Kinetics of Supercritical Drying of Gels
Abstract
:1. Introduction
1.1. Drying Techniques of Wet Gels
- ambient pressure drying with matrix strengthening
- freeze drying
- supercritical drying
1.2. Ambient Pressure Drying with Matrix Strengthening
1.3. Freeze Drying
1.4. Supercritical Drying
2. Supercritical Drying
- Scaling up of laboratory scale drying units to pilot and industrial scale.
- Assessing the economics of production of aerogels on an industrial scale.
- Speeding up aerogel research in the laboratory by shortening drying times.
- Providing information on the pore properties of aerogels such as porosity, pore volume, and tortuosity.
- Improving mass transfer models for aerogels which can also be useful for impregnation of aerogels with a variety of chemicals and also for solvent exchange.
- Providing information on the nature of capillary forces in aerogels.
- Improving our understanding of convective mass transfer in SCFs and enable the development of correlations for convective mass transfer coefficients for SCFs.
- Improving our understanding of axial dispersion in packed beds in SCFs.
- Providing information on the effective diffusivities in aerogels.
3. Kinetics of Supercritical Drying
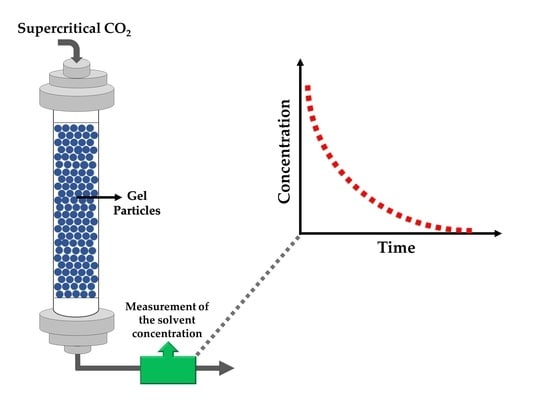
3.1. Experimental Techniques to Investigate Drying Kinetics
- A CO2 feeding system consisting of a CO2 tank and a high pressure-pump to feed CO2.
- A temperature and pressure controlled high pressure vessel in which gel to be dried is placed, valves to start/stop the CO2 flow into the vessel and to adjust the flow rate, and pressure transducers and thermocouples to monitor operating conditions.
- A back pressure regulator or a needle valve to control the pressure in the vessel.
- Flowmeters to measure the flow rate of the inlet and/or exit streams.
- A system to measure the composition of the exit stream from the vessel as a function of time.
3.1.1. Techniques Used to Measure Concentration of the Solvent in CO2
- chromatography
- gravimetric determination
- in situ spectroscopy
3.1.2. Further Considerations
3.2. Mass Transfer Mechanisms in Supercritical Drying of Gels
3.2.1. Diffusion
3.2.2. Convective Mass Transfer
3.2.3. Spillage by Volume Expansion
3.2.4. Axial Dispersion
3.3. Models for Kinetics of Supercritical Drying
3.4. Review of Studies on Drying Kinetics
4. Conclusions and Future Research Directions
Acknowledgments
Conflicts of Interest
References
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon N. Y. 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Özbakir, Y.; Erkey, C. Experimental and theoretical investigation of supercritical drying of silica alcogels. J. Supercrit. Fluids 2015, 98, 153–166. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Review: Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Nanostructure of calcium alginate aerogels obtained from multistep solvent exchange route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Angelescu, D.G.; Anastasescu, M.; Anghel, D.F. Synthesis and modeling of calcium alginate nanoparticles in quaternary water-in-oil microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 95–103. [Google Scholar] [CrossRef]
