Effect of Xanthan Gum on the Rheological Behavior and Microstructure of Sodium Caseinate Acid Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermodynamic Compatibility of NaCAS–XG Blends
2.2. Protein Conformational Changes
2.3. Effect of XG on the Viscosity of the Medium
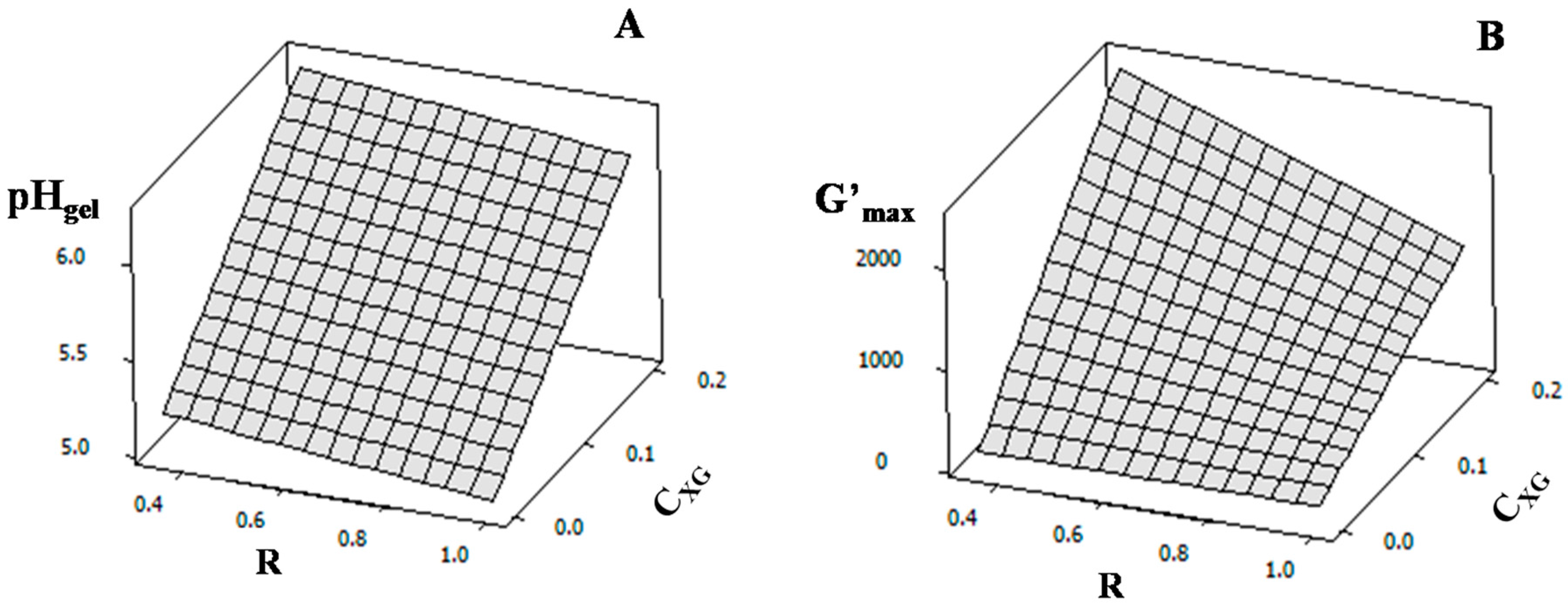
2.4. Rheological Properties of NaCAS Acid Gels in the Presence of XG
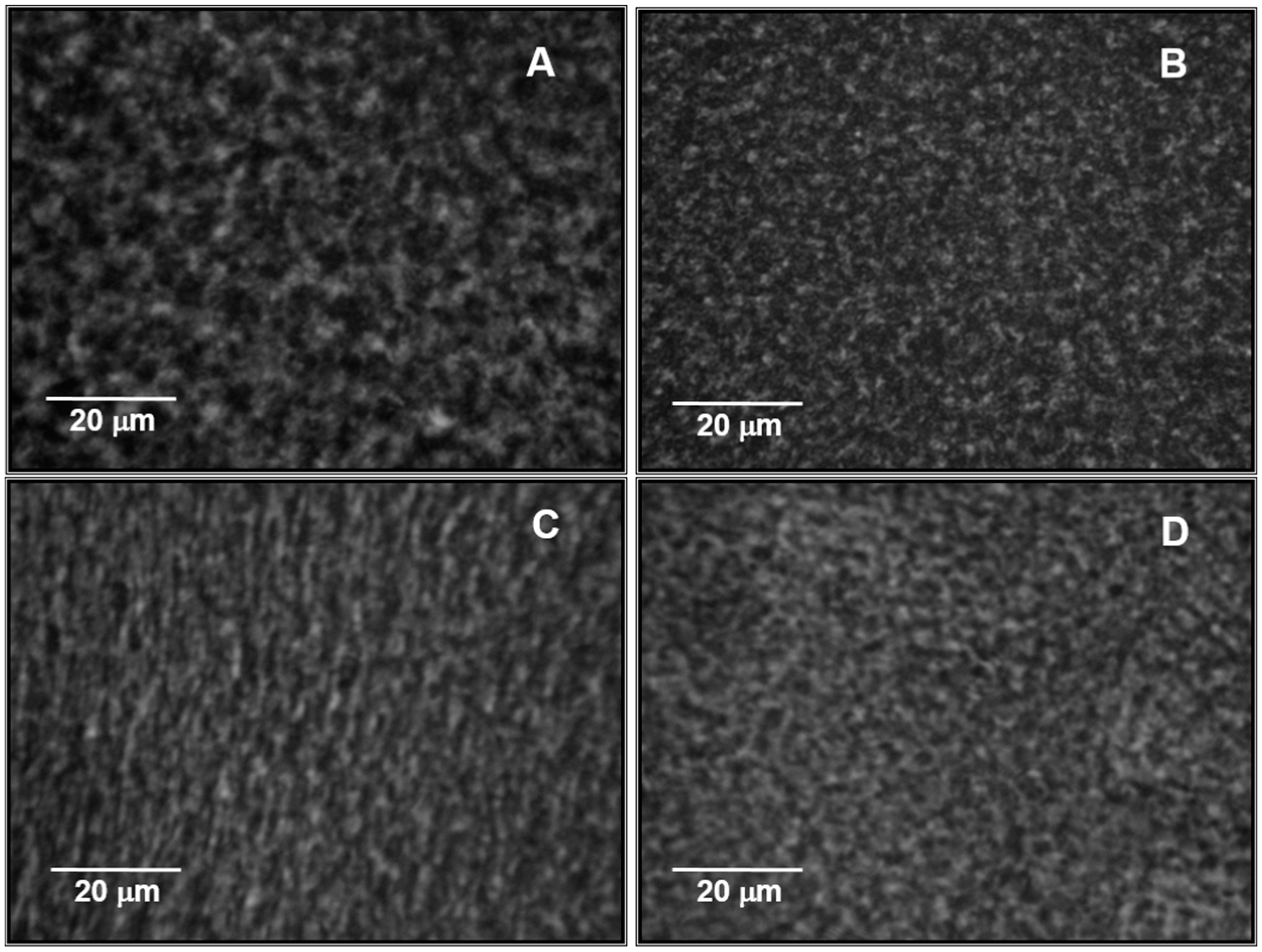
2.5. Microstructure of NaCAS Acid Gels in the Presence of XG
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Phase Diagrams
4.3. Intrinsic Fluorescence Emission Spectra
4.4. Viscometry
4.5. Rheological Properties of Acid Gels—Experimental Design
4.6. Conventional Optical Microscopy (OCM)
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dickinson, E. Emulsion stabilization by polysaccharide and protein-polysaccharide complexes. In Food Polysaccharides And Their Applications; Stephen, A.M., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 501–515. [Google Scholar]
- Tolstoguzov, V.B. Protein-polysaccharide interactions. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 171–198. [Google Scholar]
- Piculell, L.; Lindman, B. Association and segregation in aqueous polymer/polymer, polymer/surfactant, and surfactant/surfactant mixtures: Similarities and differences. Adv. Colloid Interface Sci. 1992, 41, 149–178. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Review and update of casein chemistry. J. Dairy Sci. 1993, 76, 3054–3061. [Google Scholar] [CrossRef]
- Casanova, H.; Dickinson, E. Influence of protein interfacial composition on salt stability of mixed casein emulsions. J. Agric. Food Chem. 1998, 46, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Morr, C.V.; Ha, E.Y.W. Structural and functional properties of caseinate and whey protein isolate as affected by temperature and pH. J. Food Sci. 1992, 57, 1210–1229. [Google Scholar] [CrossRef]
- Lieske, B.; Konrad, G. Thermal modification of sodium-caseinate. Influence of temperature and pH on selected physicochemical and functional properties. Milchwissenschaft 1994, 49, 16–20. [Google Scholar]
