.; Sun, X.; Li, M. Injectable hydrogel nanoarchitectonics with near-infrared controlled drug delivery for in situ photothermal/endocrine synergistic endometriosis therapy. Biomater. Res. 2023, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, E.; Rosa, E.; Ferrauto, G.; Diaferia, C.; Gallo, E.; Accardo, A.; Terreno, E. Development of cationic peptide-based hydrogels loaded with iopamidol for CEST-MRI detection. J. Mater. Chem. B 2023, 11, 7435–7441. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Han, H.; Xu, Z.; Li, S.; Zhu, Y.; Chen, Y.; Ge, L.; Zhang, Y. Short and simple peptide-based pH-sensitive hydrogel for antitumor drug delivery. Chin. Chem. Lett. 2022, 33, 1936–1940. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, M.; Huang, Y.; Liu, Y.; Liu, X.; Zhu, L.; Zhu, X.; Guo, Y.; Zhang, C. In Situ Vaccination with An Injectable Nucleic Acid Hydrogel for Synergistic Cancer Immunotherapy. Angew. Chem. Int. Ed. 2024, 63, e202315282. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, X.; Chen, Q.; Zhu, Y.; Shi, Z.; Deng, X.; Wang, C.; Chen, H. Injectable Responsive Hydrogel Delivery Platform: Enabling High Tissue Penetration and Sonogenetic-Like Potentiating Anti-Tumor Immunotherapy. Adv. Funct. Mater. 2024, 1, 2313723. [Google Scholar] [CrossRef]
- Weiss, S.C. Conventional topical delivery systems. Dermatol. Ther. 2011, 24, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Patents 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Pranantyo, D.; Yeo, C.K.; Wu, Y.; Fan, C.; Xu, X.; Yip, Y.S.; Vos, M.I.G.; Mahadevegowda, S.H.; Lim, P.L.K.; Yang, L.; et al. Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing. Nat. Commun. 2024, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, Z.; Cong, M.; Zhong, X.; Mao, Y.; Fan, M.; Jiao, F.; Qiao, H. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J. Control. Release 2024, 368, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Jing, L.; Zhou, M.; Gao, J.; Shi, Y.; Zhang, S. Multifunctional nanocomposite hydrogel dressing with low-temperature photothermal and controlled antibiotics release for combating bacterial infection. Mater. Des. 2024, 239, 112812. [Google Scholar] [CrossRef]
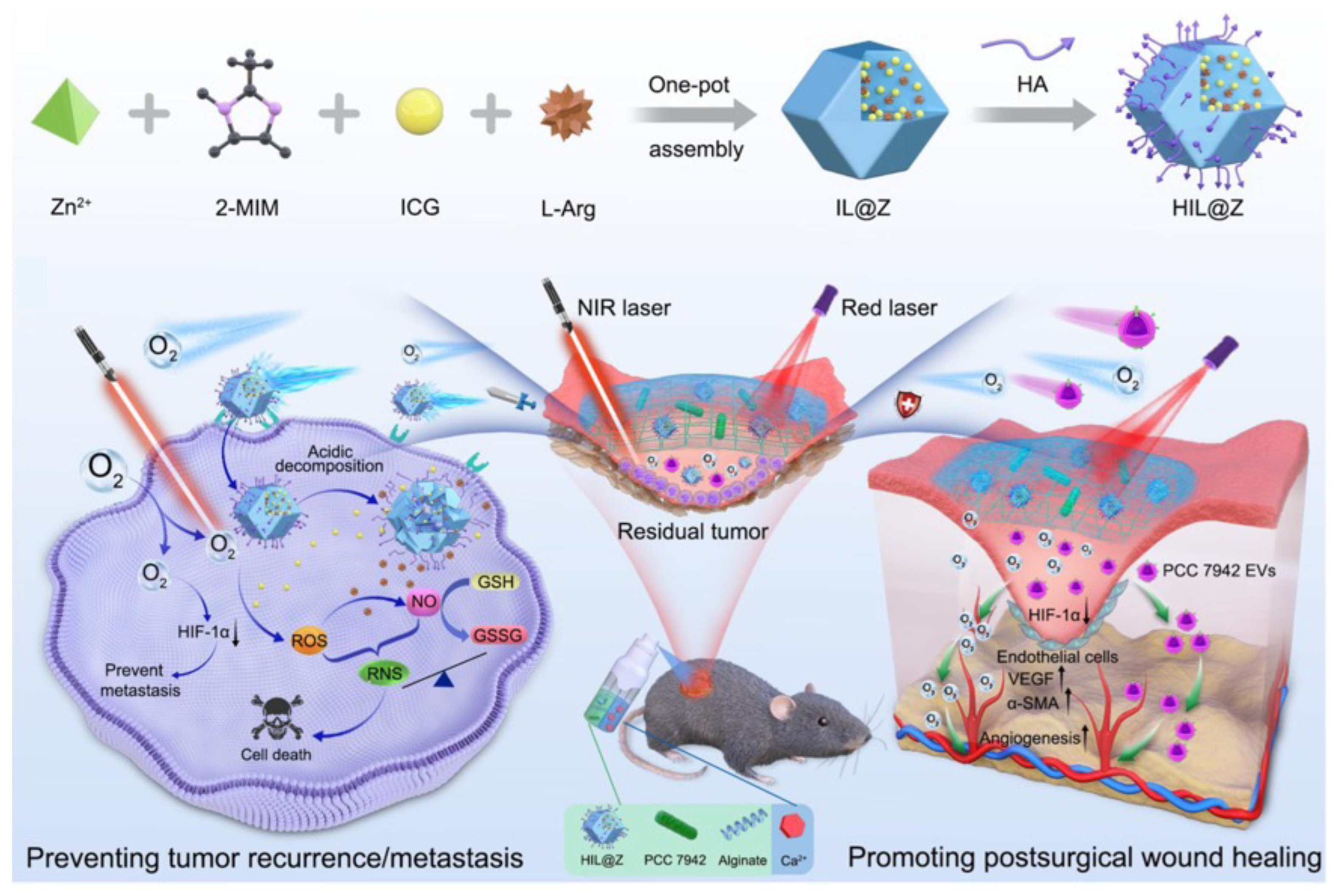
- Chen, S.; Luo, Y.; He, Y.; Li, M.; Liu, Y.; Zhou, X.; Hou, J.; Zhou, S. In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing. Nat. Commun. 2024, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Huo, S.; Wang, Z.; Yang, S.; Dou, L.; Liu, Y.; Huang, J.; Cai, C.; Fang, B.; Xu, G. Multifunctional biomimetic hydrogel dressing provides anti-infection treatment and improves immunotherapy by reprogramming the infection-related wound microenvironment. J. Nanobiotechnol. 2024, 22, 80. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Zdravković, N.M.; Budimir Filimonović, M.D.; Pavlović, V.B.; Butulija, S.V.; Milivojević, D.D.; Marković, Z.M.; Todorović Marković, B.M. Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light. J. Funct. Biomater. 2024, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Zdravković, N.M.; Trišić, D.D.; Budimir, M.D.; Marković, Z.M.; Kozyrovska, N.O.; Marković, B.M.T. Chronic wound dressings–pathogenic bacteria anti-biofilm treatment with bacterial cellulose-chitosan polymer or bacterial cellulose-chitosan dots composite hydrogels. Int. J. Biol. Macromol. 