Photo-Crosslinkable Colloids: From Fluid Structure and Dynamics of Spheres to Suspensions of Ellipsoids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Characterization
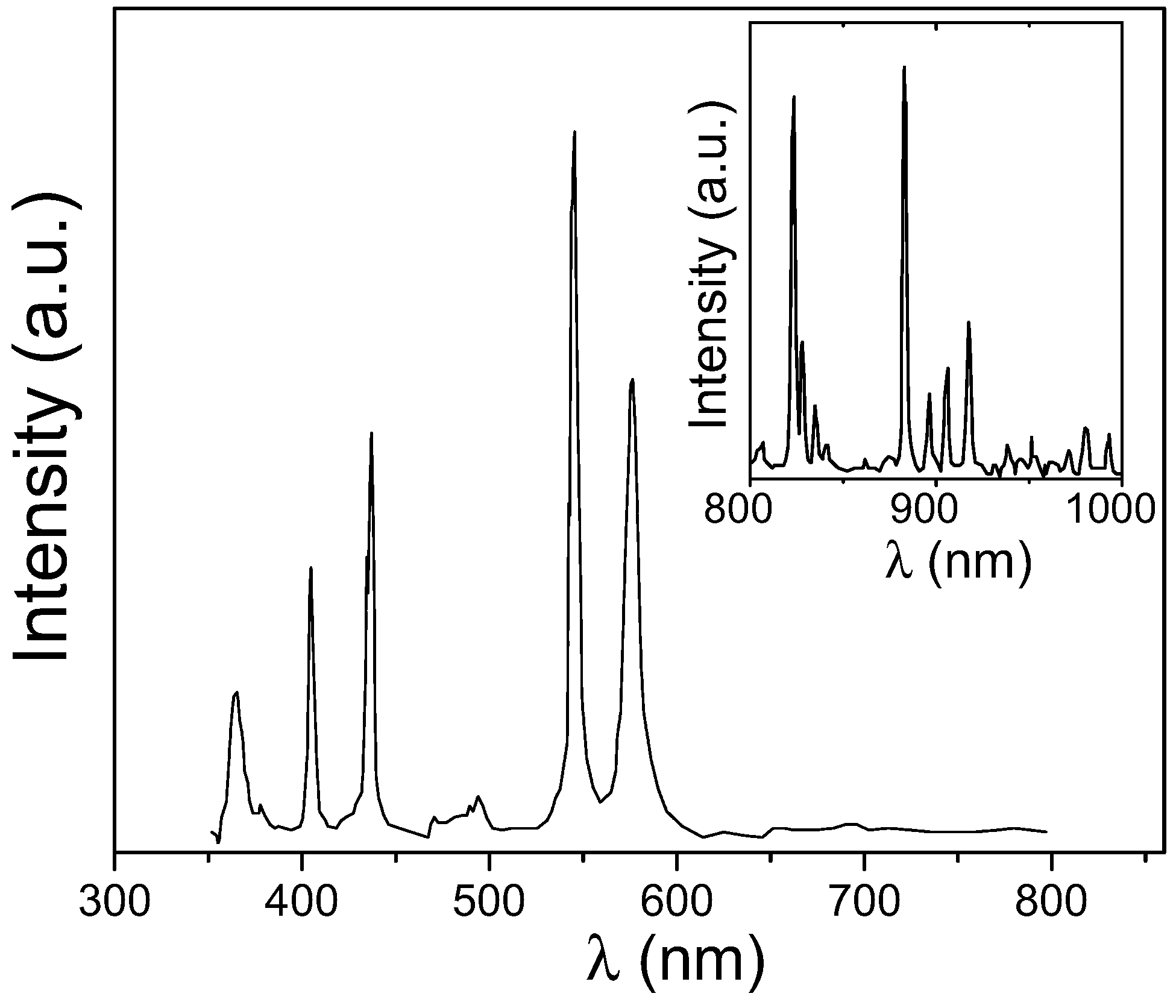
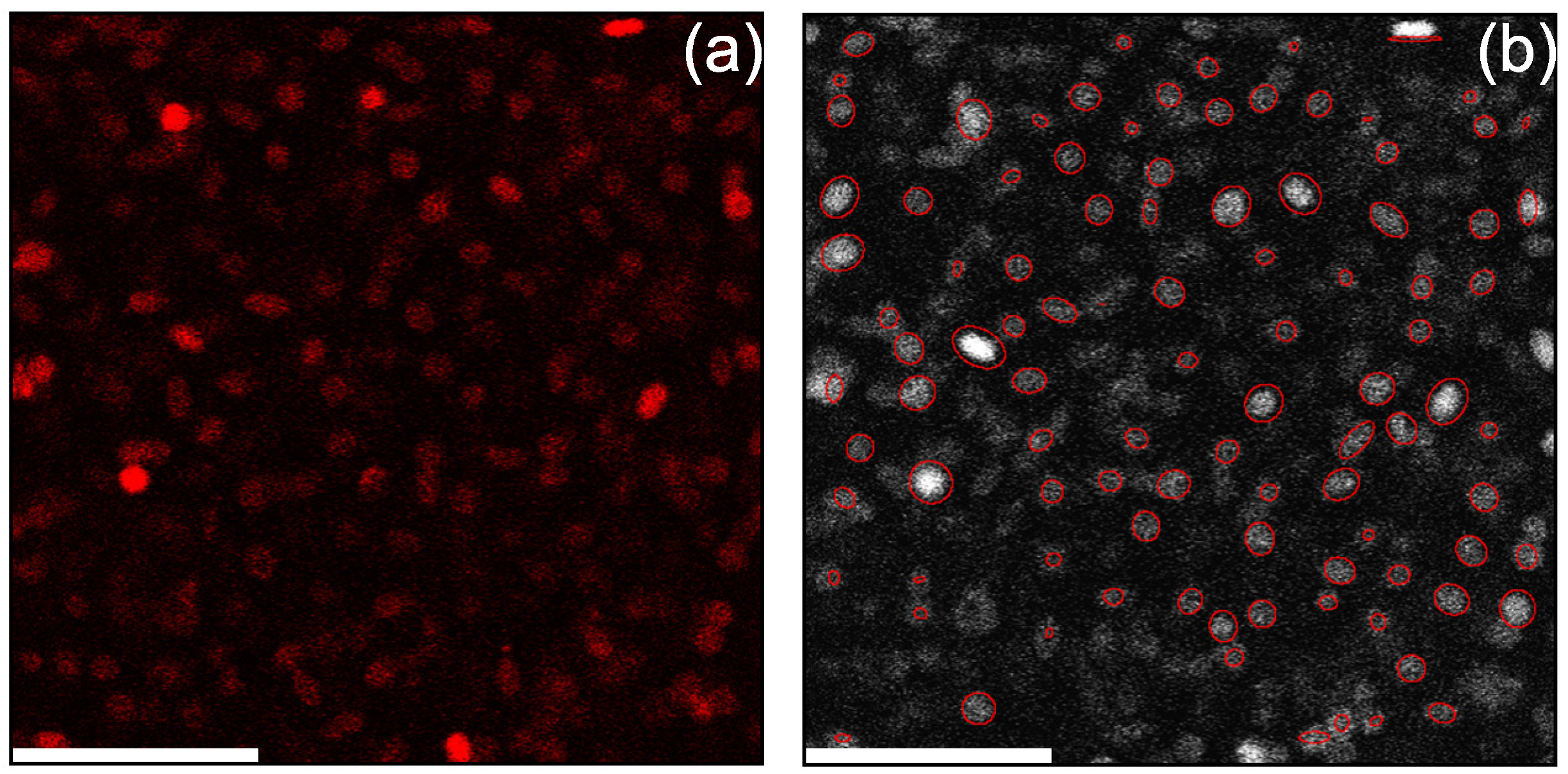
2.1.1. Electron Microscopy
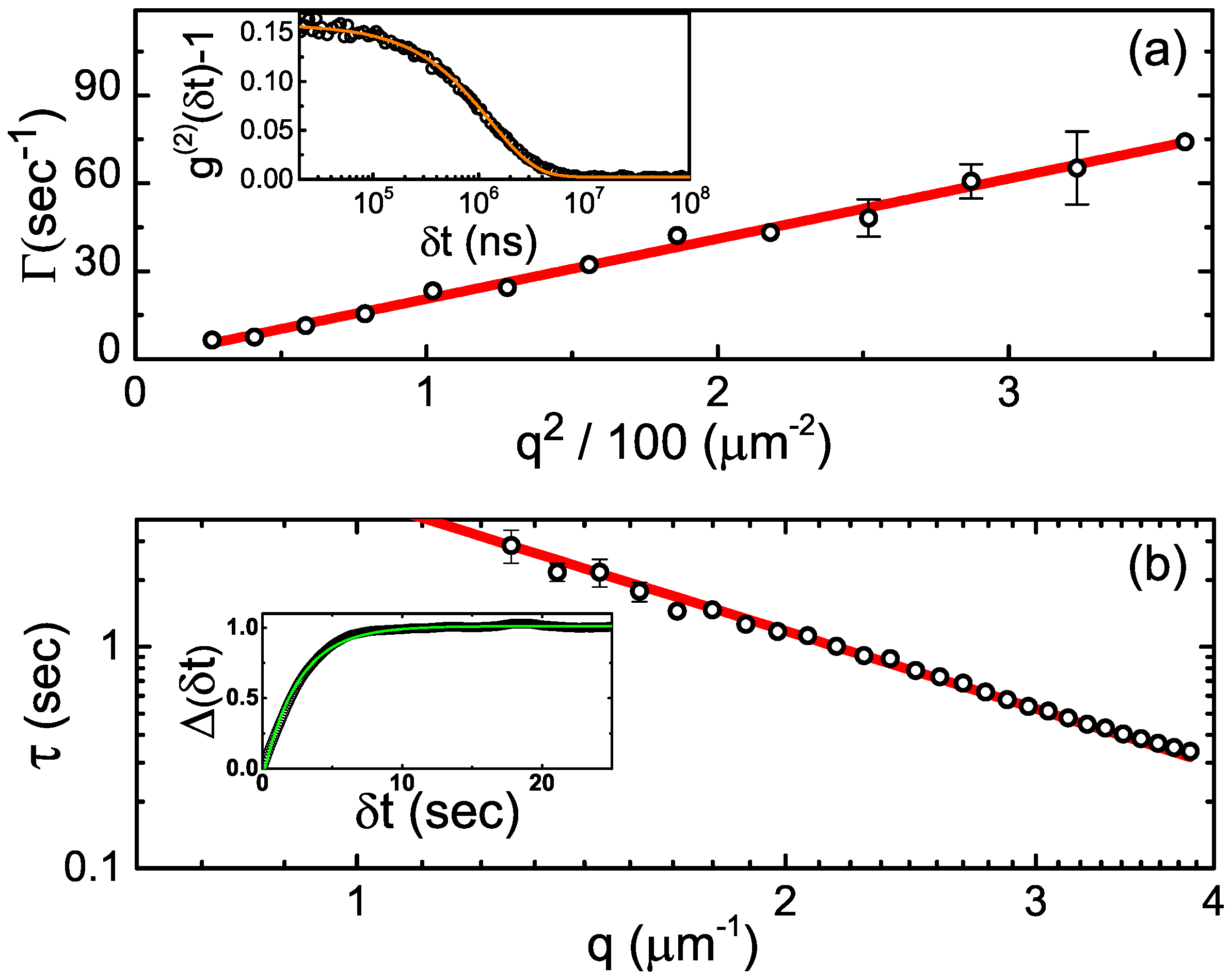
2.1.2. Dynamic Light Scattering
2.1.3. Confocal Differential Dynamic Microscopy
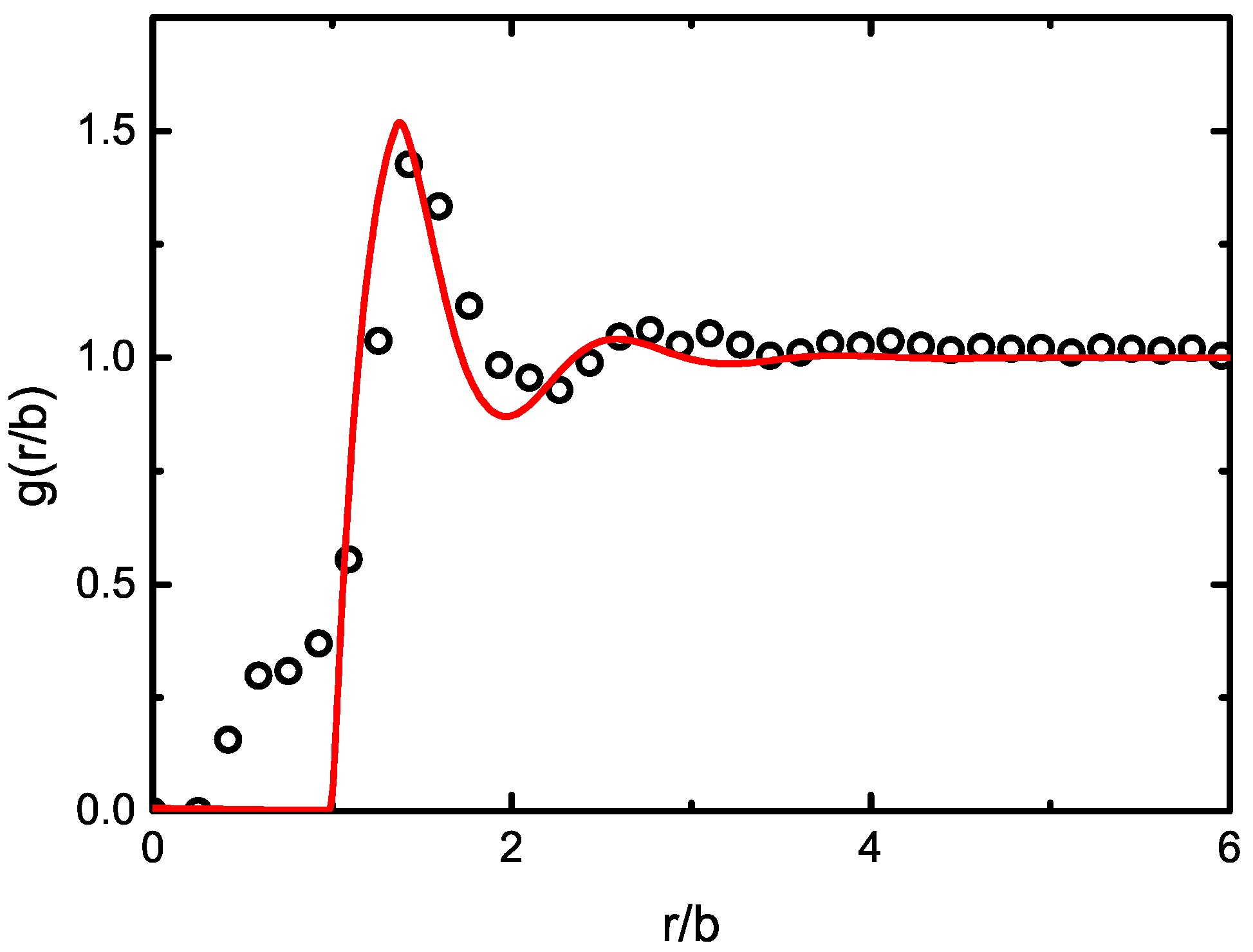
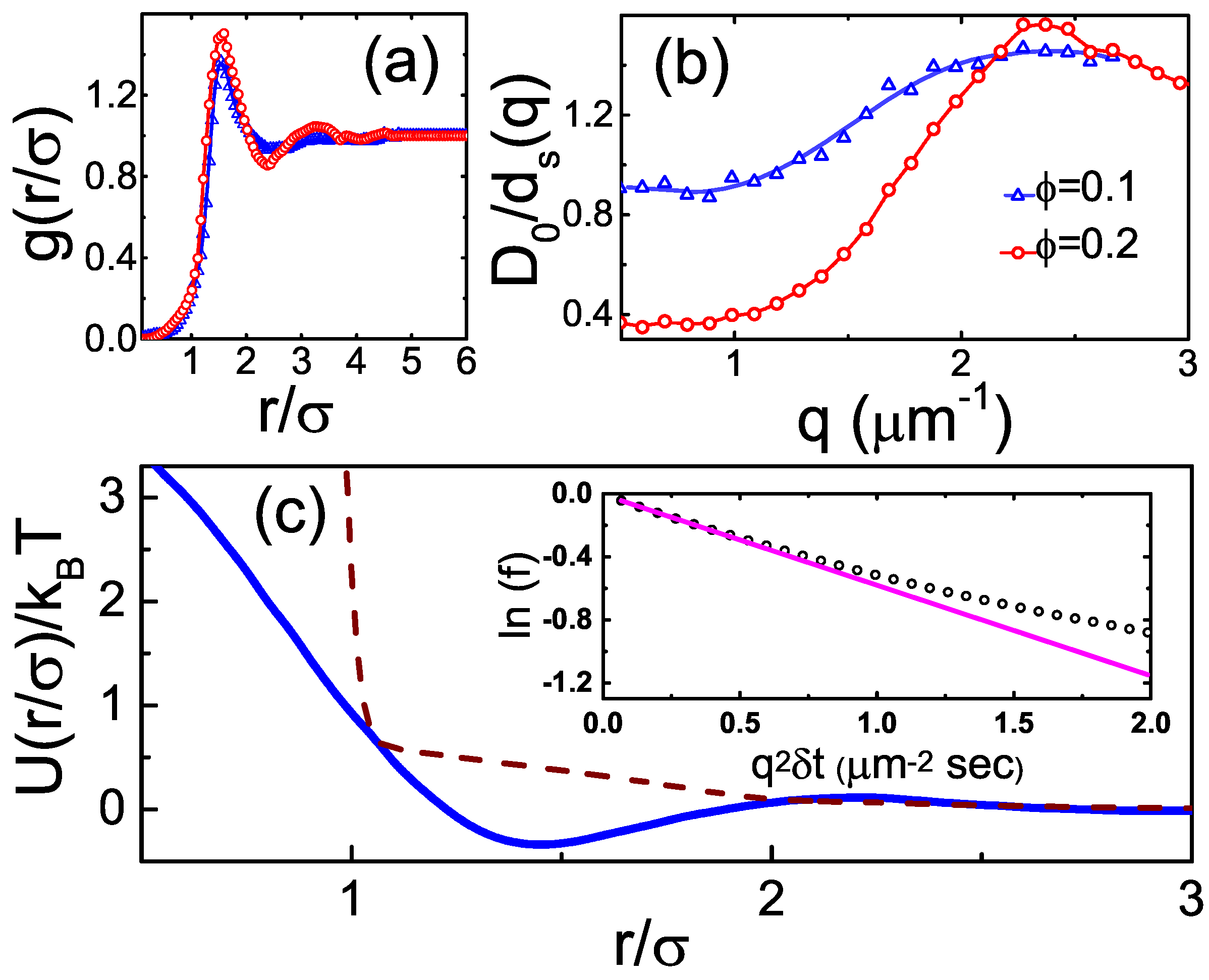
2.2. Dense Fluids: Structure
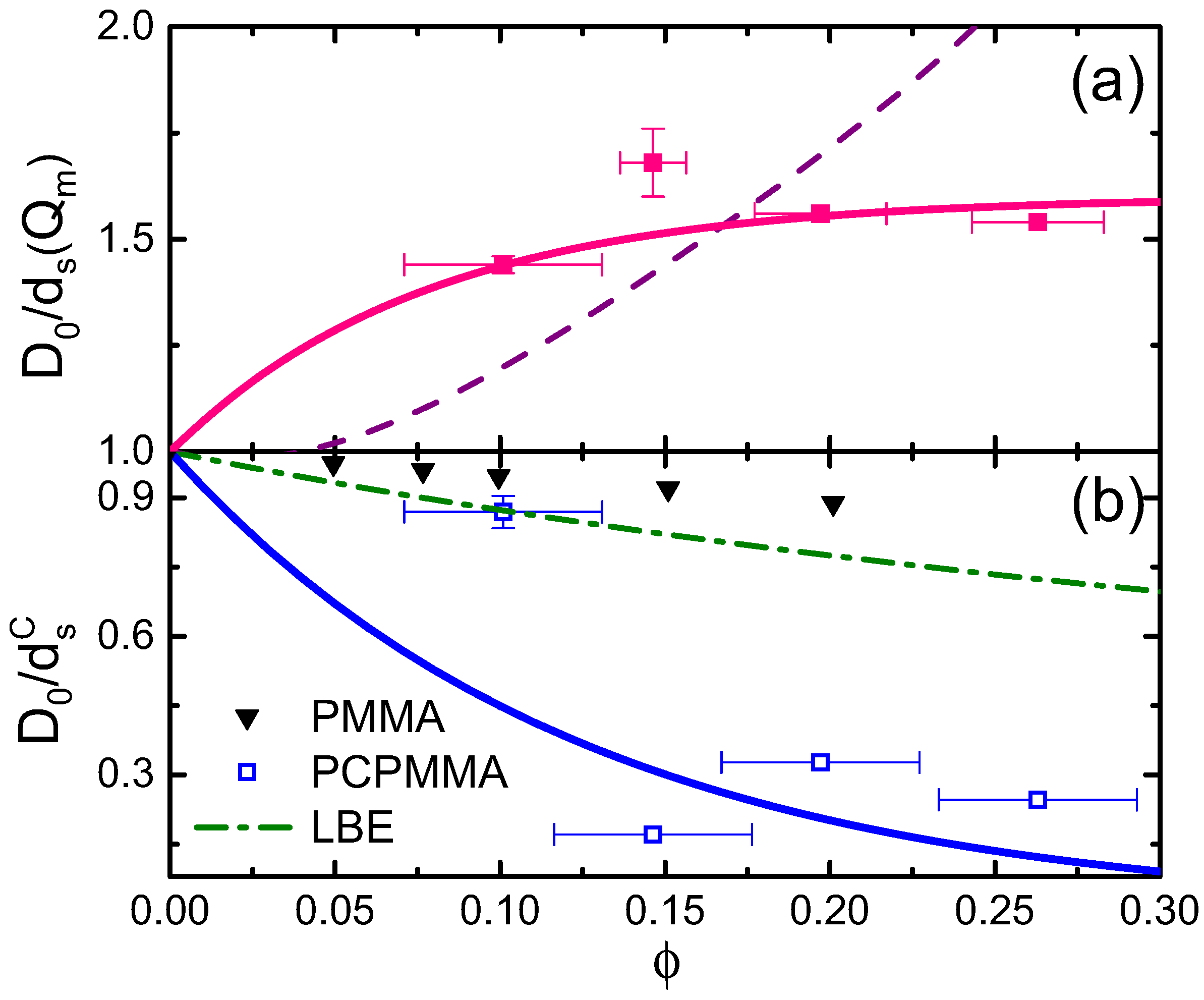
2.3. Dense Fluids: Dynamics
2.4. PCPMMA Ellipsoids
3. Experimental
3.1. Materials
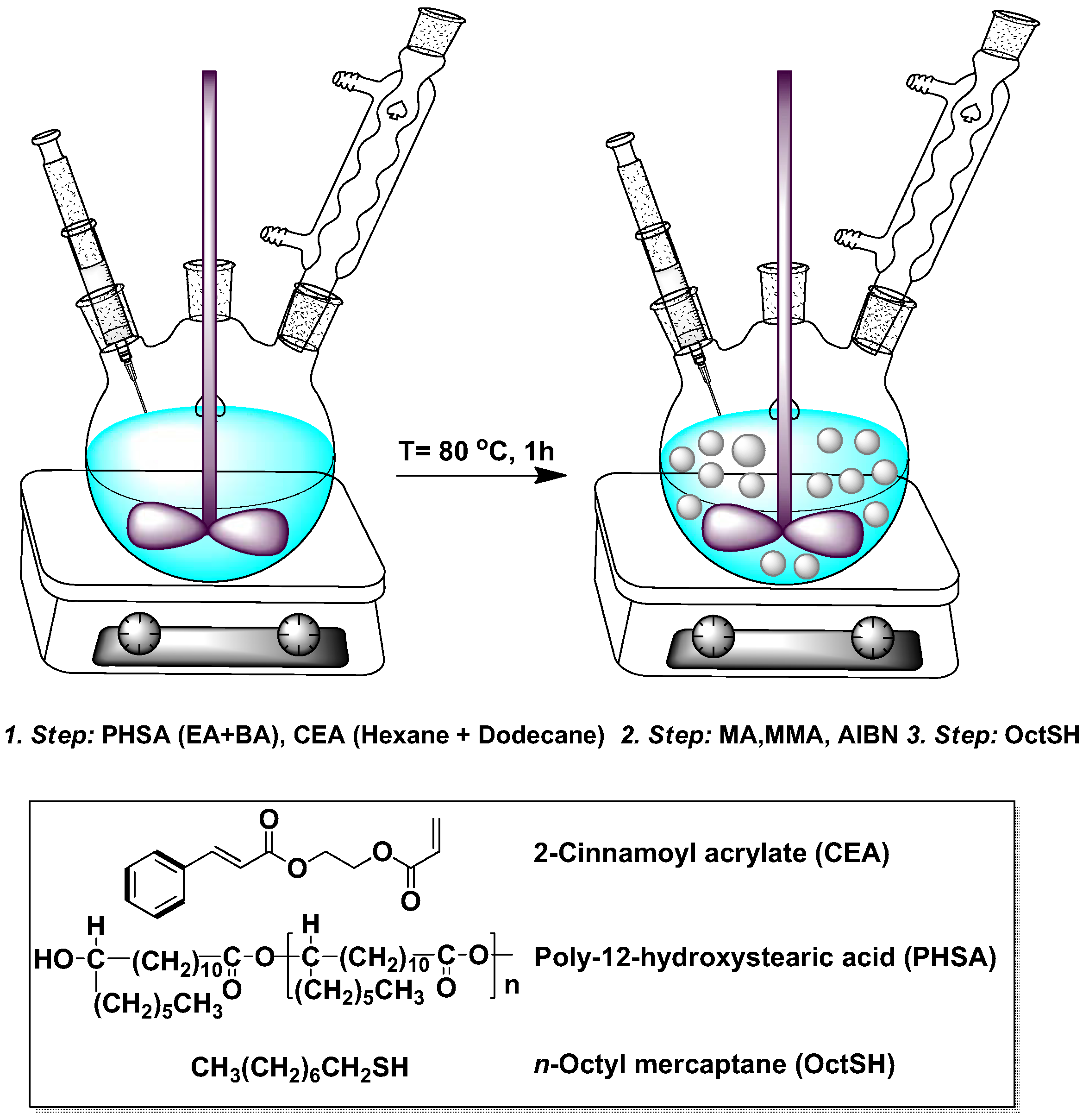
3.2. Particle Synthesis
3.3. Photo-Crosslinking of the Particles
3.4. Stretching of Colloidal PCPMMA Spheres to Form Colloidal Ellipsoids
3.5. Fluorescent Staining of the Colloids
3.6. Formation and Imaging of Fluid Suspensions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. PCPMMA Ellipsoids: Preliminary Determination of the Fluid Structure
References and Notes
- Perrin, J. Mouvement brownien et réalité moléculaire. Ann. Chim. Phys. 1909, 18, 1–144. [Google Scholar]
