Micromechanical Characterization of Hydrogels Undergoing Swelling and Dissolution at Alkaline pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Whey Protein Hydrogels
2.2. Dynamic Indentation Testing
2.3. Data Analysis
3. Results and Discussions
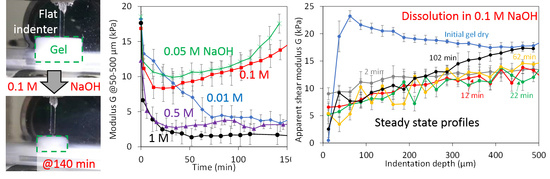
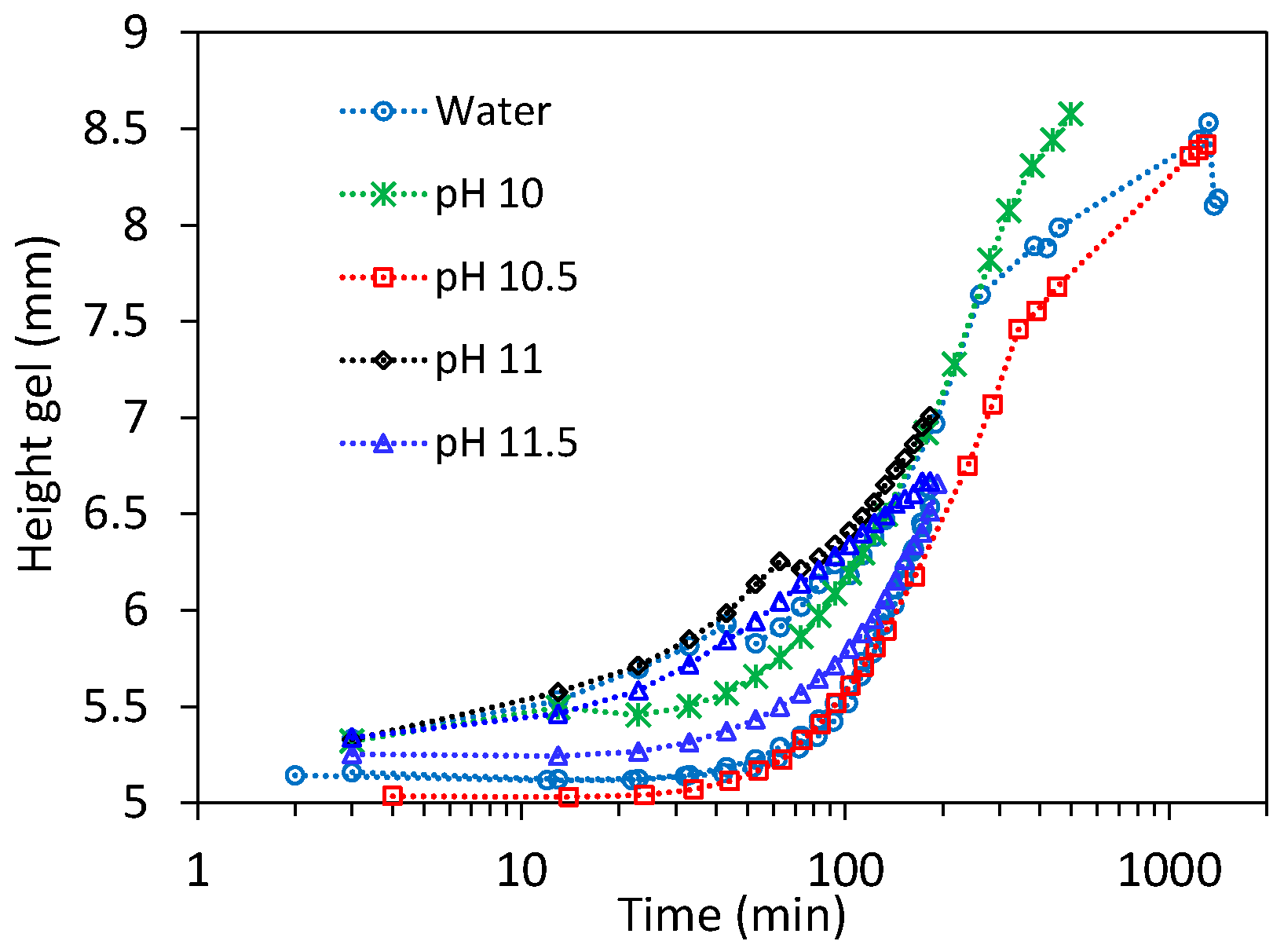
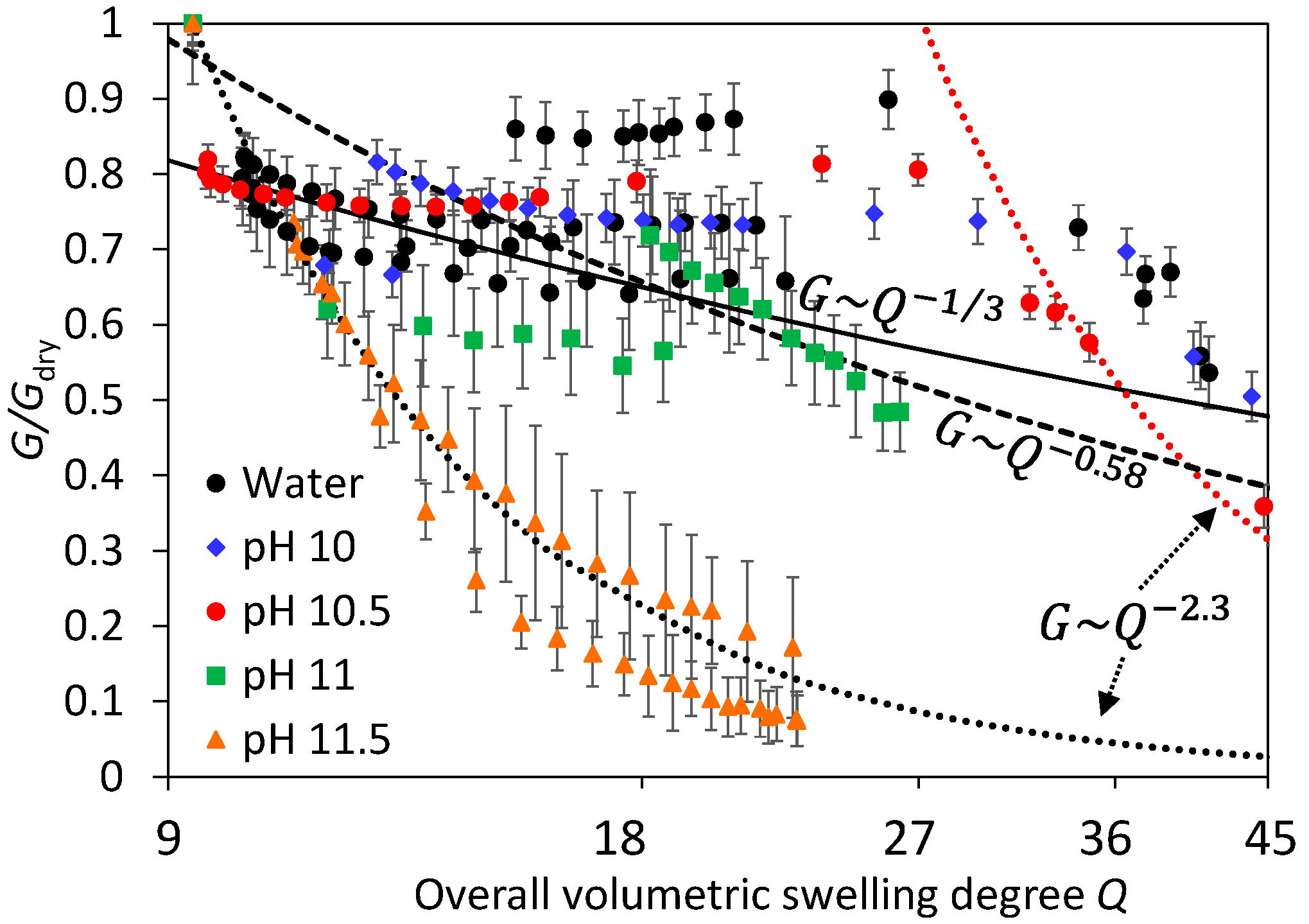
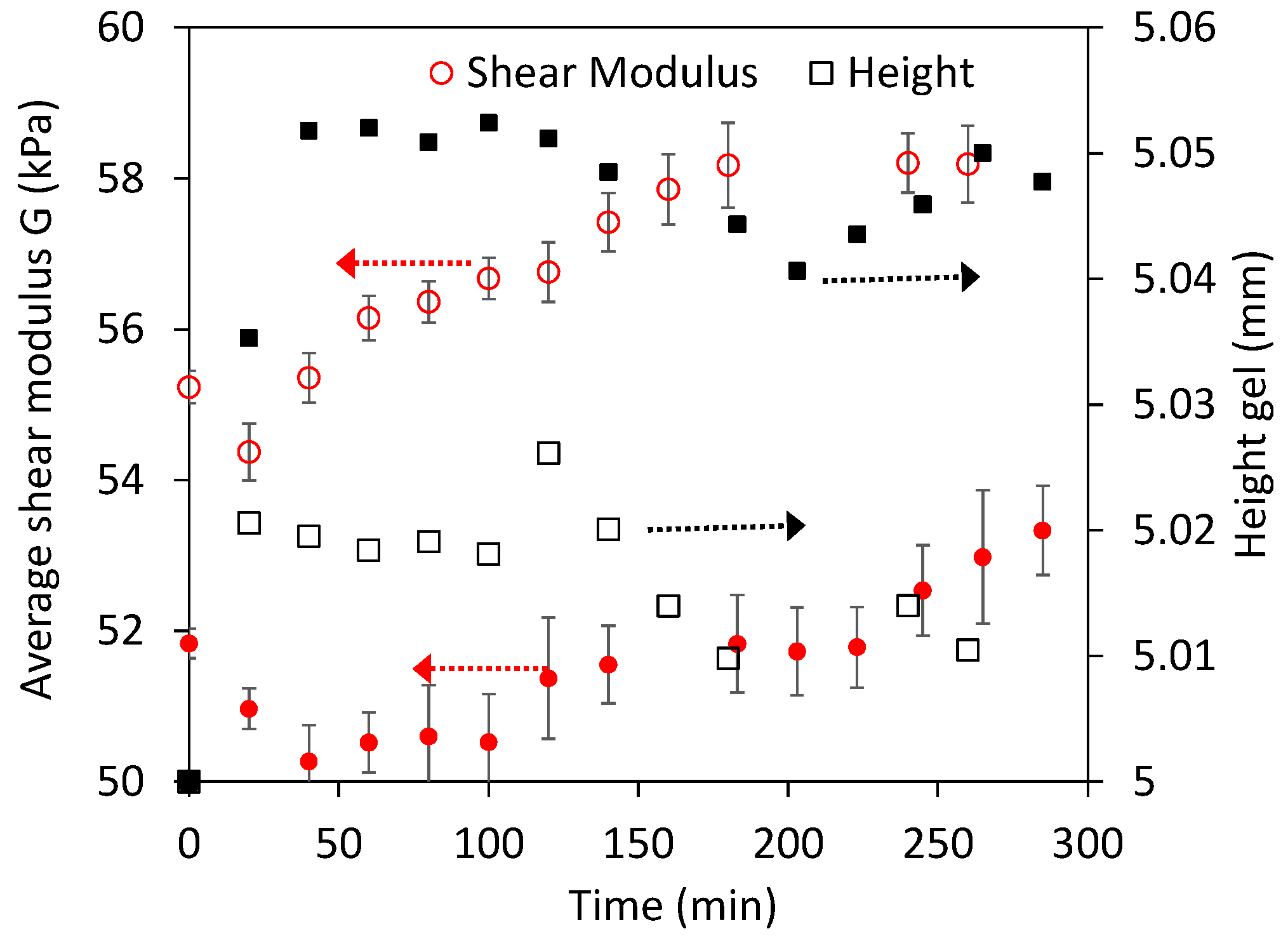
3.1. Swelling Experiments at Low NaOH Concentrations
3.2. Shrinking Experiments at High NaCl Concentrations
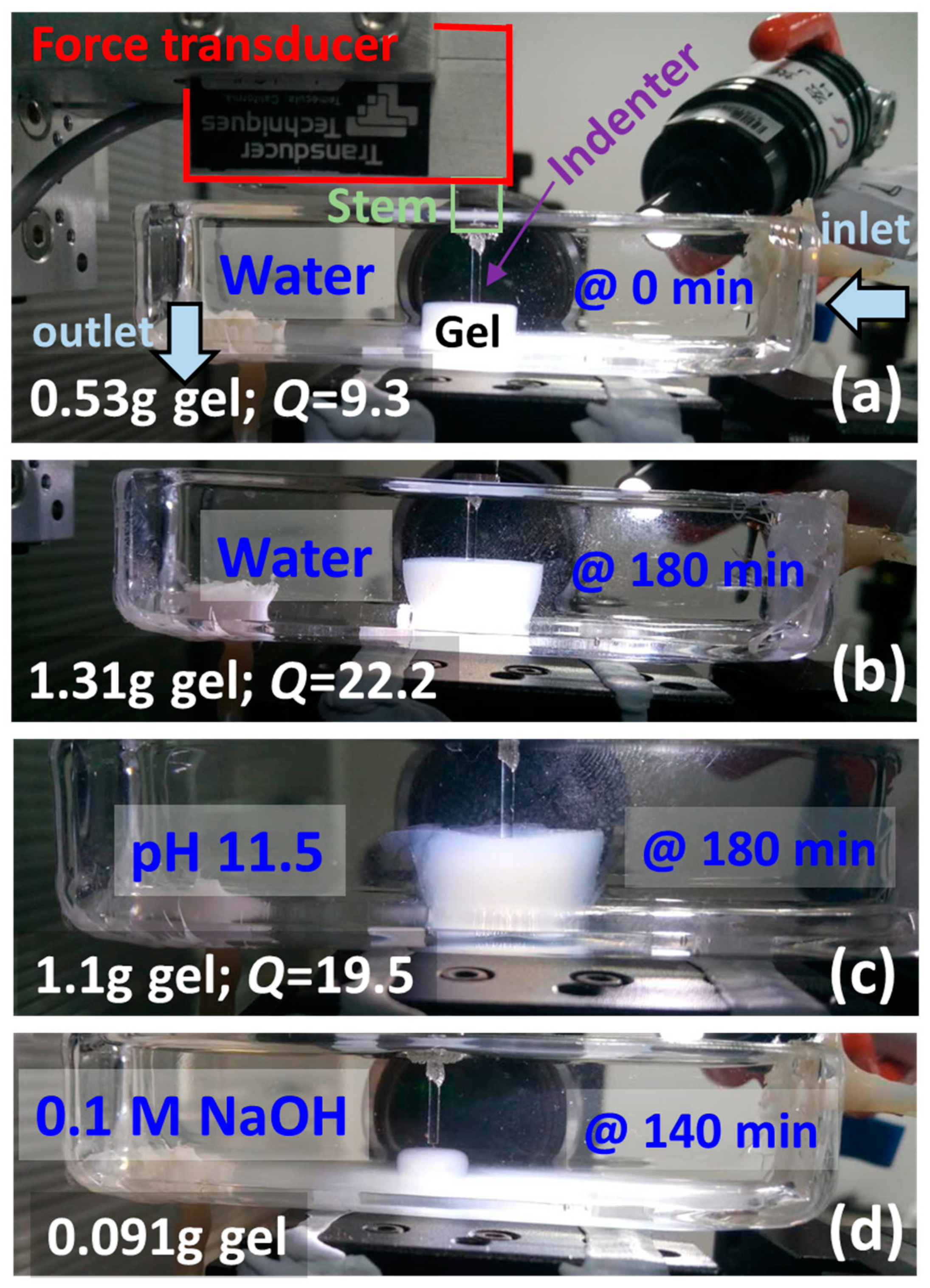
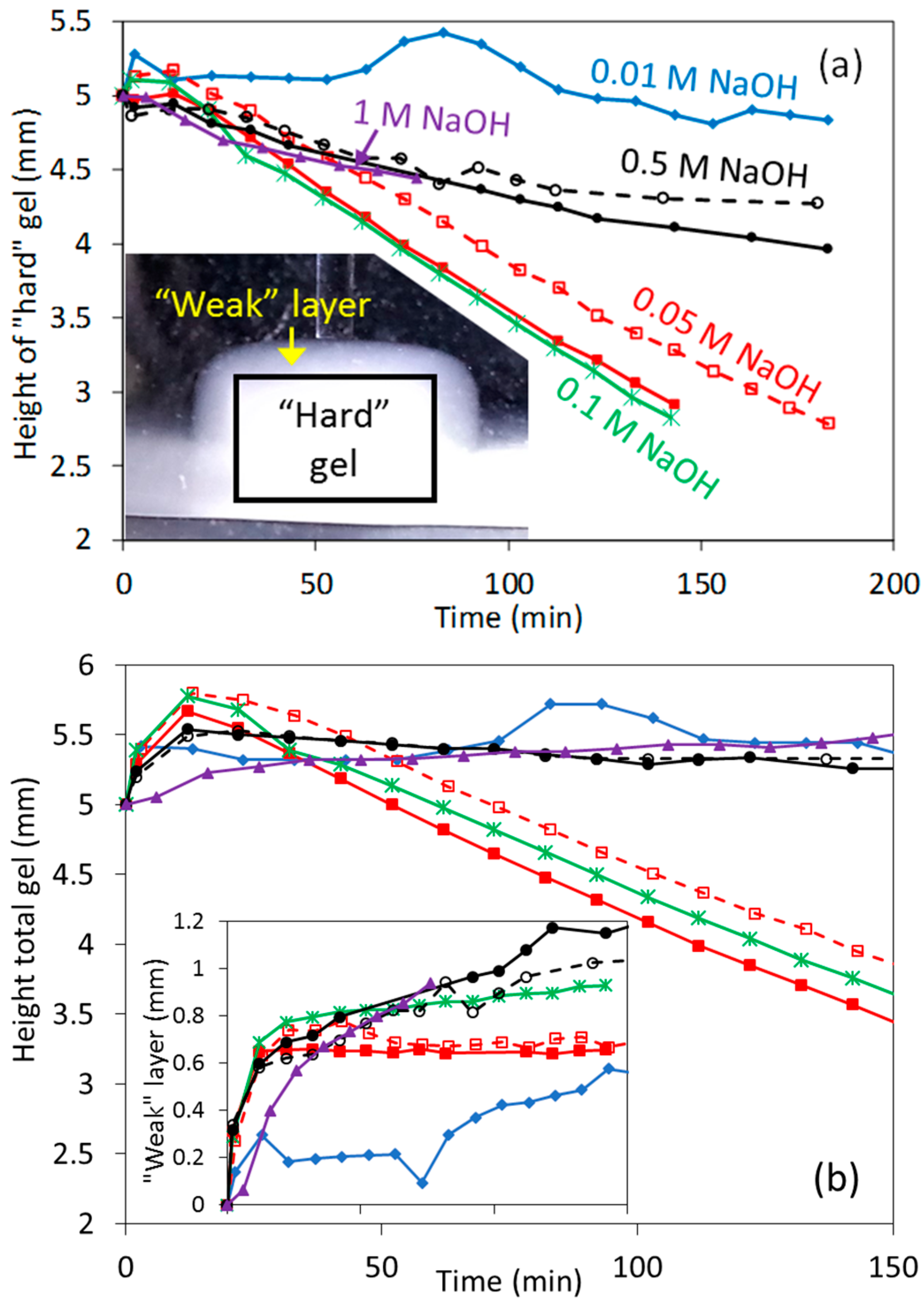
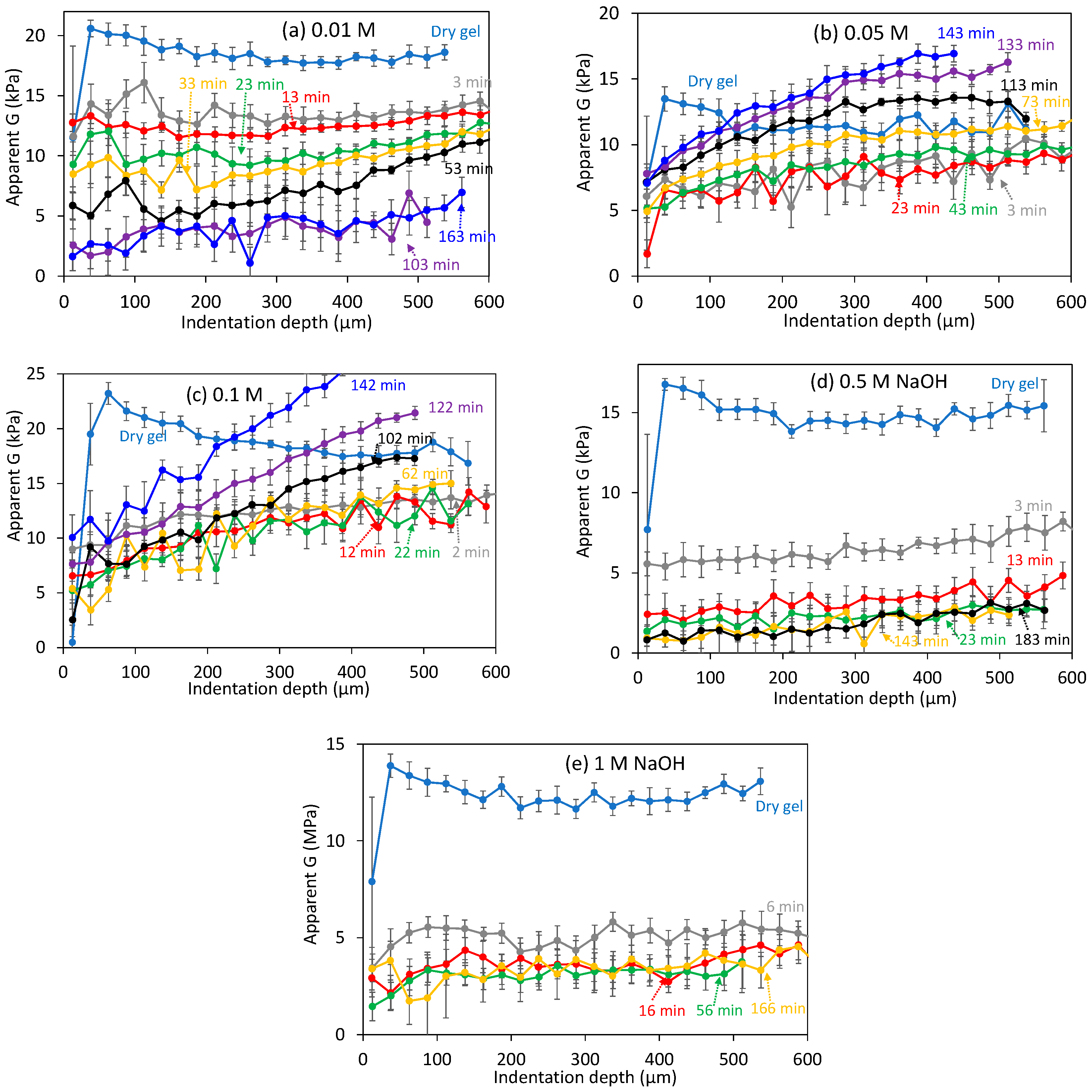
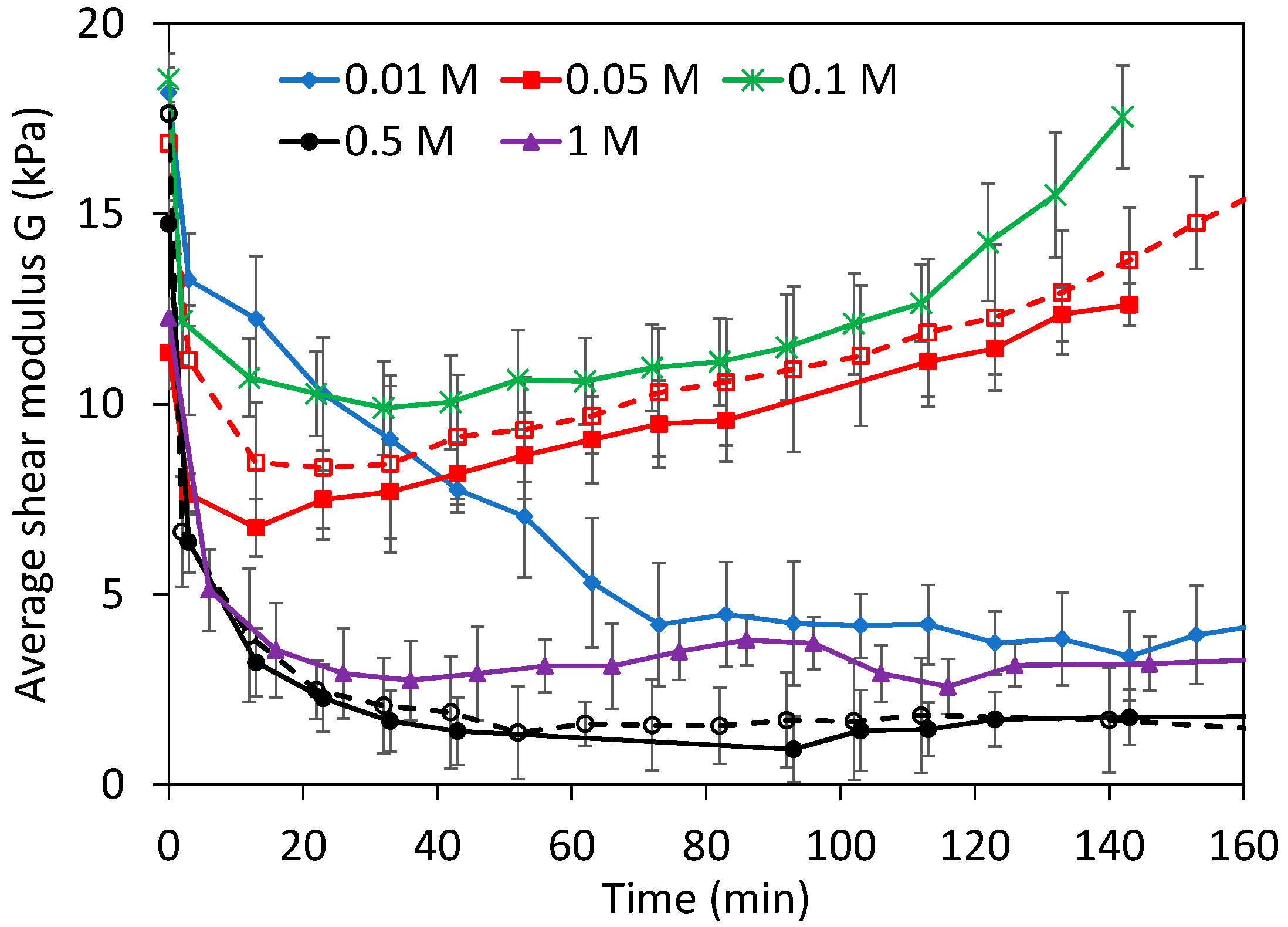
3.3. Dissolution Experiments at High NaOH Concentrations