2021, 191, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Xu, H.; Ding, P.T.; Li, S.M.; Zheng, J.M. Thermosetting gels with modulated gelation temperature for ophthalmic use: The rheological and gamma scintigraphic studies. J. Control. Release 2002, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wu, Z.; Hu, X.; Huang, J.; Yi, Z.; Gong, Z.; Li, H.; Peng, K.; Shu, C.; Koole, L.H. A tissue-adhesive F127 hydrogel delivers antioxidative copper-selenide nanoparticles for the treatment of dry eye disease. Acta Biomater. 2024, 175, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Qi, X.; Ma, L.; Zhao, L.; Dou, S.; Wang, Y.; Zhou, Q.; Zhang, Y.; Yang, C.; Wang, H.; et al. Fabrication of nanozyme-thixotropic anionic hydrogel coating with multi-enzyme-mimicking activity for the treatment of fungal keratitis. Chem. Eng. J. 2024, 486, 150264. [Google Scholar] [CrossRef]
- Liu, H.; Bi, X.; Wu, Y.; Pan, M.; Ma, X.; Mo, L.; Wang, J.; Li, X. Cationic self-assembled peptide-based molecular hydrogels for extended ocular drug delivery. Acta Biomater. 2021, 131, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]

| Property | Methods | Advantages | Disadvantages |
|---|---|---|---|
| Mesh size | Rheological testing | Non-destructive, suitable for varied hydrogels, time-dependence analysis. | Specialized equipment needed, complex interpretation. |
| Estimation from swelling ratio | Straightforward, informative. | Limited by the drawbacks of swelling ratio methods. | |
| Mechanical testing | Correlates with mechanical properties, diverse application. | Indirect estimation, sample preparation can be complex. | |
| Scattering techniques | Broad applicability, non-destructive, in-situ analysis possible. | Advanced equipment required, sensitive to sample preparation. | |
| Swelling ratio | Weight change | Simple, cost-effective, broadly applicable. | Influenced by environmental factors, not for fast-swelling gels. |
| Volumetric change | Direct measurement, effective for significant swelling. | Challenging for irregular shapes or small samples. | |
| Differential scanning calorimetry (DSC) | Quantitative, non-destructive. | Specialist interpretation needed, higher equipment cost. | |
| Porosity | Estimation from swelling ratio | Straightforward, informative. | Limited by the drawbacks of swelling ratio methods. |
| Mercury intrusion porosimetry | Accurate pore size and distribution, reproducible. | May alter structure, mercury is hazardous. | |
| SEM | Detailed surface imagery, pore size and distribution. | Dry samples only, surface-level. | |
| Gas adsorption | Non-destructive, good for surface area and microporosity. | Limited to surface, not suitable for all types. | |
| Capillary flow porometry | Provides thorough porosity data. | Requires careful choice of phases. | |
| Microstructure | SEM | Detailed surface imagery, pore size and distribution. | Dry samples only, surface-level. |
| TEM | High-resolution internal images. | Requires meticulous preparation, small coverage. | |
| NMR | Molecular insights, non-destructive. | Expensive, complex data interpretation. | |
| AFM | Nanoscale surface topology. | Surface-specific, complex sample prep. | |
| Confocal microscopy | 3D imaging, non-destructive. | Limited penetration, fluorescence needed. | |
| X-ray CT | Non-destructive 3D internal imaging, high resolution. | High cost, requires contrast agents for some hydrogels. | |
