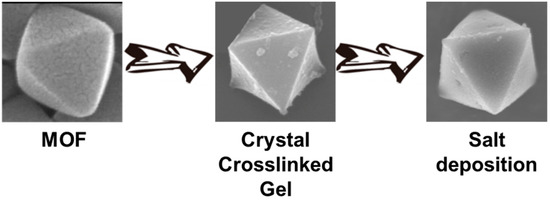
Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes
Abstract
:1. Introduction
2. Results and Discussion
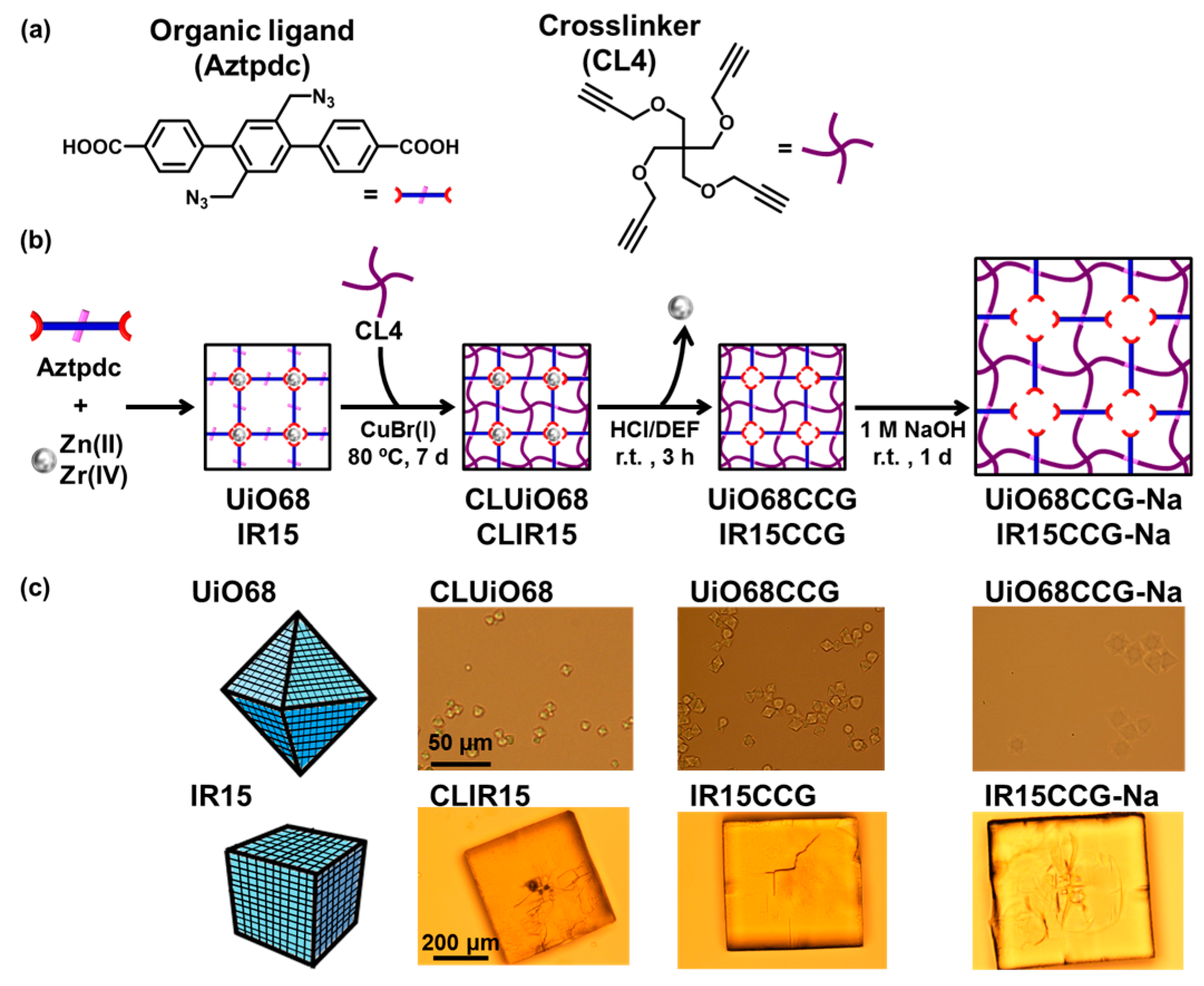
2.1. Preparation
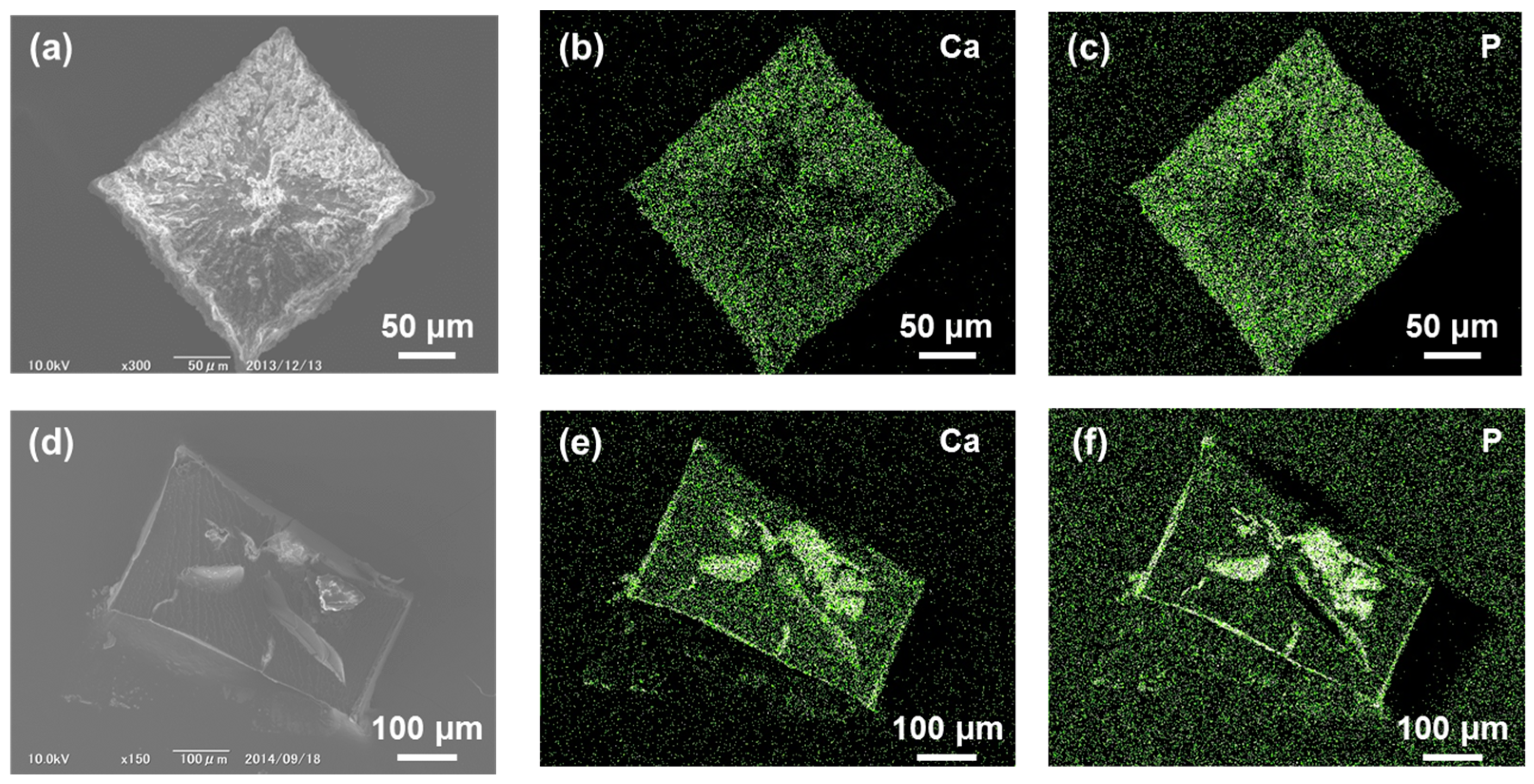
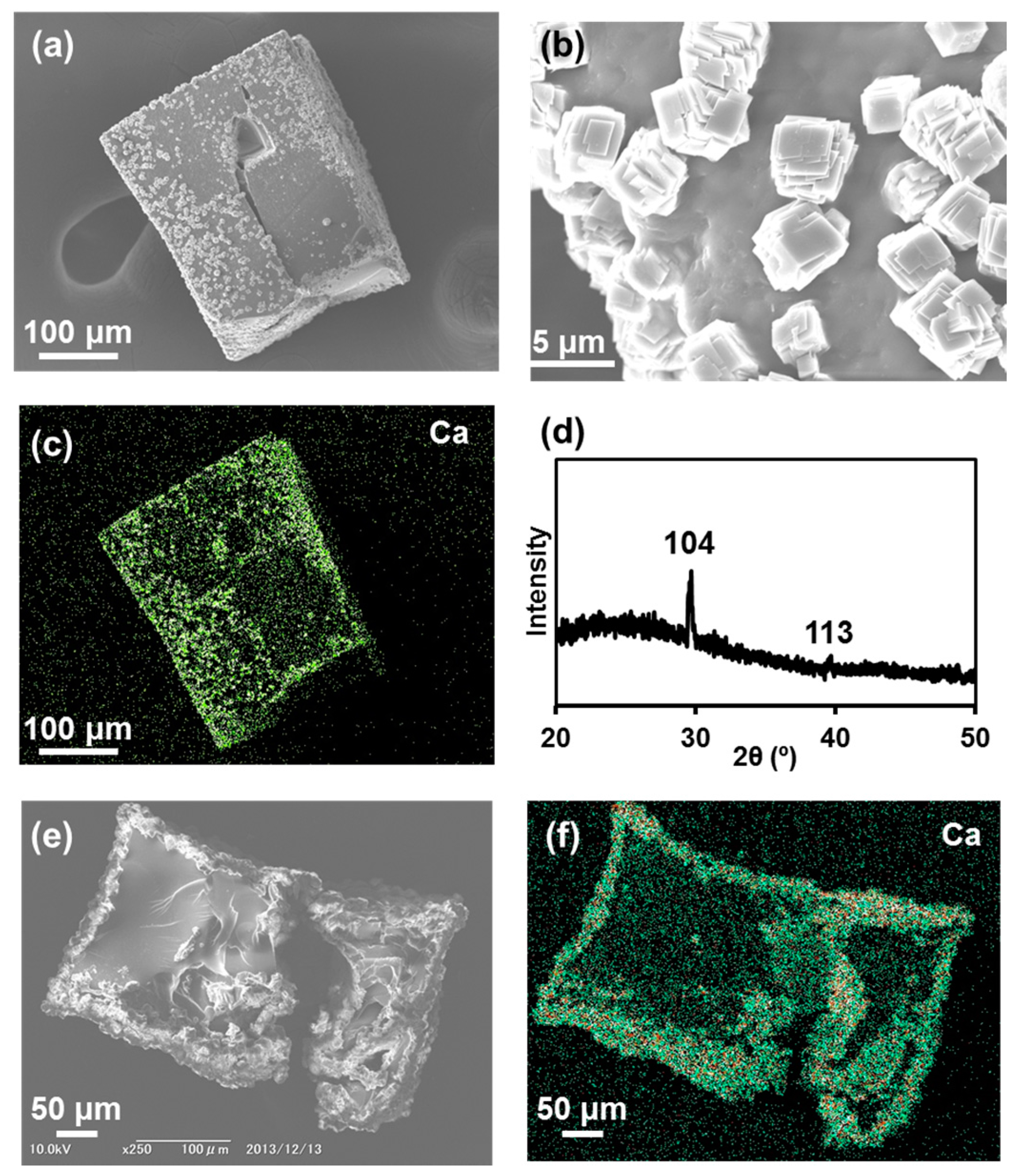
2.2. Calcium Phosphate Deposited on CCG-Nas
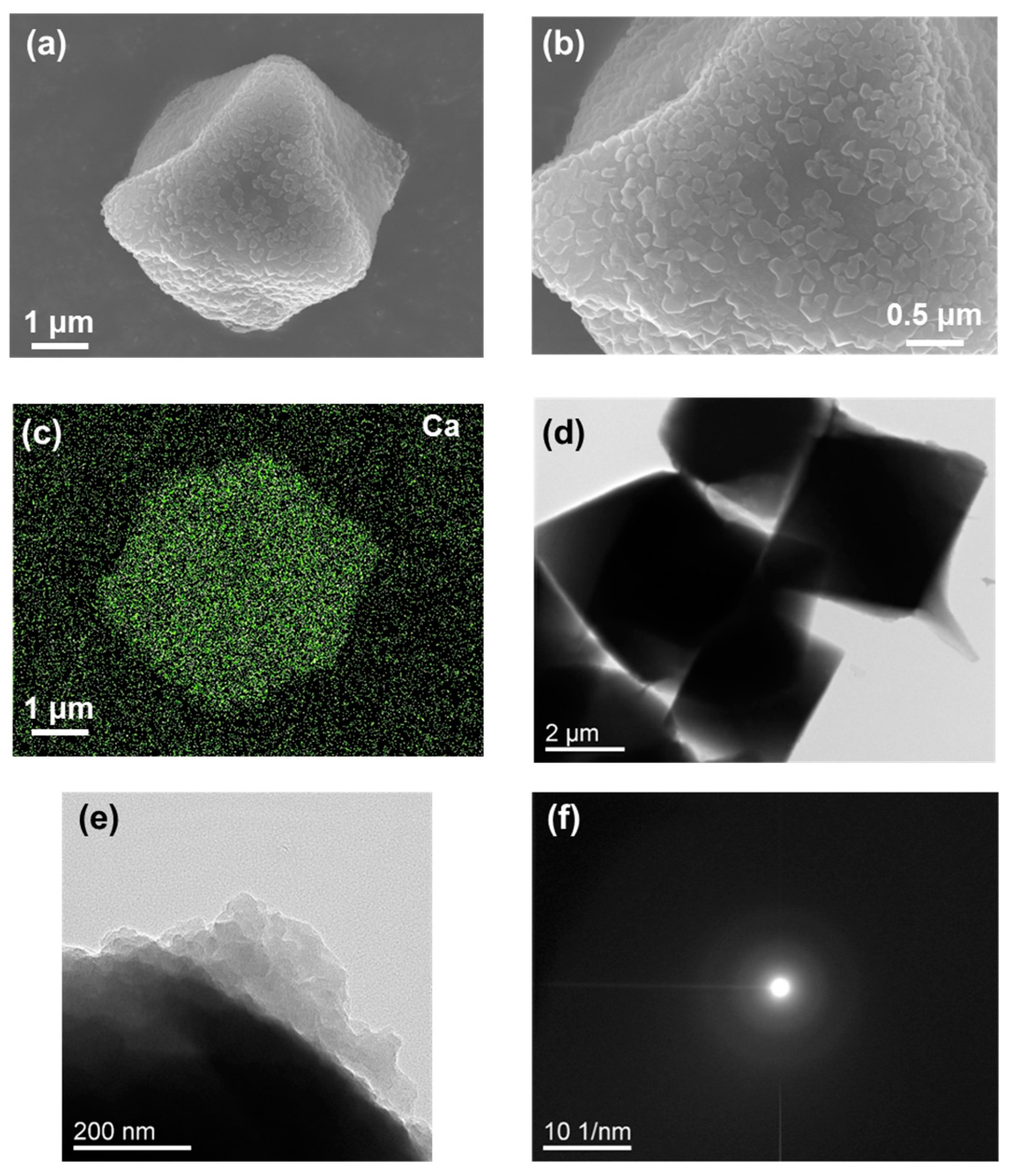
2.3. Calcium Carbonate Deposited on CCG-Nas
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Preparation of UiO68CCG-Na
4.3. Preparation of IR15CCG-Na
4.4. Preparation of UiO68CCG-Na@CP
4.5. Preparation of IR15CCG-Na@CP
4.6. Preparation of UiO68CCG-Na@CC
4.7. Preparation of IR15CCG-Na@CC
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mann, S. Molecular recognition in biomineralization. Nature 1988, 332, 119–124. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Control and Design Principles in Biological Mineralization. Angew. Chem. Int. Ed. Engl. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Bruet, B.J.F.; Qi, H.J.; Boyce, M.C.; Panas, R.; Tai, K.; Frick, L.; Ortiz, C. Nanoscale morphology and indentation of individual nacre tablets from the gastropod mollusc Trochus niloticus. J. Mater. Res. 2005, 20, 2400–2419. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Ribeiro, L.; Maillot, F.; Ward, O.; Eichhorn, S.J.; Meldrum, F.C. Bio-Inspired Synthesis and Mechanical Properties of Calcite–Polymer Particle Composites. Adv. Mater. 2010, 22, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.S.; Cantaert, B.; Kim, Y.-Y.; Li, Y.; Read, E.S.; Semsarilar, M.; Armes, S.P.; Meldrum, F.C. Systematic Study of the Effects of Polyamines on Calcium Carbonate Precipitation. Chem. Mater. 2014, 26, 2703–2711. [Google Scholar] [CrossRef]
- Song, J.; Saiz, E.; Bertozzi, C.R. A New Approach to Mineralization of Biocompatible Hydrogel Scaffolds: An Efficient Process toward 3-Dimensional Bonelike Composites. J. Am. Chem. Soc. 2003, 125, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Frith, W.J.; Rawlins, C.; Weaver, A.C.; Heppenstall-Butler, M. Hollow Calcium Carbonate Microsphere Formation in the Presence of Biopolymers and Additives. Cryst. Growth Des. 2009, 9, 534–545. [Google Scholar] [CrossRef]
- Xu, X.-R.; Cai, A.-H.; Liu, R.; Pan, H.-H.; Tang, R.-K.; Cho, K. The roles of water and polyelectrolytes in the phase transformation of amorphous calcium carbonate. J. Cryst. Growth 2008, 310, 3779–3787. [Google Scholar] [CrossRef]