3.4. Non-Elastic Deformation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvarez, N.; Daufin, G.; Gésan-Guiziou, G. Recommendations for rationalizing cleaning-in-place in the dairy industry: Case study of an ultra-high temperature heat exchanger. J. Dairy Sci. 2010, 93, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Colby, R.H.; Dobrynin, A.V.; Joanny, J.-F. Elastic modulus and equilibrium swelling of polyelectrolyte gels. Macromolecules 1996, 29, 398–406. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Chen, X.D. Dissolution of whey protein concentrate gels in alkali. AIChE J. 2006, 52, 792–803. [Google Scholar] [CrossRef]
- Gordon, P.W.; Brooker, A.D.; Chew, Y.J.; Wilson, D.I.; York, D.W. A scanning fluid dynamic gauging technique for probing surface layers. Meas. Sci. Technol. 2010, 21, 085103. [Google Scholar] [CrossRef]
- Chew, J.Y.M.; Paterson, W.R.; Wilson, D.I. Fluid dynamic gauging for measuring the strength of soft deposits. J. Food Eng. 2004, 65, 175–187. [Google Scholar] [CrossRef]
- Möhle, R.B.; Langemann, T.; Haesner, M.; Augustin, W.; Scholl, S.; Neu, T.R.; Hempel, D.C.; Horn, H. Structure and shear strength of microbial biofilms as determined with confocal laser scanning microscopy and fluid dynamic gauging using a novel rotating disc biofilm reactor. Biotechnol. Bioeng. 