- Bisson, A.; Rigacci, A.; Lecomte, D.; Rodier, E.; Achard, P. Drying of Silica Gels to Obtain Aerogels: Phenomenology and Basic Techniques. Dry. Technol. 2003, 21, 593–628. [Google Scholar] [CrossRef]
- Aegerter, M.; Leventis, N.; Koebel, M. Advances in Sol-Gel Derived Materials and Technologies. In Aerogels Handbook; Springer: New York, NY, USA, 2011; ISBN 9781441974778. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, Surface Area and Porosity. 2. Auflage, Academic Press, London 1982. 303 Seiten, Preis: $ 49.50. Berichte der Bunsengesellschaft für Phys. Chemie 1982, 86, 957. [Google Scholar] [CrossRef]
- Smith, D.M.; Deshpande, R.; Jeffrey Brinke, C. Preparation of Low-Density Aerogels at Ambient Pressure. MRS Proc. 1992, 271, 567–572. [Google Scholar] [CrossRef]
- Smith, D.M.; Stein, D.; Anderson, J.M.; Ackerman, W. Preparation of low-density xerogels at ambient pressure. J. Non-Cryst. Solids 1995, 186, 104–112. [Google Scholar] [CrossRef]
- Hæreid, S.; Anderson, J.; Einarsrud, M.A.; Hua, D.W.; Smith, D.M. Thermal and temporal aging of TMOS-based aerogel precursors in water. J. Non-Cryst. Solids 1995, 185, 221–226. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldaña, M.D.A. Lupin hull cellulose nanofiber aerogel preparation by supercritical CO2 and freeze drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Sanli, D.; Bozbag, S.E.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Chang, C.J.; Day, C.-Y.; Ko, C.-M.; Chiu, K.-L. Densities and P-x-y diagrams for carbon dioxide dissolution in methanol, ethanol, and acetone mixtures. Fluid Phase Equilib. 1997, 131, 243–258. [Google Scholar] [CrossRef]
- Day, C.-Y.; Chang, C.J.; Chen, C.-Y. Phase Equilibrium of Ethanol + CO2 and Acetone + CO2 at Elevated Pressures. J. Chem. Eng. Data 1996, 41, 839–843. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Hatakeyama, Y.; Kawai, H.; Morita, T.; Sumiyoshi, T. Large-area silica aerogel for use as Cherenkov radiators with high refractive index, developed by supercritical carbon dioxide drying. J. Supercrit. Fluids 2016, 110, 183–192. [Google Scholar] [CrossRef]
- Aerogels.org Supercritical Drying with Liquid Carbon Dioxide Part 2 of 2. Available online: http://www.aerogel.org/?p=1344 (accessed on 26 December 2017).
- Wawrzyniak, P.; Rogacki, G.; Pruba, J.; Bartczak, Z. Diffusion of ethanol-carbon dioxide in silica gel. J. Non-Cryst. Solids 1998, 225, 86–90. [Google Scholar] [CrossRef]
- Van Bommel, M.J.; de Haan, A.B. Drying of silica aerogel with supercritical carbon dioxide. J. Non-Cryst. Solids 1995, 186, 78–82. [Google Scholar] [CrossRef]
- Lebedev, A.E.; Katalevich, A.M.; Menshutina, N.V. Modeling and scale-up of supercritical fluid processes. Part I: Supercritical drying. J. Supercrit. Fluids 2015, 106, 122–132. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Rueda, M.; Mato, R.; Martín, Á. View cell investigation of silica aerogels during supercritical drying: Analysis of size variation and mass transfer mechanisms. J. Supercrit. Fluids 2014, 92, 24–30. [Google Scholar] [CrossRef]