- Ford, W.E.; Yasuda, A.; Wessels, J.M. Microcrystalline Composite Particles of Carbon Nanotubes and Calcium Carbonate. Langmuir 2008, 24, 3479–3485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qi, L. Bioinspired Fabrication of 3D Ordered Macroporous Single Crystals of Calcite from a Transient Amorphous Phase. Angew. Chem. Int. Ed. 2008, 47, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-Q.; Cölfen, H.; Xu, A.-W.; Hartmann, J.; Antonietti, M. Aggregation: Systematic Morphogenesis of Calcium Carbonate by Nonclassical Crystallization. ACS Nano 2017, 3, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, N.; Sugawara, A.; Kato, T. Template Effect of Crystalline Poly(vinyl alcohol) for Selective Formation of Aragonite and Vaterite CaCO3 Thin Films. Macromolecules 2003, 36, 6449–6452. [Google Scholar] [CrossRef]
- Kato, T.; Sugawata, A.; Hosoda, N. Calcium Carbonate–Organic Hybrid Materials. Adv. Mater. 2002, 14, 869–877. [Google Scholar] [CrossRef]
- Kato, T.; Suzuki, T.; Amamiya, T.; Irie, T. Makoto Komiyama Effects of macromolecules on the crystallization of CaCO3 the Formation of Organic/Inorganic Composites. Supramol. Sci. 1998, 5, 411–415. [Google Scholar] [CrossRef]