2007, 98, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Saikhwan, P.; Mercadé-Prieto, R.; Chew, Y.; Gunasekaran, S.; Paterson, W.; Wilson, D.I. Swelling and dissolution in cleaning of whey protein gels. Food Bioprod. Process. 2010, 88, 375–383. [Google Scholar] [CrossRef]
- Dumitru, A.C.; Espinosa, F.M.; Garcia, R.; Foschi, G.; Tortorella, S.; Valle, F.; Dallavalle, M.; Zerbetto, F.; Biscarini, F. In situ nanomechanical characterization of the early stages of swelling and degradation of a biodegradable polymer. Nanoscale 2015, 7, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Boukari, H.; Leach, J.B. Solute diffusion and interactions in cross-linked poly (ethylene glycol) hydrogels studied by fluorescence correlation spectroscopy. Soft Matter 2010, 6, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Vauthier, C.; Huang, N.; Grossiord, J.-L.; Moine, L.; Agnely, F. Degradation of hydrolyzable hydrogel microspheres. Soft Matter 2013, 9, 1929–1936. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Chen, X.D.; Mercadé-Prieto, R. Swelling of whey and egg white protein hydrogels with stranded and particulate microstructures. Int. J. Biol. Macromol. 2016, 83, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Hu, B.-W. Load relaxation of a flat rigid circular indenter on a gel half space. J. Non-Cryst. Solids 2006, 352, 4034–4040. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Vlassak, J.J.; Suo, Z. Using indentation to characterize the poroelasticity of gels. Appl. Phys. Lett. 2010, 96, 121904. [Google Scholar] [CrossRef]
