Non-Conventional Methods for Gelation of Alginate
Abstract
:1. Introduction
2. Cryogelation of Alginate
2.1. Cation-Free Cryogelation
2.2. Ionotropic Cryogelation
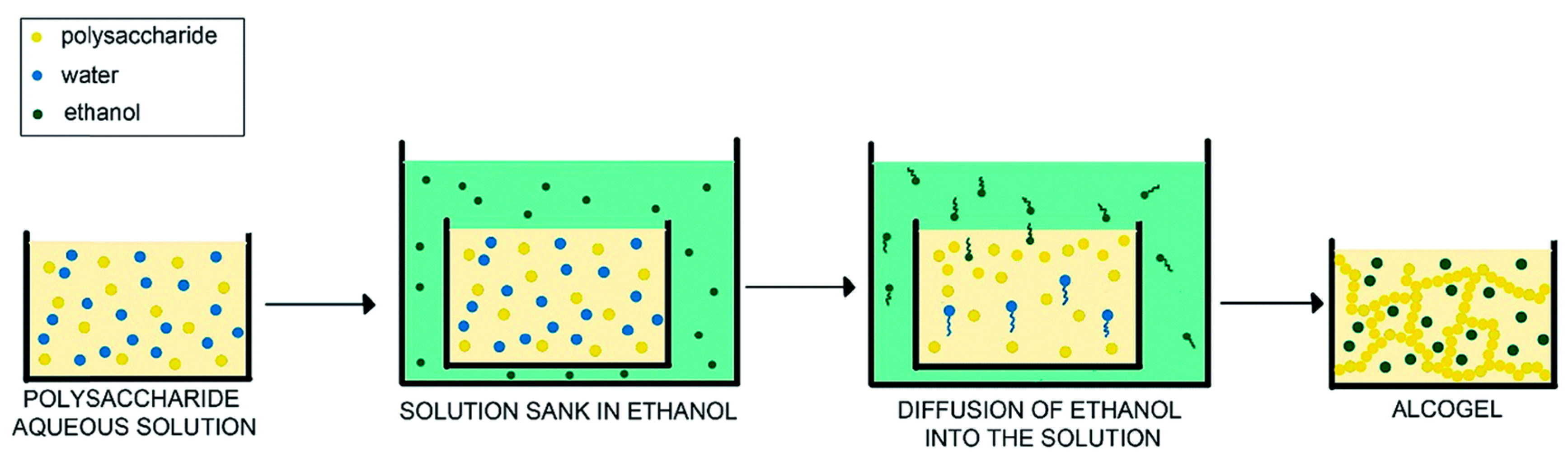
3. Non-Solvent Induced Phase Separation
4. Carbon Dioxide Induced Gelation
5. Other Gelation Methods
6. Drying of Alginate Gels
7. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donati, I.; Paoletti, S. Material Properties of Alginates. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–53. ISBN 978-3-540-92678-8. [Google Scholar]
- Yuguchi, Y.; Hasegawa, A.; Padoł, A.M.; Draget, K.I.; Stokke, B.T. Local structure of Ca2+ induced hydrogels of alginate-oligoguluronate blends determined by small-angle-X-ray scattering. Carbohydr. Polym. 2016, 152, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Drach, M. Calcium-α-l-Guluronate Complexes: Ca2+ Binding Modes from DFT-MD Simulations. J. Phys. Chem. B 2013, 117, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Draget, K.I. Alginate Gelation Technologies. In Food Colloids; Bergenståhl, B., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2004; pp. 279–293. ISBN 978-1-85573-783-9. [Google Scholar]
- Lozinsky, V.I. Polymeric cryogels as a new family of macroporous and supermacroporous materials for biotechnological purposes. Russ. Chem. Bull. 2008, 57, 1015–1032. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Giannouli, P.; Morris, E.R. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze-thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Florián-Algarín, V.; Acevedo, A. Rheology and Thermotropic Gelation of Aqueous Sodium Alginate Solutions. J. Pharm. Innov. 