- Lucey, J.A.; Tamehana, M.; Singh, H.; Munro, P.A. A comparison of the formation, rheological properties and microstructure of acid skim milk gels made with a bacterial culture or glucono-[delta]-lactone. Food Res. Int. 1998, 31, 147–155. [Google Scholar] [CrossRef]
- Kobori, T.; Matsumoto, A.; Sugiyama, S. Ph-dependent interaction between sodium caseinate and xanthan gum. Carbohydr. Polym. 2009, 75, 719–723. [Google Scholar] [CrossRef]
- Jansson, P.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Quinn, F.X.; Hatakeyama, T.; Takahashi, M.; Hatakeyama, H. The effect of annealing on the conformational properties of xanthan hydrogels. Polymer 1994, 35, 1248–1252. [Google Scholar] [CrossRef]
- Yoshida, T.; Takahashi, M.; Hatakeyama, T.; Hatakeyama, H. Annealing induced gelation of xanthan/water systems. Polymer 1998, 39, 1119–1122. [Google Scholar] [CrossRef]
- Jensen, S.; Rolin, C.; Ipsena, R. Stabilisation of acidified skimmed milk with hm pectin. Food Hydrocoll. 2010, 24, 291–299. [Google Scholar] [CrossRef]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of emulsions containing sodium caseinate and dextran sulfate: Relationship to complexation in solution. Food Hydrocoll. 2008, 22, 647–659. [Google Scholar] [CrossRef]
- Koupantsis, T.; Kiosseoglou, V. Whey protein-carboxymethylcellulose interaction in solution and in oil-in-water emulsion systems. Effect on emulsion stability. Food Hydrocoll. 2009, 23, 1156–1163. [Google Scholar] [CrossRef]
- Liu, J.; Verespej, E.; Alexander, M.; Corredig, M. Comparison on the effect of high-methoxyl pectin or soybean-soluble polysaccharide on the stability of sodium caseinate-stabilized oil/water emulsions. J. Agric. Food Chem. 2007, 55, 6270–6278. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Tamehana, M.; Hemar, Y.; Munro, P. Interfacial compositions, microstructures and properties of oil-in-water emulsions formed with mixtures of milk proteins and k-carrageenan: 1. Sodium caseinate. Food Hydrocoll. 2003, 17, 539–548. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Liu, T.; Long, Z.; Kong, J.; Zhao, M. Sodium caseinate/xanthan gum interactions in aqueous solution: Effect on protein adsorption at the oil–water interface. Food Hydrocoll. 2012, 27, 339–346. [Google Scholar] [CrossRef]