- Lekkerkerker, H.N.W.; de Villeneuve, V.W.A.; de Folter, J.W.J.; Schmidt, M.; Hennequin, Y.; Bonn, D.; Indekeu, J.O.; Aarts, D.G.A.L. Life at ultralow interfacial tension: Wetting, waves and droplets in demixed colloid-polymer mixtures. Eur. Phys. J. B 2008, 64, 341–347. [Google Scholar] [CrossRef]
- Cohen, A.P.; Janai, E.; Mogilko, E.; Schofield, A.B.; Sloutskin, E. Fluid suspensions of colloidal ellipsoids: Direct structural measurements. Phys. Rev. Lett. 2011, 107, 238301. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.P.; Janai, E.; Rapaport, D.C.; Schofield, A.B.; Sloutskin, E. Structure and interactions in fluids of prolate colloidal ellipsoids: Comparison between experiment, theory and simulation. J. Chem. Phys. 2012, 137, 184505. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ni, R.; Wang, F.; Dijkstra, M.; Wang, Y.; Han, Y. Structural signatures of dynamic heterogeneities in monolayers of colloidal ellipsoids. Nat. Commun. 2014, 5, 3829. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Han, Y. Self-diffusion in two-dimensional hard ellipsoid suspensions. J. Chem. Phys. 2010, 133, 124509. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.P.; Dorosz, S.; Schofield, A.B.; Schilling, T.; Sloutskin, E. Structural transition in a fluid of spheroids: A low-density vestige of jamming. Phys. Rev. Lett. 2016, 116, 098001. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Liu, Z.F.; Song, K.; Clays, K.; Tung, C.H. Photonic crystals of oblate spheroids by blown film extrusion of prefabricated colloidal crystals. Langmuir 2009, 25, 10218–10222. [Google Scholar] [CrossRef] [PubMed]
- Mohraz, A.; Solomon, M.J. Direct visualization of colloidal rod assembly by confocal microscopy. Langmuir 2005, 21, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Mukhija, D.; Solomon, M.J. Nematic order in suspensions of colloidal rods by application of a centrifugal field. Soft Matter 2011, 7, 540–545. [Google Scholar] [CrossRef]
- Ho, C.C.; Keller, A.; Odell, J.A.; Ottewill, R.H. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym. Sci. 1993, 271, 469–479. [Google Scholar] [CrossRef]
- Cohen, A.P. Fluids of Colloidal Ellipsoids: Confocal Microscopy Studies. Master’s Thesis, Bar-Ilan University, Ramat Gan, Israel, 2012. [Google Scholar]
- Zheng, Z.; Wang, F.; Han, Y. Glass transitions in quasi-two-dimensional suspensions of colloidal ellipsoids. Phys. Rev. Lett. 2011, 107, 065702. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.; Wyss, H.M. Towards the self-assembly of anisotropic colloids: Monodisperse oblate ellipsoids. J. Colloid Interface Sci. 2014, 416, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Ahn, K.H.; Lee, S.J. Film squeezing process for generating oblate spheroidal particles with high yield and uniform sizes. Colloid Polym. Sci. 2016, 294, 859–867. [Google Scholar] [CrossRef]
- Antl, L.; Goodwin, J.W.; Hill, R.D.; Ottewill, R.H.; Owens, S.M.; Papworth, S.; Waters, J.A. The preparation of poly(methyl methacrylate) latices in non-aqueous media. Colloids Surf. 1986, 17, 67–78. [Google Scholar] [CrossRef]
- Hsiao, L.C.; Schultz, B.A.; Glaser, J.; Engel, M.; Szakasits, M.E.; Glotzer, S.C.; Solomon, M.J. Metastable orientational order of colloidal discoids. Nat. Commun. 2015, 6, 8507. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.K.; Zumbusch, A.; Pfleiderer, P. Photo-crosslinkable, deformable PMMA colloids. J. Mater. Chem. C 2013, 1, 7228–7236. [Google Scholar] [CrossRef]
- Lu, P.J.; Giavazzi, F.; Angelini, T.E.; Zaccarelli, E.; Jargstorff, F.; Schofield, A.B.; Wilking, J.N.; Romanowsky, M.B.; Weitz, D.A.; Cerbino, R. Characterizing concentrated, multiply scattering, and actively driven fluorescent systems with confocal differential dynamic microscopy. Phys. Rev. Lett. 2012, 108, 218103. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, M.N.; Stiefelhagen, J.C.P.; Heessels-Gürboǧa, G.; van der Hoeven, J.E.S.; Elbers, N.A.; Dijkstra, M.; van Blaaderen, A. Charging of poly(methyl methacrylate) (PMMA) colloids in cyclohexyl bromide: Locking, size dependence, and particle mixtures. Langmuir 2015, 31, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Segrè, P.N.; Behrend, O.P.; Pusey, P.N. Short-time Brownian motion in colloidal suspensions: Experiment and simulation. Phys. Rev. E 1995, 52, 5070–5083. [Google Scholar] [CrossRef]
- Janai, E.; Cohen, A.P.; Butenko, A.V.; Schofield, A.B.; Schultz, M.; Sloutskin, E. Dipolar colloids in apolar media: Direct microscopy of two-dimensional suspensions. Sci. Rep. 2016, 6, 28578. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Shutman, M.; Sloutskin, E.; Butenko, A.V. Locating particles accurately in microscope images requires image-processing kernels to be rotationally symmetric. Opt. Express 2013, 21, 30755–30763. [Google Scholar] [CrossRef] [PubMed]
- Atherton, T.J.; Kerbyson, D.J. Size invariant circle detection. Image Vis. Comput. 1999, 17, 795–803. [Google Scholar] [CrossRef]