- Griffin, J.S.; Mills, D.H.; Cleary, M.; Nelson, R.; Manno, V.P.; Hodes, M. Continuous extraction rate measurements during supercritical CO2 drying of silica alcogel. J. Supercrit. Fluids 2014, 94, 38–47. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Quiño, J.; Ruehl, M.; Klima, T.; Ruiz, F.; Will, S.; Braeuer, A. Supercritical drying of aerogel: In situ analysis of concentration profiles inside the gel and derivation of the effective binary diffusion coefficient using Raman spectroscopy. J. Supercrit. Fluids 2016, 108, 1–12. [Google Scholar] [CrossRef]
- Rogacki, G.; Wawrzyniak, P. Diffusion of ethanol-liquid CO2 in silica aerogel. J. Non-Cryst. Solids 1995, 186, 73–77. [Google Scholar] [CrossRef]
- Bommel, M.J.; Haan, A.B. Drying of silica gels with supercritical carbon dioxide. J. Mater. Sci. 1994, 29, 943–948. [Google Scholar] [CrossRef]
- Novak, Z.; Zeljko, K. Diffusion of methanol—Liquid CO2 and methanol—Supercritical CO2 in silica aerogels. J. Non-Cryst. Solids 1997, 163–169. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Rogacki, G.; Pruba, J.; Bartczak, Z. Effective diffusion coefficient in the low temperature process of silica aerogel production. J. Non-Cryst. Solids 2001, 285, 50–56. [Google Scholar] [CrossRef]
- Masmoudi, Y.; Rigacci, A.; Ilbizian, P.; Cauneau, F.; Achard, P. Diffusion during the supercritical drying of silica gels. Dry. Technol. 2006, 24, 1121–1125. [Google Scholar] [CrossRef]
- Braeuer, A. High Pressure: Fellow and Opponent of Spectroscopic Techniques. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 7, pp. 1–40. ISBN 9780444634221. [Google Scholar]
- Baloch, S.K.; Jonáš, A.; Kiraz, A.; Alaca, B.E.; Erkey, C. Determination of composition of ethanol-CO2 mixtures at high pressures using frequency response of microcantilevers. J. Supercrit. Fluids 2017. [Google Scholar] [CrossRef]
- Eriş, G.; Baloch, S.K.; Bozkurt, A.A.; Jonáš, A.; Kiraz, A.; Alaca, B.E.; Erkey, C. Characterization of fluid mixtures at high pressures using frequency response of microcantilevers. Sens. Actuators A Phys. 2017, 261, 202–209. [Google Scholar] [CrossRef]
- Woignier, T.; Scherer, G.W.; Alaoui, A. Stress in aerogel during depressurization of autoclave: I. Theory. J. Sol-Gel Sci. Technol. 1994, 3, 127–139. [Google Scholar] [CrossRef]
- Woignier, T.; Scherer, G.W.; Alaoui, A. Stress in aerogel during depressurization of autoclave: II. Silica gels. J. Sol-Gel Sci. Technol. 1994, 3, 141–150. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Masson, E.; Pizzi, A.; Celzard, A. Impact of depressurizing rate on the porosity of aerogels. Microporous Mesoporous Mater. 2012, 152, 240–251. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Rao, B.S. Modeling of supercritical drying of ethanol-soaked silica aerogels with carbon dioxide. J. Chem. Technol. Biotechnol. 2008, 83, 1101–1109. [Google Scholar] [CrossRef]
- Özbakir, Y.; Ulker, Z.; Erkey, C. Monolithic composites of silica aerogel with poly(methyl vinyl ether) and the effect of polymer on supercritical drying. J. Supercrit. Fluids 2014, 105, 108–118. [Google Scholar] [CrossRef]
- Mueller, R.; Zhang, S.; Klink, M.; Bä, M.; Vasenkov, S. The origin of a large apparent tortuosity factor for the Knudsen diffusion inside monoliths of a samaria-alumina aerogel catalyst: A diffusion NMR study. Phys. Chem. Chem. Phys. 2015, 17, 27481–27487. [Google Scholar] [CrossRef] [PubMed]
- Cussler, E.L. Fundamentals of Mass Transfer. In Diffusion; Cambridge University Press: Cambridge, UK; pp. 237–273.