2010, 5, 37–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-thaw induced gelation of alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chhatri, A.; Bajpai, J.; Bajpai, A.K.; Sandhu, S.S.; Jain, N.; Biswas, J. Cryogenic fabrication of savlon loaded macroporous blends of alginate and polyvinyl alcohol (PVA). Swelling, deswelling and antibacterial behaviors. Carbohydr. Polym. 2011, 83, 876–882. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze–thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tripathi, A.; Zo, S.; Singh, D.; Han, S.S. Synthesis of composite gelatin-hyaluronic acid-alginate porous scaffold and evaluation for in vitro stem cell growth and in vivo tissue integration. Colloids Surf. B Biointerfaces 2014, 116, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Gao, Y.; Zhang, Y.; Yu, W.; Yang, Y.; Shen, S.; Zhang, S.; Zhu, L.; Xu, L.; Tian, B.; et al. Fabrication and Use of Alginate-Based Cryogel Delivery Beads Loaded with Urea and Phosphates as Potential Carriers for Bioremediation. Ind. Eng. Chem. Res. 2016, 55, 7655–7660. [Google Scholar] [CrossRef]
- Ho, M.-H.; Kuo, P.-Y.; Hsieh, H.-J.; Hsien, T.-Y.; Hou, L.-T.; Lai, J.-Y.; Wang, D.-M. Preparation of porous scaffolds by using freeze–extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Mattiasson, B.; Kumar, A.; Galeaev, I.Y. Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-8462-7. [Google Scholar]
