Computer Simulations of Static and Dynamical Properties of Weak Polyelectrolyte Nanogels in Salty Solutions
Abstract
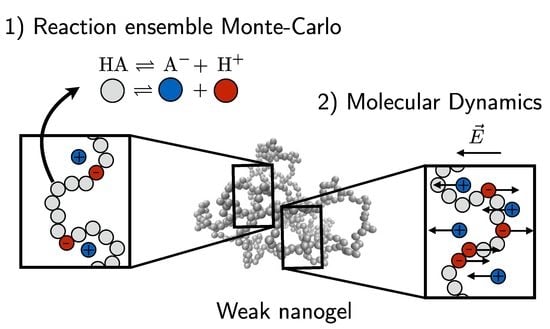
:1. Introduction
2. Results and Discussion
2.1. Static Properties
2.1.1. Monomer Charge
2.1.2. Nanogel Size
2.1.3. Ion Distribution
2.2. Dynamic Properties
2.2.1. Diffusion
2.2.2. Electrophoretic Mobility
3. Materials and Methods
3.1. Reaction Ensemble Monte Carlo
3.2. MD Simulations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, A.; Winkler, R.G. Solvent Induced Inversion of Core–Shell Microgels. ACS Macro Lett. 2017, 6, 721–725. [Google Scholar] [CrossRef]
- Ghavami, A.; Kobayashi, H.; Winkler, R.G. Internal dynamics of microgels: A mesoscale hydrodynamic simulation study. J. Chem. Phys. 2016, 145, 244902. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, A.M.; Gumerov, R.A.; Potemkin, I.I. A polymer microgel at a liquid–liquid interface: Theory vs. computer simulations. Soft Matter 2016, 12, 6799–6811. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, S.; Ghavami, A.; Holderer, O.; Scherzinger, C.; Lindner, P.; Richtering, W.; Richter, D.; Winkler, R.G. Dynamic structure factor of core-shell microgels: A neutron scattering and mesoscale hydrodynamic simulation study. Macromolecules 2016, 49, 3608–3618. [Google Scholar] [CrossRef]
- Schroeder, R.; Rudov, A.A.; Lyon, L.A.; Richtering, W.; Pich, A.; Potemkin, I.I. Electrostatic interactions and osmotic pressure of counterions control the pH-dependent swelling and collapse of polyampholyte microgels with random distribution of ionizable groups. Macromolecules 2015, 48, 5914–5927. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Martín-Molina, A. Monte Carlo simulation of thermo-responsive charged nanogels in salt-free solutions. Soft Matter 2013, 9, 7086–7094. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Ahualli, S.; Martín-Molina, A. Thermo-responsive gels in the presence of monovalent salt at physiological concentrations: A Monte Carlo simulation study. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1403–1411. [Google Scholar] [CrossRef]
- Ohshima, H. Approximate analytic expression for the pH-dependent electrophoretic mobility of soft particles. Colloid Polym. Sci. 2016, 294, 1997–2003. [Google Scholar] [CrossRef]
- Gopmandal, P.P.; Ohshima, H. Importance of pH-regulated charge density on the electrophoresis of soft particles. Chem. Phys. 2017, 483, 165–171. [Google Scholar] [CrossRef]
- Claudio, G.C.; Kremer, K.; Holm, C. Comparison of a hydrogel model to the Poisson-Boltzmann cell model. J. Chem. Phys. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Zwanikken, J.W.; Detcheverry, F.A.; de Pablo, J.J.; Olvera de la Cruz, M. Study of volume phase transitions in polymeric nanogels by theoretically informed coarse-grained simulations. Soft Matter 2011, 7, 5965–5975. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Ahualli, S.; Martín-Molina, A. Temperature-sensitive nanogels in the presence of salt: Explicit coarse-grained simulations. J. Chem. Phys. 2014, 141, 124903. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Winkler, R.G. Structure of microgels with Debye–Hückel interactions. Polymers 2014, 6, 1602–1617. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Winkler, R.G. Universal conformational properties of polymers in ionic nanogels. Sci. Rep. 2015, 6, 198316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adroher-Benítez, I.; Ahualli, S.; Martín-Molina, A.; Quesada-Pérez, M.; Moncho-Jordá, A. Role of Steric Interactions on the Ionic Permeation inside Charged Microgels: Theory and Simulations. Macromolecules 2015, 48, 4645–4656. [Google Scholar] [CrossRef]