- Jarzbski, A.B.; Lorenc, J. Pore network connectivity and effective diffusivity of silica aerogels. Chem. Eng. Sci. 1995, 50, 357–360. [Google Scholar] [CrossRef]
- Norris, P.M.; Shrinivasan, S. Aerogels: Unique Material, Fascinating Properties and Unlimited Applications. Annu. Rev. Heat Transf. 2005, 14, 385–408. [Google Scholar]
- Lawrence, M.; Jiang, Y. Porosity, Pore Size Distribution, Micro-structure BT—Bio-Aggregates Based Building Materials: State-of-the-Art Report of the RILEM Technical Committee 236-BBM; Amziane, S., Collet, F., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 39–71. ISBN 978-94-024-1031-0. [Google Scholar]
- Giesche, H. Mercury porosimetry: A general (practical) overview. Part. Part. Syst. Charact. 2006, 23, 9–19. [Google Scholar] [CrossRef]
- Ashton, C.E.; Mulders, N.; Golov, A.I. Tortuosity of 4He films on aerogel. AIP Conf. Proc. 2006, 850, 257–258. [Google Scholar] [CrossRef]
- Pisani, L. Simple Expression for the Tortuosity of Porous Media. Transp. Porous Media 2011, 88, 193–203. [Google Scholar] [CrossRef]
- Armatas, G.S. Determination of the effects of the pore size distribution and pore connectivity distribution on the pore tortuosity and diffusive transport in model porous networks. Chem. Eng. Sci. 2006, 61, 4662–4675. [Google Scholar] [CrossRef]
- Vignes, A. Diffusion in Binary Solutions. Variation of Diffusion Coefficient with Composition. Ind. Eng. Chem. Fundam. 1966, 5, 189–199. [Google Scholar] [CrossRef]
- Cullinan, H.T. Composition Dependence of Binary Diffusion Coefficient. Ind. Eng. Chem. Fundam. 1968, 7, 519–520. [Google Scholar] [CrossRef]
- Funazukuri, T.; Kong, C.Y.; Kagei, S. Binary diffusion coefficients of acetone in carbon dioxide at 308.2 and 313.2 K in the pressure range from 7.9 to 40 MPa. Int. J. Thermophys. 2000, 21, 651–669. [Google Scholar] [CrossRef]
- Funazukuri, T.; Kong, C.Y.; Kagei, S. Infinite-Dilution Binary Diffusion Coefficients of 2-Propanone, 2-Butanone, 2-Pentanone, and 3-Pentanone in CO2 by the Taylor Dispersion Technique from 308.15 to 328.15 K in the Pressure Range from 8 to 35 MPa. Int. J. Thermophys. 2000, 21, 1279–1290. [Google Scholar] [CrossRef]
- Kong, C.Y.; Funazukuri, T.; Kagei, S. Binary diffusion coefficients and retention factors for polar compounds in supercritical carbon dioxide by chromatographic impulse response method. J. Supercrit. Fluids 2006, 37, 359–366. [Google Scholar] [CrossRef]
- Medina, I. Determination of diffusion coefficients for supercritical fluids. J. Chromatogr. A 2012, 1250, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Bosma, J.C.; Wesselingh, J.A. Estimation of Diffusion Coefficients in Dilute Liquid Mixtures. Chem. Eng. Res. Des. 1999, 77, 325–328. [Google Scholar] [CrossRef]
- Frank, M.J.W.; Kuipers, J.A.M.; van Swaaij, W.P.M. Diffusion Coefficients and Viscosities of CO2 + H2O, CO2 + CH3OH, NH3 + H2O, and NH3 + CH3OH Liquid Mixtures. J. Chem. Eng. Data 1996, 41, 297–302. [Google Scholar] [CrossRef]
- Tyn, M.T.; Calus, W.F. Diffusion Coefficients in Dilute Binary Liquid Mixtures. J. Chem. Eng. Data 1975, 20, 106–109. [Google Scholar] [CrossRef]
- Eaton, A.P.; Akgerman, A. Infinite-Dilution Diffusion Coefficients in Supercritical Fluids. Ind. Eng. Chem. Res. 1997, 36, 923–931. [Google Scholar] [CrossRef]