- Langley, K.R.; Green, M.L. Compression and impact strength of gels, prepared from fractionated whey proteins, in relation to composition and microstructure. J. Dairy Res. 1989, 56, 275–284. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, D.; Raabe, D. The use of flat punch indentation to determine the viscoelastic properties in the time and frequency domains of a soft layer bonded to a rigid substrate. Acta Biomater. 2009, 5, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Mercadé-Prieto, R. Spatial quantification of hydrogels swelling using wide-field fluorescence microscopy. Chem. Eng. Sci. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Lee, D.; Barber, J.R.; Thouless, M.D. Indentation of an elastic half space with material properties varying with depth. Int. J. Eng. Sci. 2009, 47, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Agee, P.; Herbert, E. Continuous stiffness measurement during instrumented indentation testing. Exp. Tech. 2010, 34, 86–94. [Google Scholar] [CrossRef]
- Long, R.; Hall, M.S.; Wu, M.; Hui, C.-Y. Effects of Gel Thickness on Microscopic Indentation Measurements of Gel Modulus. Biophys. J. 2011, 101, 643–650. [Google Scholar] [CrossRef] [PubMed]
- ASTM 2015 Standard Test Method for Compressive Properties of Rigid Plastics ASTM D695-15, (n.d.). Available online: http://dx.doi.org/10.1520/D0695-15 (accessed on 30 September 2017).