- Tkalec, G.; Kranvogl, R.; Perva Uzunalić, A.; Knez, Ž.; Novak, Z. Optimisation of critical parameters during alginate aerogels’ production. J. Non-Cryst. Solids 2016, 443, 112–117. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; De Marco, I.; Reverchon, E. Chitosan scaffolds formation by a supercritical freeze extraction process. J. Supercrit. Fluids 2014, 90, 27–34. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. In Saline Water Conversion—II; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1963; Volume 38, pp. 117–132. ISBN 978-0-8412-0039-5. [Google Scholar]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Wan, L.S.C.; Heng, P.W.S.; Chan, L.W. Drug encapsulation in alginate microspheres by emulsification. J. Microencapsul. 1992, 9, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 2007, 8, E87–E93. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, D.; Scott, A. Hydrophobic interaction in the gelation of high methoxyl pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast production of high-methoxyl pectin aerogels for enhancing the bioavailability of low-soluble drugs. J. Supercrit. Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
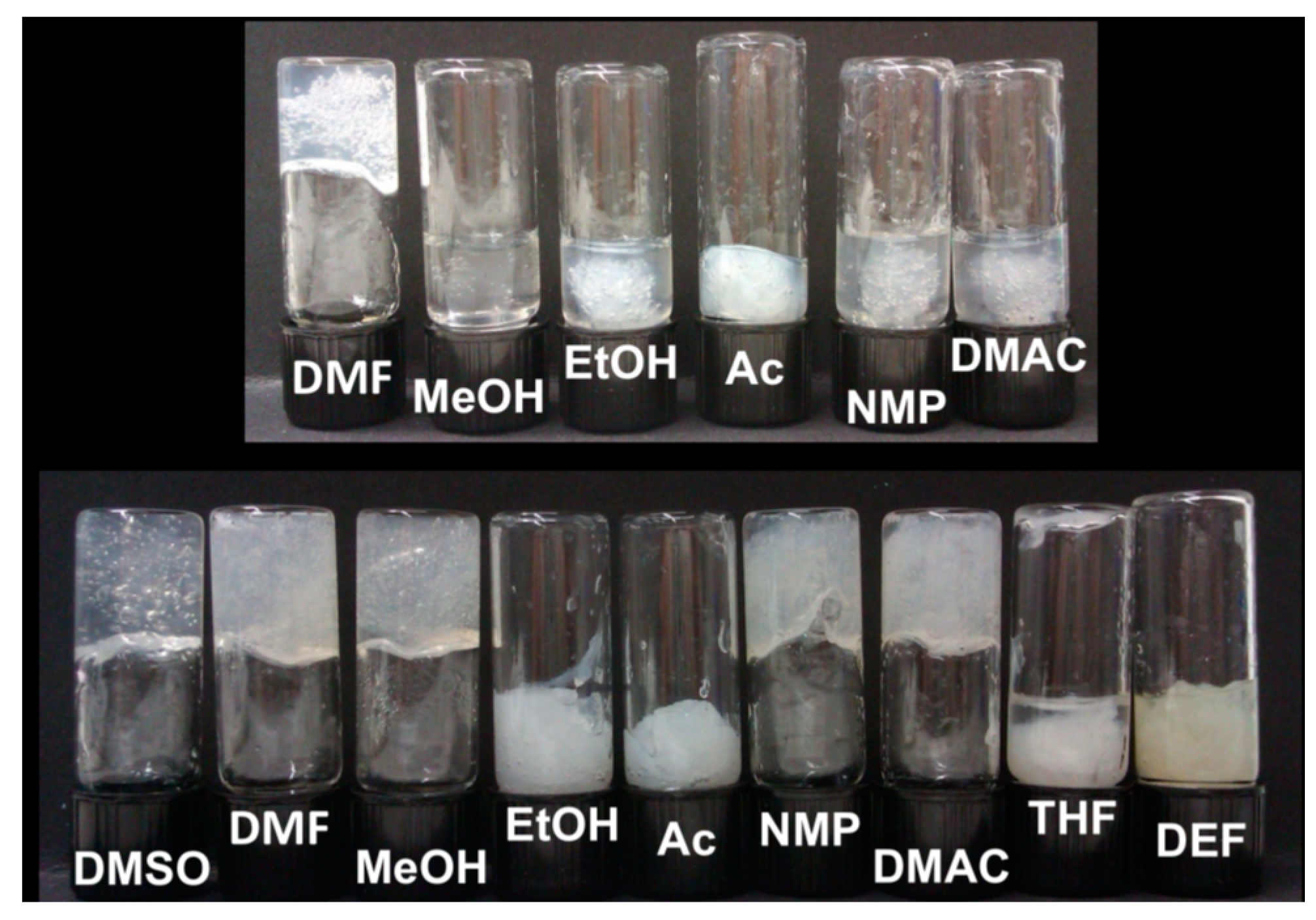
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Ghafar, A.; Gurikov, P.; Subrahmanyam, R.; Parikka, K.; Tenkanen, M.; Smirnova, I.; Mikkonen, K.S. Mesoporous guar galactomannan based biocomposite aerogels through enzymatic crosslinking. Compos. Part A Appl. Sci. Manuf. 2017, 94, 93–103. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Renzo, F.D.; Quignard, F. Nanostructure of Calcium Alginate Aerogels Obtained from Multistep Solvent Exchange Route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