- Funazukuri, T.; Hachisu, S.; Wakao, N. Measurement of diffusion coefficients of C18 unsaturated fatty acid methyl esters, naphthalene, and benzene in supercritical carbon dioxide by a tracer response technique. Anal. Chem. 1989, 61, 118–122. [Google Scholar] [CrossRef]
- He, C.-H.; Yu, Y.-S. New Equation for Infinite-Dilution Diffusion Coefficients in Supercritical and High-Temperature Liquid Solvents. Ind. Eng. Chem. Res. 1998, 37, 3793–3798. [Google Scholar] [CrossRef]
- Funazukuri, T.; Kong, C.Y.; Murooka, N.; Kagei, S. Measurements of Binary Diffusion Coefficients and Partition Ratios for Acetone, Phenol, alpha-Tocopherol, and beta-Carotene in Supercritical Carbon Dioxide with a Poly(ethylene glycol)-Coated Capillary Column. Ind. Eng. Chem. Res. 2000, 39, 4462–4469. [Google Scholar] [CrossRef]
- Funazukuri, T.; Kong, C.Y.; Kagei, S. Binary diffusion coefficients in supercritical fluids: Recent progress in measurements and correlations for binary diffusion coefficients. J. Supercrit. Fluids 2006, 38, 201–210. [Google Scholar] [CrossRef]
- Snijder, E.D.; Te Riele, M.J.M.; Versteeg, G.F.; Van Swaaij, W.P.M. Diffusion Coefficients of CO, CO2, N2O, and N2 in Ethanol and Toluene. J. Chem. Eng. Data 1995, 40, 37–39. [Google Scholar] [CrossRef]
- Hiroshi, T.; Iichihiko, F.M.; Sato Tetsushi, O.K. Simultaneous determination of diffusion coefficient and solubility of gas in liquid by a diaphragm cell. J. Chem. Eng. Jpn. 1975, 8, 252–253. [Google Scholar] [CrossRef]
- Chen, B.H.C.; Chen, S.H. Diffusion of slightly soluble gases in liquids: Measurement and correlation with implications on liquid structures. Chem. Eng. Sci. 1985, 40, 1735–1741. [Google Scholar] [CrossRef]
- Stüber, F.; Vazquez, A.M.; Larrayoz, M.A.; Recasens, F. Supercritical Fluid Extraction of Packed Beds: External Mass Transfer in Upflow and Downflow Operation. Ind. Eng. Chem. Res. 1996, 35, 3618–3628. [Google Scholar] [CrossRef]
- Puiggené, J.; Larrayoz, M.A.; Recasens, F. Free liquid-to-supercritical fluid mass transfer in packed beds. Chem. Eng. Sci. 1997, 52, 195–212. [Google Scholar] [CrossRef]
- Tan, C.-S.; Liang, S.-K.; Liou, D.-C. Fluid—Solid mass transfer in a supercritical fluid extractor. Chem. Eng. J. 1988, 38, 17–22. [Google Scholar] [CrossRef]
- Wakao, N.; Kagei, S. Heat and Mass Transfer in Packed Beds; Studies in Cybernetics; Gordon and Breach Science Publishers: Philadelphia, PA, USA, 1982; ISBN 9780677058603. [Google Scholar]
- Zheng, S.; Hu, X.; Ibrahim, A.-R.; Tang, D.; Tan, Y.; Li, J. Supercritical Fluid Drying: Classification and Applications. Recent Patents Chem. Eng. 2010, 3, 230–244. [Google Scholar] [CrossRef]
- Kordikowski, A.; Schenk, A.P.; Van Nielen, R.M.; Peters, C.J. Volume expansions and vapor-liquid equilibria of binary mixtures of a variety of polar solvents and certain near-critical solvents. J. Supercrit. Fluids 1995, 8, 205–216. [Google Scholar] [CrossRef]
- De La Fuente Badilla, J.C.; Peters, C.J.; De Swaan Arons, J. Volume expansion in relation to the gas-antisolvent process. J. Supercrit. Fluids 2000, 17, 13–23. [Google Scholar] [CrossRef]
- Kato, M.; Kodama, D.; Ono, T.; Kokubo, M. Volumetric Properties of Carbon Dioxide + Ethanol at 313.15 K. J. Chem. Eng. Data 2009, 54, 2953–2956. [Google Scholar] [CrossRef]
- Liu, K.; Kiran, E. Viscosity, Density and Excess Volume of Acetone + Carbon Dioxide Mixtures at High Pressures. Ind. Eng. Chem. Res. 2007, 46, 5453–5462. [Google Scholar] [CrossRef]