- Horkay, F.; Zrinyi, M. Studies on the mechanical and swelling behavior of polymer networks based on the scaling concept. 4. Extension of the scaling approach to gels swollen to equilibrium in a diluent of arbitrary activity. Macromolecules 1982, 15, 1306–1310. [Google Scholar] [CrossRef]
- Lin, D.C.; Douglas, J.F.; Horkay, F. Development of minimal models of the elastic properties of flexible and stiff polymer networks with permanent and thermoreversible cross-links. Soft Matter 2010, 6, 3548–3561. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.F.; McKenna, G.B. The effect of swelling on the elasticity of rubber: Localization model description. Macromolecules 1993, 26, 3282–3288. [Google Scholar] [CrossRef]
- Obukhov, S.P.; Rubinstein, M.; Colby, R.H. Network Modulus and Superelasticity. Macromolecules 1994, 27, 3191–3198. [Google Scholar] [CrossRef]
- Horkay, F.; Basser, P.J. Osmotic Observations on Chemically Cross-Linked DNA Gels in Physiological Salt Solutions. Biomacromolecules 2004, 5, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Menut, P.; Seiffert, S.; Sprakel, J.; Weitz, D.A. Does size matter? Elasticity of compressed suspensions of colloidal- and granular-scale microgels. Soft Matter 2012, 8, 156–164. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Biopolymer gel swelling analysed with scaling laws and Flory–Rehner theory. Food Hydrocoll. 2015, 48, 94–101. [Google Scholar] [CrossRef]
- Urayama, K.; Kawamura, T.; Kohjiya, S. Elastic modulus and equilibrium swelling of networks crosslinked by end-linking oligodimethylsiloxane at solution state. J. Chem. Phys. 1996, 105, 4833–4840. [Google Scholar] [CrossRef]
- Horkay, F.; McKenna, G.B. Polymer Networks and Gels. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 497–523. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Paterson, W.R.; Wilson, D.I. The pH threshold in the dissolution of β-lactoglobulin gels and aggregates in alkali. Biomacromolecules 2007, 8, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.M.; Pool, M.D.; Metters, A.T. Influence of Network Structure on the Degradation of Photo-Cross-Linked PLA-b-PEG-b-PLA Hydrogels. Biomacromolecules 2006, 7, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Havea, P.; Watkinson, P.; Kuhn-Sherlock, B. Heat-Induced Whey Protein Gels: Protein− Protein Interactions and Functional Properties. J. Agric. Food Chem. 2009, 57, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Mercadé-Prieto, R.; Falconer, R.J.; Paterson, W.R.; Wilson, D.I. Effect of gel structure on the dissolution of heat-induced β-lactoglobulin gels in alkali. J. Agric. Food Chem. 2006, 54, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Horkay, F.; Lin, D.C. Mapping the Local Osmotic Modulus of Polymer Gels. Langmuir 2009, 25, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Mercadé-Prieto, R.; Paterson, W.R.; Wilson, D.I. Effect of salts on the alkaline degradation of β-lactoglobulin gels and aggregates: Existence of a dissolution threshold. Food Hydrocoll. 2009, 23, 1587–1595. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Paterson, W.R.; Chen, X.D.; Wilson, D.I. Diffusion of NaOH into a protein gel. Chem. Eng. Sci. 2008, 63, 2763–2772. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Lopez, J.; Chen, X.D. Poroelastic relaxation indentation of whey protein hydrogels. Food Hydrocoll. 2016, 54, 221–226. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Martin, F.; Jeantet, R.; Chen, X.D.; Mercadé-Prieto, R. Micromechanical Characterization of Hydrogels Undergoing Swelling and Dissolution at Alkaline pH. Gels 2017, 3, 44. https://doi.org/10.3390/gels3040044
Hu W, Martin F, Jeantet R, Chen XD, Mercadé-Prieto R. Micromechanical Characterization of Hydrogels Undergoing Swelling and Dissolution at Alkaline pH. Gels. 2017; 3(4):44. https://doi.org/10.3390/gels3040044
Chicago/Turabian StyleHu, Wei, Francois Martin, Romain Jeantet, Xiao Dong Chen, and Ruben Mercadé-Prieto. 2017. "Micromechanical Characterization of Hydrogels Undergoing Swelling and Dissolution at Alkaline pH" Gels 3, no. 4: 44. https://doi.org/10.3390/gels3040044