- Moschakis, T.; Murray, B.S.; Dickinson, E. Microstructural evolution of viscoelastic emulsions stabilised by sodium caseinate and xanthan gum. J. Colloid Interface Sci. 2005, 284, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, M.; Yang, B.; Cui, C. Effect of xanthan gum on the physical properties and textural characteristics of whipped cream. Food Chem. 2009, 116, 624–628. [Google Scholar] [CrossRef]
- Hemar, Y.; Tamehana, M.; Munro, P.A.; Singh, H. Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocoll. 2001, 15, 565–574. [Google Scholar] [CrossRef]
- Braga, A.L.M.; Cunha, R.L. The effects of xanthan conformation and sucrose concentration on the rheological properties of acidified sodium caseinate-xanthan gels. Food Hydrocoll. 2004, 18, 977–986. [Google Scholar] [CrossRef]
- Nash, W.; Pinder, D.N.; Hemar, Y.; Singh, H. Dynamic light scattering investigation of sodium caseinate and xanthan mixtures. Int. J. Biol. Macromol. 2002, 30, 269–271. [Google Scholar] [CrossRef]
- Sanchez, C.; Zuniga-Lopez, R.; Schmitt, C.; Despond, S.; Hardy, J. Microstructure of acid–induced skim milk–locust bean gum–xanthan gels. Int. Dairy J. 2000, 10, 199–212. [Google Scholar] [CrossRef]
- Rohart, A.; Bouveresse, J.; Rutledge, D.N.; Michon, C. Spectrophotometric analysis of polysaccharide/milk protein interactions with methylene blue using independent components analysis. Food Hydrocoll. 2014, 43, 769–776. [Google Scholar] [CrossRef]
- Rodd, A.B.; Dunstan, D.E.; Boger, D.V. Characterisation of xanthan gum solutions using dynamic light scattering and rheology. Carbohydr. Polym. 2000, 42, 159–174. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer US: New York, NY, USA, 1986. [Google Scholar]
- Qi, P.X.; Brown, E.M.; Farrell, H.M. New views’ on structure-function relationships in milk proteins. Trends Food Sci. Technol. 2001, 12, 339–346. [Google Scholar] [CrossRef]