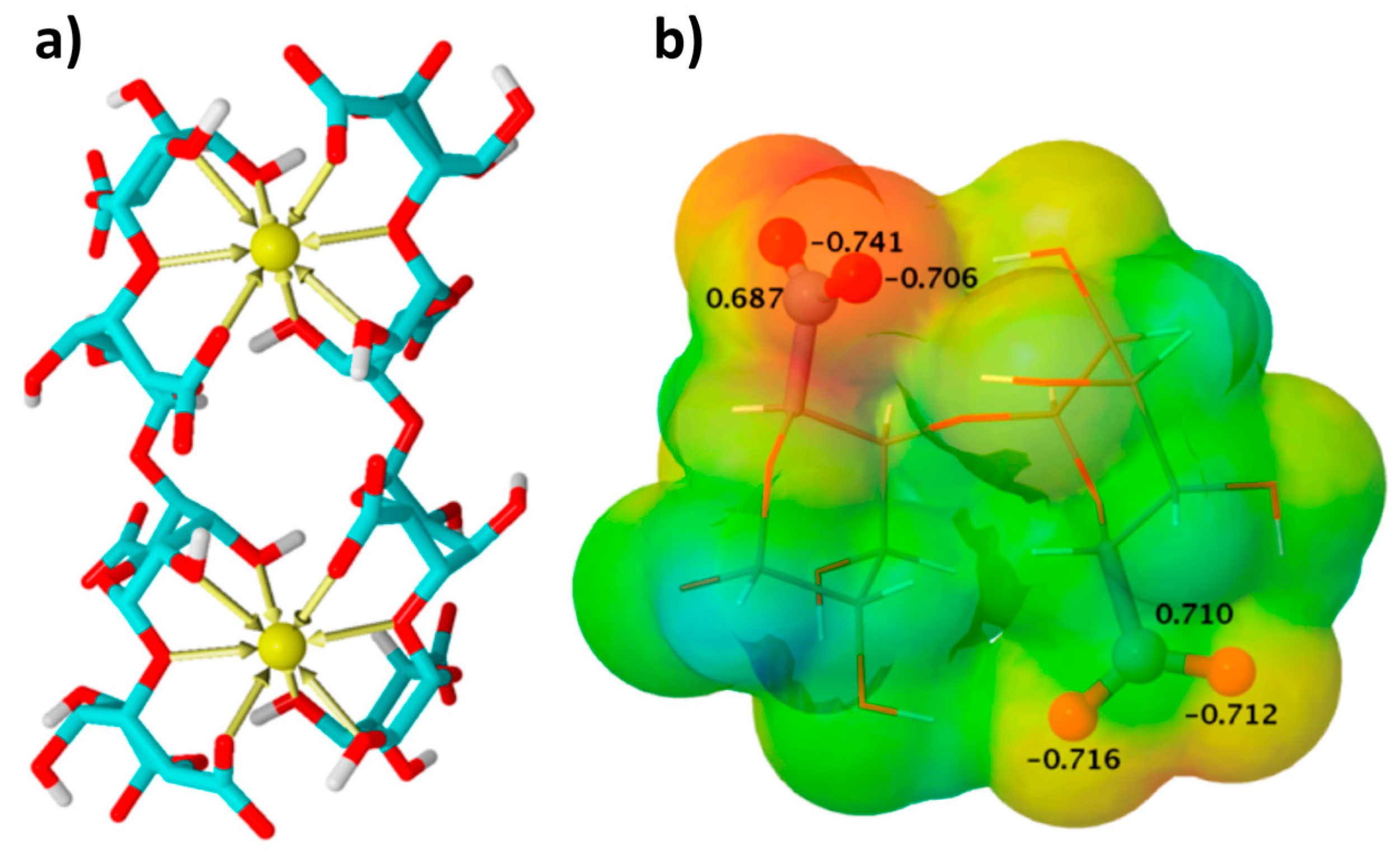
- Pérez-Madrigal, M.M.; Torras, J.; Casanovas, J.; Häring, M.; Aleman, C.; Díaz Díaz, D. A paradigm shift for preparing versatile M2+-free gels from unmodified sodium alginate. Biomacromolecules 2017, 18, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Themistou, E.; Sarkar, B.; Tsianou, M.; Alexandridis, P. Structure and dynamics of dextran in binary mixtures of a good and a bad solvent. Colloid Polym. Sci. 2010, 288, 1301–1312. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple Steps and Critical Behaviors of the Binding of Calcium to Alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, J.; Huang, Y.; Li, D.; Chen, X. Improving surface and mechanical properties of alginate films by using ethanol as a co-solvent during external gelation. Carbohydr. Polym. 2015, 123, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Vicini, S.; Mauri, M.; Wichert, J.; Castellano, M. Alginate gelling process: Use of bivalent ions rich microspheres. Polym. Eng. Sci. 2017, 57, 531–536. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Borisova, A.; De Bruyn, M.; Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Segatto, M.L.; Clark, J.H. A Sustainable Freeze-Drying Route to Porous Polysaccharides with Tailored Hierarchical Meso- and Macroporosity. Macromol. Rapid Commun. 2015, 36, 774–779. [Google Scholar] [CrossRef] [PubMed]
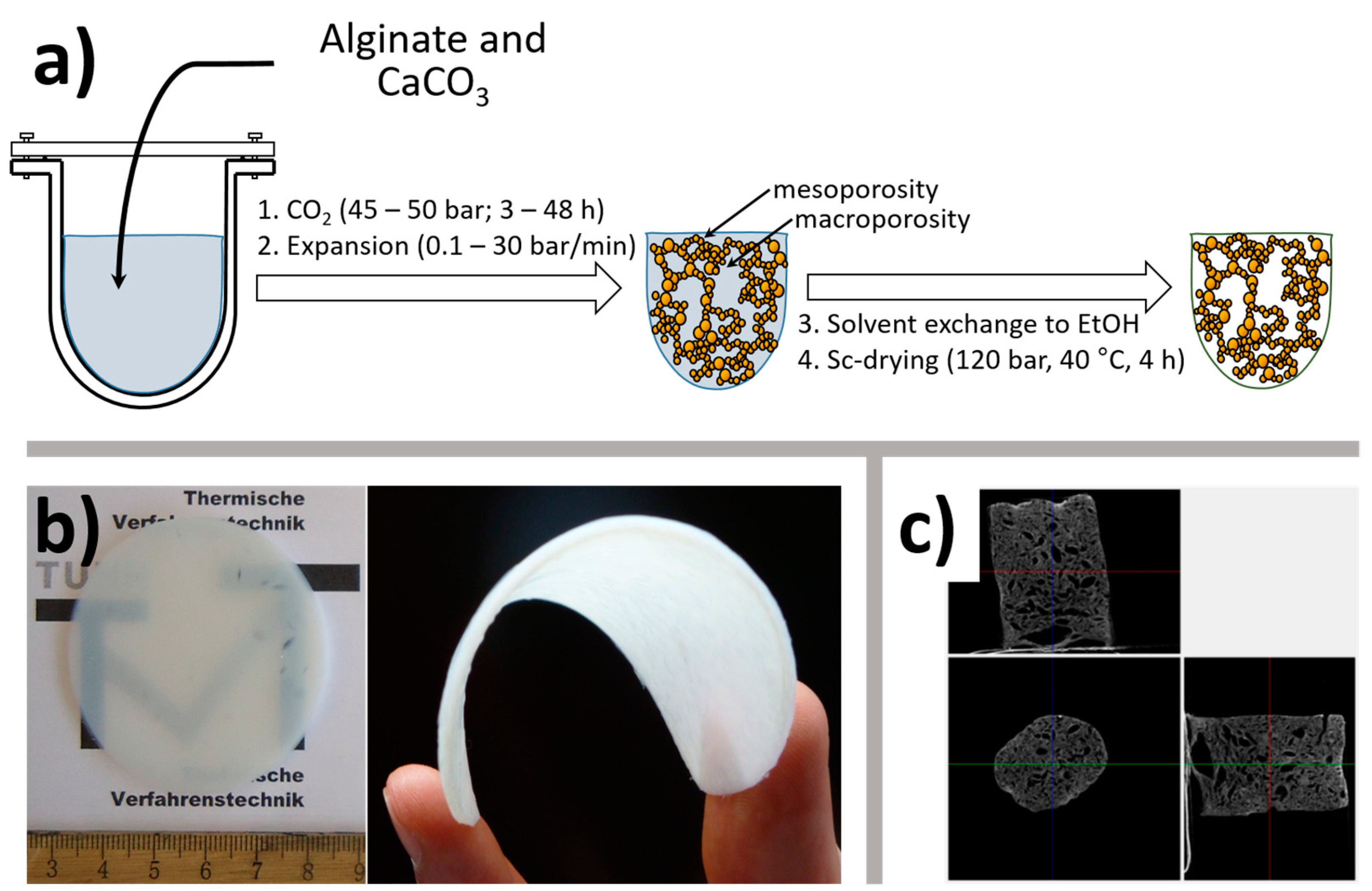