- Han, N.-W.; Bhakta, J.; Carbonell, R.G. Longitudinal and lateral dispersion in packed beds: Effect of column length and particle size distribution. AIChE J. 1985, 31, 277–288. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, X.; Jiang, W. Theoretical models for supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-S.; Liou, D.-C. Axial Dispersion of Supercritical Carbon Dioxide in Packed Beds. Ind. Eng. Chem. Res. 1989, 28, 1246–1250. [Google Scholar] [CrossRef]
- Orlović, A.M.; Petrović, S.; Skala, D.U. Mathematical modeling and simulation of gel drying with supercritical carbon dioxide. J. Serbian Chem. Soc. 2005, 70, 125–136. [Google Scholar] [CrossRef]




















| Solvents | γLV * in 10−3 Nm−1 | Pc in Bar for d = 5 nm | Pc in Bar for d = 50 nm | Pc in Bar for d = 500 nm |
|---|---|---|---|---|
| Water | 73.1 ** | 974 | 60.9 | 5.9 |
| Ethanol | 22.8 | 303 | 19.0 | 1.8 |
| Methanol | 22.6 | 301 | 18.8 | 1.8 |
| Acetone | 23.7 | 316 | 19.8 | 1.9 |
| Hexane | 18.4 | 246 | 15.4 | 1.5 |
| Isopropanol | 21.7 | 289 | 18.1 | 1.7 |
| Reference | Equation |
|---|---|
| Funazukuri, Hachisu, and Wakao [62,67] | |
| V2 is molar volume of solvent 2 (m3 mol−1), is solvent molar volume at which viscous flow stops, (m3 mol−1), η is visocity of solvent 2 (kg·m−1·s−1), T is absolute temperature (K), Sc is Schmidt number, is density of solvent 2 (kg·m−3). The superscript o indicates that the parameter is evaluated at atmospheric pressure. | |
| He-Yu [68] | For and |
| is reduced density of solvent 2 (kg m−3), is molar mass of solute 1 (kg mol−1), is critical molar volume of solvent 2 (m3 mol−1), is critical temperature (K). | |
| Funazukuri-Kong-Kagei [69,70] | is calculated as: |
| is molar volume of solvent 2 (m3 mol−1), T is absolute temperature (K), Sc is Schmidt number, Nav is avogadro number (6.022 . The superscript o indicates that the parameter is evaluated at atmospheric pressure. | |
| Eaton and Akgerman [66] | It is valid in the range 0.35 3.10 and 0.8 is reduced density of 2 (kg m−3) and is reduced temperature of 2 (K). |
| System | D21 (10−9 m2 s−1) | Method |
|---|---|---|
| CO2 + ethanol at 25 °C | 4.04 | Diaphragm cell [72] |
| CO2 + ethanol at 25 °C | 4.5 | Wetted wall column [71] |
| CO2 + ethanol at 25 °C | 4.11 | Taylor dispersion [71] |
| CO2 + acetone at 30 °C | 6.08 | Diaphragm cell [73] |
| CO2 + methanol at 30 °C | 4.95 | Diaphragm cell [64] |
| CO2 + methanol at 25 °C | 5.55 ± 0.09 | Taylor dispersion [73] |
| Sample | De (10−9 m2 s−1) |
|---|---|
| Silica aerogel | 5.75 |
| IPA-CO2 system at 37.5 °C and 80 bar [31] | |
| Silica gel | 3.05–5.52 |
| Ethanol-CO2 system in the range from 20 °C to 42 °C at 90 bar [27] | |
| Silica aerogel | 4.7–5.1 |
| Ethanol-CO2 system at 40 °C 100 bar [5] | |
| Silica aerogel | 5.5 |
| Ethanol-CO2 system at 42 °C and 90 bar [46] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2018, 4, 3. https://doi.org/10.3390/gels4010003
Şahin İ, Özbakır Y, İnönü Z, Ulker Z, Erkey C. Kinetics of Supercritical Drying of Gels. Gels. 2018; 4(1):3. https://doi.org/10.3390/gels4010003
Chicago/Turabian StyleŞahin, İbrahim, Yaprak Özbakır, Zeynep İnönü, Zeynep Ulker, and Can Erkey. 2018. "Kinetics of Supercritical Drying of Gels" Gels 4, no. 1: 3. https://doi.org/10.3390/gels4010003