- Nanikashvili, P.M.; Butenko, A.V.; Liber, S.R.; Zitoun, D.; Sloutskin, E. Denser fluids of charge-stabilized colloids form denser sediments. Soft Matter 2014, 10, 4913–4921. [Google Scholar] [CrossRef] [PubMed]
- Lange, H. Comparative test of methods to determine particle size and particle size distribution in the submicron range. Part. Part. Syst. Charact. 1995, 12, 148–157. [Google Scholar] [CrossRef]
- Patty, P.J.; Frisken, B.J. Direct determination of the number-weighted mean radius and polydispersity from dynamic light-scattering data. Appl. Opt. 2006, 45, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N.; van Megen, W. Detection of small polydispersities by photon correlation spectroscopy. J. Chem. Phys. 1984, 80, 3513–3520. [Google Scholar] [CrossRef]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Expansion microscopy. Science 2015, 347, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, F.; Cerbino, R. Digital Fourier microscopy for soft matter dynamics. J. Opt. 2014, 16, 083001. [Google Scholar] [CrossRef]
- Sentjabrskaja, T.; Zaccarelli, E.; de Michele, C.; Sciortino, F.; Tartaglia, P.; Voigtmann, T.; Egelhaaf, S.U.; Laurati, M. Anomalous dynamics of intruders in a crowded environment of mobile obstacles. Nat. Commun. 2016, 7, 11133. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.W.; Sainis, S.K.; Dufresne, E.R. Many-body electrostatic forces between colloidal particles at vanishing ionic strength. Phys. Rev. Lett. 2009, 103, 138301. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.C.; Grier, D.G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 1996, 179, 298–310. [Google Scholar] [CrossRef]
- Han, Y.; Grier, D.G. Configurational temperatures and interactions in charge-stabilized colloid. J. Chem. Phys. 2005, 122, 064907. [Google Scholar] [CrossRef] [PubMed] Note that Equation (28) is fully correct in this work, while a typo appears in this equation in some other papers by the same group (the calculations were not affected by this typo). In addition, we note that spurious oscillations of U(r) may occasionally occur when the iteration procedure starts with I(r) = 0; for example, this is the case for the g(r) of ideal hard disks. Therefore, we start the iteration procedure with I(r) = (UWCA(r) + log[g(r)])/n, where UWCA is the purely–repulsive and continuous Weeks–Chandler–Andersen potential and kBT = 1.