- Gurikov, P.; Raman, S.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Raman, S.P.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- Draget, K.I.; Østgaard, K.; Smidsrød, O. Homogeneous alginate gels: A technical approach. Carbohydr. Polym. 1990, 14, 159–178. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate-lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Barbetta, A.; Barigelli, E.; Dentini, M. Porous alginate hydrogels: Synthetic methods for tailoring the porous texture. Biomacromolecules 2009, 10, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Fricke, M.; Weinrich, D.; Lölsberg, W.; Subrahmanyam, R.; Smirnova, I.; Gurikov, P. Process for Producing Porous Alginate-Based Aerogels. Patent WO2015177081 A1, 26 November 2015. [Google Scholar]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of Biopolymer Aerogels Using Green Solvents. J. Vis. Exp. 2016, e54116. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon Dioxide Induced Silk Protein Gelation for Biomedical Applications. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high pressure CO2. Biomaterials 2010, 31, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lu, A.X.; Javvaji, V.; DeVoe, D.L.; Raghavan, S.R. Light-Directed Self-Assembly of Robust Alginate Gels at Precise Locations in Microfluidic Channels. ACS Appl. Mater. Interfaces 2016, 8, 17529–17538. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Qu, X.; Lei, M.; Zhang, C.; Hong, H.; Payne, G.F.; Liu, C. Electrical signals triggered controllable formation of calcium-alginate film for wound treatment. J. Mater. Sci. Mater. Med. 2017, 28, 146. [Google Scholar] [CrossRef] [PubMed]