- Kobayashi, H.; Halver, R.; Sutmann, G.; Winkler, R.G. Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results. Polymers 2017, 9, 15. [Google Scholar] [CrossRef]
- Reed, C.E.; Reed, W.F. Monte Carlo study of titration of linear polyelectrolytes. J. Chem. Phys. 1992, 96, 1609–1620. [Google Scholar] [CrossRef]
- Ullner, M.; Jönsson, B.; Widmark, P.O. Conformational properties and apparent dissociation constants of titrating polyelectrolytes: Monte Carlo simulation and scaling arguments. J. Chem. Phys. 1994, 100, 3365–3366. [Google Scholar] [CrossRef]
- Ullner, M.; Jönsson, B.; Söderberg, B.; Peterson, C. A Monte Carlo study of titrating polyelectrolytes. J. Chem. Phys. 1996, 104, 3048–3057. [Google Scholar] [CrossRef]
- Ullner, M.; Jönsson, B. A Monte Carlo study of titrating polyelectrolytes in the presence of salt. Macromolecules 1996, 29, 6645–6655. [Google Scholar] [CrossRef]
- Ullner, M.; Woodward, C.E. Simulations of the titration of linear polyelectrolytes with explicit simple ions: Comparisons with screened Coulomb models and experiments. Macromolecules 2000, 33, 7144–7156. [Google Scholar] [CrossRef]
- Ulrich, S.; Laguecir, A.; Stoll, S. Titration of hydrophobic polyelectrolytes using Monte Carlo simulations. J. Chem. Phys. 2005, 122, 094911. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.R.; Triska, B. The reaction ensemble method for the computer simulation of chemical and phase equilibria. I. Theory and basic examples. J. Chem. Phys. 1994, 100, 3019–3027. [Google Scholar] [CrossRef]
- Johnson, J.K.; Panagiotopoulos, A.Z.; Gubbins, K.E. Reactive canonical Monte Carlo: A new simulation technique for reacting or associating fluids. Mol. Phys. 1994, 81, 717–733. [Google Scholar] [CrossRef]
- Heath Turner, C.; Brennan, J.K.; Lisal, M.; Smith, W.R.; Karl Johnson, J.; Gubbins, K.E. Simulation of chemical reaction equilibria by the reaction ensemble Monte Carlo method: A review. Mol. Simul. 2008, 34, 119–146. [Google Scholar] [CrossRef]
- Uhlík, F.; Košovan, P.; Limpouchova, Z.; Procházka, K.; Borisov, O.V.; Leermakers, F.A. Modeling of ionization and conformations of starlike weak polyelectrolytes. Macromolecules 2014, 47, 4004–4016. [Google Scholar] [CrossRef]
- Uhlík, F.; Košovan, P.; Zhulina, E.B.; Borisov, O.V. Charge-controlled nano-structuring in partially collapsed star-shaped macromolecules. Soft Matter 2016, 12, 4846–4852. [Google Scholar] [CrossRef] [PubMed]
- Landsgesell, J.; Holm, C.; Smiatek, J. Simulation of weak polyelectrolytes: A comparison between the constant pH and the reaction ensemble method. Eur. Phys. J. Spec. Top. 2017, 226, 725–736. [Google Scholar] [CrossRef]
- Nová, L.; Uhlík, F.; Košovan, P. Local pH and effective p KA of weak polyelectrolytes–insights from computer simulations. Phys. Chem. Chem. Phys. 2017, 19, 14376. [Google Scholar]
- Castelnovo, M.; Sens, P.; Joanny, J.F. Charge distribution on annealed polyelectrolytes. Eur. Phys. J. E 2000, 1, 115–125. [Google Scholar] [CrossRef]