- Naka, K.; Tanaka, Y.; Chujo, Y. Effect of Anionic Starburst Dendrimers on the Crystallization of CaCO3 in Aqueous Solution: Size Control of Spherical Vaterite Particles. Langmuir 2002, 18, 3655–3658. [Google Scholar] [CrossRef]
- Naka, K.; Chujo, Y. Control of Crystal Nucleation and Growth of Calcium Carbonate by Synthetic Substrates. Chem. Mater. 2001, 13, 3245–3259. [Google Scholar] [CrossRef]
- Naka, K.; Tanaka, Y.; Chujo, Y.; Ito, Y. The effect of an anionic starburst dendrimer on the crystallization of CaCO3 in aqueous solution. Chem. Commun. 1999, 0, 1931–1932. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Li, H.; Keene, E.C.; Seh, Z.W.; Estroff, L.A. Crystal Growth of Calcium Carbonate in Hydrogels as a Model of Biomineralization. Adv. Funct. Mater. 2012, 22, 2891–2914. [Google Scholar] [CrossRef]
- Li, H.; Estroff, L.A. Calcite Growth in Hydrogels: Assessing the Mechanism of Polymer-Network Incorporation into Single Crystals. Adv. Mater. 2009, 21, 470–473. [Google Scholar] [CrossRef]
- Li, H.; Estroff, L.A. Porous calcite single crystals grown from a hydrogel medium. CrystEngComm 2007, 9, 1153–1155. [Google Scholar] [CrossRef]