| Mechanical strength | Dynamic Mechanical Analysis (DMA) | Frequency-dependent properties. | Requires specific equipment and sample shapes. |
| Rheological testing | Measures viscoelastic properties, non-destructive. | Complex interpretation, condition-sensitive. | |
| Tensile and compression testing | Direct strength and elasticity measurement. | Destructive, specific sample shapes necessary. | |
| Degradability | Mass loss measurement | Direct, simple quantification. | May overlook subtle changes. |
| Gel Permeation Chromatography (GPC) | Detailed profile, molecular weight insight. | Complex, requires solubilization. | |
| NMR | Degradation pathways at the molecular level. | Requires expensive equipment, expertise for data interpretation. | |
| Viscosity measurement | Indicates molecular weight changes. | Indirect, requires careful interpretation. |
| Delivery Route | Hydrogels | Formulation | Active Reagent | Application |
|---|---|---|---|---|
| Oral | Sodium alginate hydrogel | Attach medium-chain lipids (C10) onto the surface of zeolitic imidazole framework-90 (ZIF-90), then encapsulated the nanoparticles with sodium alginate [130]. | Protein. | Protein therapeutics. |
| Calcium alginate hydrogel | Coating hyaluronic acid-modified selenium nanoparticles with a protective shell of calcium alginate (SA) hydrogel [131]. | Selenoprotein. | Inflammatory bowel disease. | |
| Hyaluronic acid hydrogel | Insulin-loaded glucose-responsive nanocarriers were encapsulated into a three-dimensional hyaluronic acid hydroge [134]. | Insulin. | Diabetes. | |
| Sodium alginate hydrogel | Zein/sodium alginate-based core-shell microspheres (Zein/SA/BG) are developed for oral delivery of Bioactive glass [135]. | Bioactive glass. | Inflammatory bowel disease. | |
| PEG hydrogel | Synthesized via nanoprecipitation using the pH-responsive copolymers based on poly(methacrylic acid-co-methyl methacrylate)-block-poly(ethylene glycol) [136]. | Antibody. | Antibody therapies. | |
| Sodium alginate biopolymer and poly vinyl alcohol (PVA) hydrogel | Pristine and curcumin loaded calcium alginate/poly vinyl alcohol beads (CA/PVA and CA/PVA/Cur) were prepared by an ionotropic gelation method of SA followed by crosslinking of PVA [137]. | Curcumin. | Colon cancer. | |
| Peptide-based hydrogel | Mucopenetrating nanoemulsions of 100 nm are embedded in a scaffold constituted of the self-assembling peptide hydrogel product [138]. | Cytokine. | Inflammatory bowel diseases. | |
| Inject | Hyaluronic acid hydrogel | Oxidized hyaluronic acid (OHA) cross-linked with hyaluronic acid-adipic acid dihydrazide (HA-ADH), further incorporated with SeNPs [146]. | Selenium. | Osteoarthritis. |
| PLA-PEG-PLA hydrogel | Drug-loaded micelles mixed with water-dispersed ferrimagnetic iron oxide nanocubes (wFIONs) [143]. | Doxorubicin. | Glioblastoma multiforme. | |
| Chitosan hydrogel | Chitosan micellar self-healing hydrogel (CM hydrogel) with comparable modulus to brain [144]. | Curcumin. | Stroke. | |
| Peptide-based hydrogel | Cross-linking oxidized dextran (Ox-Dex) with MMP-2-cleavable peptide [145]. | Antagomir-21. | Intervertebral disc degeneration. | |
| Agarose (AG) hydrogels | Polydopamine (PDA), letrozole (LTZ), and agarose (AG) hydrogels were combined to construct an near-infrared controlled drug delivery [147]. | Letrozole. | Endometriosis. | |