- Wilkinson, D.R.; Edwards, S.F. The use of stereology to determine the partial two-body correlation functions for hard sphere ensembles. J. Phys. D Appl. Phys. 1982, 15, 551–562. [Google Scholar] [CrossRef]
- Han, Y.; Grier, D.G. Confinement-induced colloidal attractions in equilibrium. Phys. Rev. Lett. 2003, 91, 038302. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N. Microscopy of soft materials. In Liquids, Freezing and the Glass Transition; Hansen, J.P., Levesque, D., Zinn-Justin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Chapter 10; p. 763. [Google Scholar]
- The current definition is adopted from Segre et al. [21]. Slightly different definitions of τR are occasionally used in the literature, see e.g. Lu et al. [19]. In our case, the exact definition does not matter, as we are only interested in the t ≪ τR limit.
- Gapinski, J.; Patkowski, A.; Banchio, A.J.; Holmqvist, P.; Meier, G.; Lettinga, M.P.; Nägele, G. Collective diffusion in charge-stabilized suspensions: Concentration and salt effects. J. Chem. Phys. 2007, 126, 104905. [Google Scholar] [CrossRef] [PubMed]
- Gapinski, J.; Patkowski, A.; Banchio, A.J.; Buitenhuis, J.; Holmqvist, P.; Lettinga, M.P.; Meier, G.; Nägele, G. Structure and short-time dynamics in suspensions of charged silica spheres in the entire fluid regime. J. Chem. Phys. 2009, 130, 084503. [Google Scholar] [CrossRef] [PubMed]
- Gapinski, J.; Patkowski, A.; Nägele, G. Generic behavior of the hydrodynamic function of charged colloidal suspensions. J. Chem. Phys. 2010, 132, 054510. [Google Scholar] [CrossRef] [PubMed]
- Abade, G.C.; Cichocki, B.; Ekiel-Jezewska, M.L.; Nägele, G.; Wajnryb, E. Short-time dynamics of permeable particles in concentrated suspensions. J. Chem. Phys. 2010, 132, 014503. [Google Scholar] [CrossRef] [PubMed]
- Ladd, A.J.C. Numerical simulations of particulate suspensions via a discretized Boltzmann equation. Part I. Theoretical foundation. J. Fluid Mech. 1994, 271, 285–309. [Google Scholar] [CrossRef]
- Behrend, O.P. Solid-fluid boundaries in particle suspension simulations via the lattice Boltzmann method. Phys. Rev. E 1995, 52, 1164–1175. [Google Scholar] [CrossRef]
- Kanai, T.; Boon, N.; Lu, P.J.; Sloutskin, E.; Schofield, A.B.; Smallenburg, F.; van Roij, R.; Dijkstra, M.; Weitz, D.A. Crystallization and reentrant melting of charged colloids in nonpolar solvents. Phys. Rev. E 2015, 91, 030301(R). [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pfleiderer, P.; Schofield, A.B.; Clasen, C.; Vermant, J. Synthesis and directed self-assembly of patterned anisometric polymeric particles. J. Am. Chem. Soc. 2011, 133, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Dinsmore, A.D.; Weeks, E.R.; Prasad, V.; Levitt, A.C.; Weitz, D.A. Three-dimensional confocal microscopy of colloids. Appl. Opt. 2001, 40, 4152–4159. [Google Scholar] [CrossRef] [PubMed]
- Butenko, A.V.; Mogilko, E.; Amitai, L.; Pokroy, B.; Sloutskin, E. Coiled to diffuse: Brownian motion of a helical bacterium. Langmuir 2012, 28, 12941–12947. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Sims, P.A.; Oki, H.; Macarthur, J.B.; Weitz, D.A. Target-locking acquisition with real-time confocal (TARC) microscopy. Opt. Express 2007, 15, 8702–8712. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.P.; Alesker, M.; Schofield, A.B.; Zitoun, D.; Sloutskin, E. Photo-Crosslinkable Colloids: From Fluid Structure and Dynamics of Spheres to Suspensions of Ellipsoids. Gels 2016, 2, 29. https://doi.org/10.3390/gels2040029
Cohen AP, Alesker M, Schofield AB, Zitoun D, Sloutskin E. Photo-Crosslinkable Colloids: From Fluid Structure and Dynamics of Spheres to Suspensions of Ellipsoids. Gels. 2016; 2(4):29. https://doi.org/10.3390/gels2040029
Chicago/Turabian StyleCohen, Avner P., Maria Alesker, Andrew B. Schofield, David Zitoun, and Eli Sloutskin. 2016. "Photo-Crosslinkable Colloids: From Fluid Structure and Dynamics of Spheres to Suspensions of Ellipsoids" Gels 2, no. 4: 29. https://doi.org/10.3390/gels2040029
APA StyleCohen, A. P., Alesker, M., Schofield, A. B., Zitoun, D., & Sloutskin, E. (2016). Photo-Crosslinkable Colloids: From Fluid Structure and Dynamics of Spheres to Suspensions of Ellipsoids. Gels, 2(4), 29. https://doi.org/10.3390/gels2040029