- Li, H.; Estroff, L.A. Hydrogels Coupled with Self-Assembled Monolayers: An in Vitro Matrix To Study Calcite Biomineralization. J. Am. Chem. Soc. 2007, 129, 5480–5483. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Nakada, S.; Fujimoto, K. Preparation of nanometre-sized spiral mineral via controlled mineralization using a gel particle as a template. RSC Adv. 2014, 4, 6027–6030. [Google Scholar] [CrossRef]
- Ishiwata, T.; Michibata, A.; Kokado, K.; Ferlay, S.; Hosseini, M.W.; Sada, K. Box-like gel capsule from heterostructure based on a core-shell MOF as template of crystal crosslinking. Chem. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kokado, K.; Ishiwata, T.; Anan, S.; Sada, K. Unidirectional compression and expansion of a crosslinked MOF crystal prepared via axis-dependent crosslinking and ligand exchange. Polym. J. 2017, 49, 685–689. [Google Scholar] [CrossRef]
- Ishiwata, T.; Kokado, K.; Sada, K. Anisotropically Swelling Gels Attained through Axis-Dependent Crosslinking of MOF Crystals. Angew. Chem. Int. Ed. 2017, 56, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Oura, T.; Taniguchi, R.; Kokado, K.; Sada, K. Crystal Crosslinked Gels with Aggregation-Induced Emissive Crosslinker Exhibiting Swelling Degree-Dependent Photoluminescence. Polymers 2017, 9, 19. [Google Scholar] [CrossRef]