- Braga, A.L.M.; Menossi, M.; Cunha, R.L. The effect of the glucono-[delta]-lactone/caseinate ratio on sodium caseinate gelation. Int. Dairy J. 2006, 16, 389–398. [Google Scholar] [CrossRef]
- Cavallieri, A.L.F.; da Cunha, R.L. The effects of acidification rate, pH and ageing time on the acidic cold set gelation of whey proteins. Food Hydrocoll. 2008, 22, 439–448. [Google Scholar] [CrossRef]
- Rohart, A.; Michon, C. Designing microstructure into xanthan gum-enriched acid milk gels. Innov. Food Sci. Emerg. Technol. 2014, 25, 53–57. [Google Scholar] [CrossRef]
- Kuaye, A.Y. An ultraviolet spectrophotometric method to determine milk protein content in alkaline medium. Food Chem. 1994, 49, 207–211. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Portsch, A.; Norton, I.T. Effect of sucrose on the phase and flow behaviour of polysaccharide/protein aqueous two-phase systems. Food Hydrocoll. 2010, 24, 217–226. [Google Scholar] [CrossRef]
- Curcio, S.; Gabriele, D.; Giordano, V.; Calabrò, V.; de Cindio, B.; Iorio, G. A rheological approach to the study of concentrated milk clotting. Rheol. Acta 2001, 40, 154–161. [Google Scholar] [CrossRef]
- Burkert, J.F.M.; Maugeri, F.; Rodrigues, M.I. Optimization of extracellular lipase production by geotrichum sp. Using factorial design. Bioresour. Technol. 2004, 91, 77–84. [Google Scholar] [CrossRef]




| Independent variables | Responses | |||||
|---|---|---|---|---|---|---|
| CNaCAS (wt %) | CXG (wt %) | R | T (°C) | tgel (min) a | pHgel a | G’max (Pa) b |
| 3 (−1) | 0.2 (+1) | 1.00 (+1) | 15 (−1) | 3.04 | 6.11 | 1070.00 |
| 3 (−1) | 0.1 (0) | 0.35 (−1) | 15 (−1) | 26.92 | 5.77 | 1346.00 |
| 3 (−1) | 0 (−1) | 1.00 (+1) | 15 (−1) | 17.53 | 5.17 | 109.30 |
| 3 (−1) | 0 (−1) | 0.35 (−1) | 15 (−1) | 76.04 | 4.97 | 228.20 |
| 5 (+1) | 0.2 (+1) | 0.35 (−1) | 15 (−1) | 3.39 | 6.39 | 2733.00 |
| 5 (+1) | 0.1 (0) | 1.00 (+1) | 15 (−1) | 10.90 | 5.59 | 1236.00 |
| 5 (+1) | 0 (−1) | 0.35 (−1) | 15 (−1) | 68.00 | 5.12 | 781.80 |
| 5 (+1) | 0 (−1) | 1.00 (+1) | 15 (−1) | 19.99 | 4.95 | 495.00 |
| 3 (−1) | 0.2 (+1) | 0.35 (−1) | 35 (+1) | 3.23 | 6.18 | 2496.25 |
| 3 (−1) | 0.1 (0) | 1.00 (+1) | 35 (+1) | 5.33 | 5.65 | 218.65 |