| Peptide-based hydrogel | Iopamidol was loaded into Ac-K1/Ac-K2 HGs [148]. | Iopamidol. | Imaging agents. | |
| Peptide-based hydrogel | pH-responsive ionic-complementary octapeptide FOE to delivery DOX [149]. | Doxorubicin. | Cancer. | |
| Nucleic acid hydrogel | Crosslinking PD-L1 siRNA with a SN38- and CpG-containing Y-motif [151]. | PD-L1 siRNA. | Cancer. | |
| Hyaluronic acid hydrogel | HA-F127@Ti-MOF-Au/PEG-TK-DOX/PFD (abbr. HFTiDP) encapsulates sonosensitizer (Ti-MOF-Au), chemotherapeutic prodrug (PEG-TK-DOX), and ECM-solubilizing drug pirfenidone (PFD) [152]. | Doxorubicin. | Triple-negative breast cancer. | |
| Topical | PEG hydrogel | Crosslinked polyethylene glycol (PEG) hydrogel tethered with highly potent antibacterial cationic polymer, polyimidazolium (PIM), and the antioxidant N-acetylcysteine (NAC) [156]. | N-acetylcysteine. | Wound healing. |
| Gelatin methacryloyl hydrogel | Gelatin methacryloyl hydrogel loaded dandelion-derived extracellular vesicle-like nanoparticles [157]. | Dandelion-derived extracellular vesicle-like nanoparticles. | Wound healing. | |
| Hyaluronic acid hydrogel | Near-infrared (NIR) light-responsive multifunctional hydrogel system (PDA/Mup@DA-HA) consisting of mupirocin-loaded polydopamine nanoparticles (PDA) and dopamine-modified hyaluronic acid (DA-HA) hydrogel [158]. | Mupirocin. | Bacterial infection and tissue regeneration. | |
| Calcium alginate hydrogel | Sprayable calcium alginate hydrogel encapsulating HIL@Z nanodrug and photosynthetic cyanobacteria [159]. | Photosynthetic cyanobacteria. | Tumor recurrence/metastasis and wound healing. | |
| Porcine acellular dermal matrix | Hydrogel matrix is derived from porcine acellular dermal matrix and is loaded with bioactive glass nanoparticles doped with magnesium and loaded with Curcumin [160]. | Curcumin. | Antimicrobial and wound healing. | |
| Chitosan hydrogel | Nanochitosan dots (ChiDs) were synthesized using gamma rays and encapsulated in bacterial cellulose (BC) polymer matrix [161]. | Nanochitosan dots. | Chronic infections. | |
| Chitosan hydrogel | Hydrogels composed of bacterial cellulose (BC) with chitosan polymer (Chi)-BC-Chi and chitosan nanoparticles (nChiD) [162]. | Bacterial cellulose with chitosan polymer. | Chronic infections. | |
| Ocular | Aldehyde-functionalized F127 (AF127) hydrogel | Combines copper-selenium nanoparticles (Cu2-xSe NPs) AF127 [164]. | Cu2-xse nanoparticles. | Dry eye disease. |
| Hyaluronic acid hydrogel | Nanozyme-thixotropic hydrogel coating (NHC) incorporated voriconazole and copper-proanthocyanidins, self-synthesized polyaldehyde oligomer with amino functionalized hyaluronic acid [165]. | Voriconazole and copper-proanthocyanidins. | Fungal keratitis. | |
| Peptide-based hydrogel | Nap-FFKK generate supramolecular hydrogels spontaneously in a pH value of 5-7 [166]. | Nap-FFKK. | Ocular disorders. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. https://doi.org/10.3390/gels10040262
Liu B, Chen K. Advances in Hydrogel-Based Drug Delivery Systems. Gels. 2024; 10(4):262. https://doi.org/10.3390/gels10040262
Chicago/Turabian StyleLiu, Boya, and Kuo Chen. 2024. "Advances in Hydrogel-Based Drug Delivery Systems" Gels 10, no. 4: 262. https://doi.org/10.3390/gels10040262
APA StyleLiu, B., & Chen, K. (2024). Advances in Hydrogel-Based Drug Delivery Systems. Gels, 10(4), 262. https://doi.org/10.3390/gels10040262