- Ishiwata, T.; Furukawa, Y.; Sugikawa, K.; Kokado, K.; Sada, K. Transformation of Metal-Organic Framework to Polymer Gel by Cross-Linking the Organic Ligands Pre-Organized in Metal-Organic Framework. J. Am. Chem. Soc. 2013, 135, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Ishiwata, T.; Sugikawa, K.; Kokado, K.; Sada, K. Nano- and Microsized Cubic Gel Particles from Cyclodextrin Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 10566–10569. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, Y.; Oka, C.; Ishiwata, T.; Kokado, K.; Sada, K. Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes. Gels 2018, 4, 16. https://doi.org/10.3390/gels4010016
Mochizuki Y, Oka C, Ishiwata T, Kokado K, Sada K. Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes. Gels. 2018; 4(1):16. https://doi.org/10.3390/gels4010016
Chicago/Turabian StyleMochizuki, Yumi, Chihiro Oka, Takumi Ishiwata, Kenta Kokado, and Kazuki Sada. 2018. "Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes" Gels 4, no. 1: 16. https://doi.org/10.3390/gels4010016
APA StyleMochizuki, Y., Oka, C., Ishiwata, T., Kokado, K., & Sada, K. (2018). Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes. Gels, 4(1), 16. https://doi.org/10.3390/gels4010016