| 3 (−1) | 0 (−1) | 1.00 (+1) | 35 (+1) | 6.95 | 4.95 | 26.87 |
| 3 (−1) | 0 (−1) | 0.35 (−1) | 35 (+1) | 19.59 | 5.39 | 22.71 |
| 5 (+1) | 0.2 (+1) | 1.00 (+1) | 35 (+1) | 3.62 | 5.92 | 1400.00 |
| 5 (+1) | 0.1 (0) | 0.35 (−1) | 35 (+1) | 14.61 | 5.49 | 484.30 |
| 5 (+1) | 0 (−1) | 1.00 (+1) | 35 (+1) | 6.65 | 4.94 | 90.83 |
| 5 (+1) | 0 (−1) | 0.35 (−1) | 35 (+1) | 17.89 | 5.27 | 116.30 |
| Factor | tgel (min) | pHgel | G’max (Pa) | |||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Constant | 15.60 | 0.000 | 5.63 | 0.000 | 1002.2 | 0.000 |
| CNaCAS (L) | - | ― a | - | ― a | - | ― a |
| CXG (L) | −12.86 | 0.000 | 0.51 | 0.000 | 832.1 | 0.000 |
| R (L) | −7.54 | 0.002 | −0.09 | 0.024 | −290.3 | 0.011 |
| T (L) | −7.23 | 0.002 | - | ― a | - | ― a |
| CNaCAS × CNaCAS (Q) | - | ― a | - | ― a | - | ― a |
| CXG × CXG (Q) | - | ― a | - | ― a | - | ― a |
| R × R (Q) | - | ― a | - | ― a | - | ― a |
| T × T (Q) | - | ― a | - | ― a | - | ― a |
| CNaCAS × CXG | - | ― a | - | ― a | - | ― a |
| CNaCAS × R | - | ― a | - | ― a | - | ― a |
| CNaCAS × T | - | ― a | - | ― a | - | ― a |
| CXG × T | 8.72 | 0.001 | - | ― a | - | ― a |
| CXG × R | 8.16 | 0.002 | - | ― a | −301.3 | 0.019 |
| R × T | 5.78 | 0.006 | - | ― a | - | ― a |
| r2 = 89.90% | r2 = 90.79% | r2 = 79.91% | ||||
| CXG (wt %) | Average Diameter of Pores (μm) |
|---|---|
| 0 | 4.57 ± 0.04 a |
| 0.10 | 4.30 ± 0.09 |
| 0.15 | 4.07 ± 0.04 |
| 0.20 | 3.98 ± 0.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, M.E.; Armendariz, M.; Wagner, J.R.; Risso, P.H. Effect of Xanthan Gum on the Rheological Behavior and Microstructure of Sodium Caseinate Acid Gels. Gels 2016, 2, 23. https://doi.org/10.3390/gels2030023
Hidalgo ME, Armendariz M, Wagner JR, Risso PH. Effect of Xanthan Gum on the Rheological Behavior and Microstructure of Sodium Caseinate Acid Gels. Gels. 2016; 2(3):23. https://doi.org/10.3390/gels2030023
Chicago/Turabian StyleHidalgo, María E., Mirta Armendariz, Jorge R. Wagner, and Patricia H. Risso. 2016. "Effect of Xanthan Gum on the Rheological Behavior and Microstructure of Sodium Caseinate Acid Gels" Gels 2, no. 3: 23. https://doi.org/10.3390/gels2030023