- Limbach, H.J.; Holm, C. End-effects of strongly charged polyelectrolytes—A molecular dynamics study. J. Chem. Phys. 2001, 114, 9674–9682. [Google Scholar] [CrossRef]
- Ballauff, M.; Likos, C.N. Dendrimers in Solution: Insight from Theory and Simulation. Angew. Chem. Int. Ed. 2004, 43, 2998–3020. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K. PAMAM dendrimer: A pH-controlled nanosponge. Can. J. Chem. 2017, 95, 991–998. [Google Scholar] [CrossRef]
- Long, D.; Dobrynin, A.V.; Rubinstein, M.; Ajdari, A. Electrophoresis of polyampholytes. J. Chem. Phys. 1998, 108, 1234–1244. [Google Scholar] [CrossRef]
- Hickey, O.A.; Holm, C.; Harden, J.L.; Slater, G.W. Implicit Method for Simulating Electrohydrodynamics of Polyelectrolytes. Phys. Rev. Lett. 2010, 105, 148301. [Google Scholar] [CrossRef] [PubMed]
- Chubynsky, M.V.; Slater, G.W. Theory of end-labeled free-solution electrophoresis: Is the end effect important? Electrophoresis 2014, 35, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Chubynsky, M.V.; Slater, G.W. Electrophoresis of Heteropolymers. Effect of Stiffness. Macromolecules 2015, 48, 5899–5913. [Google Scholar] [CrossRef]
- Kamerlin, N.; Elvingson, C. Collapse Dynamics of Core–Shell Nanogels. Macromolecules 2016, 49, 5740–5749. [Google Scholar] [CrossRef]
- Gnan, N.; Rovigatti, L.; Bergman, M.; Zaccarelli, E. In silico synthesis of microgel particles. Macromolecules 2017, 50, 8777–8786. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.K.; Lísal, M.; Gubbins, K.E.; Rice, B.M. Reaction ensemble molecular dynamics: Direct simulation of the dynamic equilibrium properties of chemically reacting mixtures. Phys. Rev. E 2004, 70, 061103. [Google Scholar] [CrossRef] [PubMed]
- Lísal, M.; Brennan, J.K.; Smith, W.R. Mesoscale simulation of polymer reaction equilibrium: Combining dissipative particle dynamics with reaction ensemble Monte Carlo. I. Polydispersed polymer systems. J. Chem. Phys. 2006, 125, 164905. [Google Scholar] [CrossRef] [PubMed]
- Lísal, M.; Brennan, J.K.; Smith, W.R. Mesoscale simulation of polymer reaction equilibrium: Combining dissipative particle dynamics with reaction ensemble Monte Carlo. II. Supramolecular diblock copolymers. J. Chem. Phys. 2009, 130, 104902. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.S.; Olvera de La Cruz, M.; Szleifer, I. Molecular theory of weak polyelectrolyte gels: The role of pH and salt concentration. Macromolecules 2010, 44, 147–158. [Google Scholar] [CrossRef]
- Longo, G.S.; Olvera de la Cruz, M.; Szleifer, I. Non-monotonic swelling of surface grafted hydrogels induced by pH and/or salt concentration. J. Chem. Phys. 2014, 141, 124909. [Google Scholar] [CrossRef] [PubMed]
- Polotsky, A.A.; Plamper, F.A.; Borisov, O.V. Collapse-to-swelling transitions in pH-and thermoresponsive microgels in aqueous dispersions: The thermodynamic theory. Macromolecules 2013, 46, 8702–8709. [Google Scholar] [CrossRef]
- Grass, K.; Holm, C. Polyelectrolytes in electric fields: Measuring the dynamical effective charge and effective friction. Soft Matter 2009, 5, 2079–2092. [Google Scholar] [CrossRef]
- Nkodo, A.E.; Garnier, J.M.; Tinland, B.; Ren, H.; Desruisseaux, C.; McCormick, L.C.; Drouin, G.; Slater, G.W. Diffusion coefficient of DNA molecules during free solution electrophoresis. Electrophoresis 2001, 22, 2424–2432. [Google Scholar] [CrossRef]