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pour, G.; Beauger, C.; Rigacci, A.; Budtova, T. Xerocellulose: Lightweight, porous and hydrophobic cellulose prepared via ambient drying. J. Mater. Sci. 2015, 50, 4526–4535. [Google Scholar] [CrossRef]
- Zmora, S.; Glicklis, R.; Cohen, S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [CrossRef]
- Barros, A.; Quraishi, S.; Martins, M.; Gurikov, P.; Subrahmanyam, R.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Hybrid Alginate-Based Cryogels for Life Science Applications. Chem. Ing. Tech. 2016, 88, 1770–1778. [Google Scholar] [CrossRef]
- Teagarden, D.L.; Baker, D.S. Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur. J. Pharm. Sci. 2002, 15, 115–133. [Google Scholar] [CrossRef]
- Pons, A.; Casas, L.; Estop, E.; Molins, E.; Harris, K.D.M.; Xu, M. A new route to aerogels: Monolithic silica cryogels. J. Non-Cryst. Solids 2012, 358, 461–469. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in Chemical Engineering: Strategies Toward Tailor-Made Aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Gurikov, P.; Smirnova, I. Amorphization of drugs by adsorptive precipitation from supercritical solutions: A review. J. Supercrit. Fluids 2018, 132, 105–125. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. https://doi.org/10.3390/gels4010014
Gurikov P, Smirnova I. Non-Conventional Methods for Gelation of Alginate. Gels. 2018; 4(1):14. https://doi.org/10.3390/gels4010014
Chicago/Turabian StyleGurikov, Pavel, and Irina Smirnova. 2018. "Non-Conventional Methods for Gelation of Alginate" Gels 4, no. 1: 14. https://doi.org/10.3390/gels4010014