- Grass, K.; Böhme, U.; Scheler, U.; Cottet, H.; Holm, C. Importance of Hydrodynamic Shielding for the Dynamic Behavior of Short Polyelectrolyte Chains. Phys. Rev. Lett. 2008, 100, 096104. [Google Scholar] [CrossRef] [PubMed]
- Grass, K.; Holm, C. Mesoscale modelling of polyelectrolyte electrophoresis. Faraday Discuss. 2010, 144, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.W.; de Paula, J. Physical Chemistry; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Landsgesell, J.; Holm, C.; Smiatek, J. Wang-Landau Reaction Ensemble Method: Simulation of Weak Polyelectrolytes and General Acid-Base Reactions. J. Chem. Theory Comput. 2017, 13, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Madras, N.; Sokal, A.D. The pivot algorithm: A highly efficient Monte Carlo method for the self-avoiding walk. J. Stat. Phys. 1988, 50, 109–186. [Google Scholar] [CrossRef]
- Slater, G.W.; Holm, C.; Chubynsky, M.V.; de Haan, H.W.; Dube, A.; Grass, K.; Hickey, O.A.; Kingsburry, C.; Sean, D.; Shendruk, T.N.; et al. Modeling the separation of macromolecules: A review of current computer simulation methods. Electrophoresis 2009, 30, 792–818. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Edwards, B.F.; Timperman, A.T.; Carroll, R.L.; Jo, K.; Mease, J.M.; Schiffbauer, J.E. Traveling-wave electrophoresis for microfluidic separations. Phys. Rev. Lett. 2009, 102, 076103. [Google Scholar] [CrossRef] [PubMed]
- Limbach, H.J.; Arnold, A.; Mann, B.A.; Holm, C. ESPResSo—An Extensible Simulation Package for Research on Soft Matter Systems. Comput. Phys. Commun. 2006, 174, 704–727. [Google Scholar] [CrossRef]
- Arnold, A.; Lenz, O.; Kesselheim, S.; Weeber, R.; Fahrenberger, F.; Röhm, D.; Košovan, P.; Holm, C. ESPResSo 3.1—Molecular Dynamics Software for Coarse-Grained Models. In Meshfree Methods for Partial Differential Equations VI; Griebel, M., Schweitzer, M.A., Eds.; Lecture Notes in Computational Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2013; Volume 89, pp. 1–23. [Google Scholar]
- ESPResSo Homepage. Available online: http://espressomd.org (accessed on 1 December 2017).
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; IOP: London, UK, 1988. [Google Scholar]
- Deserno, M.; Holm, C. How to mesh up Ewald sums. I. A theoretical and numerical comparison of various particle mesh routines. J. Chem. Phys. 1998, 109, 7678–7693. [Google Scholar] [CrossRef]
- Roehm, D.; Arnold, A. Lattice Boltzmann simulations on GPUs with ESPResSo. Eur. Phys. J. Spec. Top. 2012, 210, 89–100. [Google Scholar] [CrossRef]
- Dünweg, B.; Schiller, U.; Ladd, A.J.C. Statistical mechanics of the fluctuating Lattice-Boltzmann equation. Phys. Rev. E 2007, 76, 036704. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sean, D.; Landsgesell, J.; Holm, C. Computer Simulations of Static and Dynamical Properties of Weak Polyelectrolyte Nanogels in Salty Solutions. Gels 2018, 4, 2. https://doi.org/10.3390/gels4010002
Sean D, Landsgesell J, Holm C. Computer Simulations of Static and Dynamical Properties of Weak Polyelectrolyte Nanogels in Salty Solutions. Gels. 2018; 4(1):2. https://doi.org/10.3390/gels4010002
Chicago/Turabian StyleSean, David, Jonas Landsgesell, and Christian Holm. 2018. "Computer Simulations of Static and Dynamical Properties of Weak Polyelectrolyte Nanogels in Salty Solutions" Gels 4, no. 1: 2. https://doi.org/10.3390/gels4010002