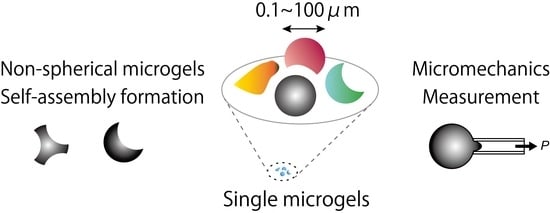
Single Micrometer-Sized Gels: Unique Mechanics and Characters for Applications
Abstract
:1. Introduction
2. Morphology of Single Microgels
2.1. Morphology Control through Phase Separation and Gelation of Polymers inside Microdroplets
2.1.1. Gelation and Wetting of Polymers on the Microdroplet Surface
2.1.2. Volume Fraction of Two Coexisting Phases and Size of the Confining Microdroplet
2.2. Morphology Control by Buckling Instability
3. Micromechanics Measurement of Single Microgels
3.1. Micromechanics of Single Microgels
3.2. Structural Differences between Single Microgels and Bulk Gels
4. Summary and Perspective
Acknowledgments
Conflicts of Interest
References
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Fernandez-Nieves, A.; Wyss, H.; Mattsson, J.; Weitz, D.A. Microgel Suspensions: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Calo, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Handore, K.; Sureshan, K.M. Soft Optical Devices from Self-Healing Gels Formed by Oil and Sugar-Based Organogelators. Angew. Chem. Int. Ed. 2011, 50, 8021–8024. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Fukuchi, M.; Kaji, H.; Nakanishi, K. Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew. Chem. Int. Ed. 2013, 52, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.S.; Gupta, R.B. Fast-responding bulk hydrogels with microstructure. J. Appl. Polym. Sci. 2002, 83, 169–178. [Google Scholar] [CrossRef]
- Shimanovich, U.; Efimov, I.; Mason, T.O.; Flagmeier, P.; Buell, A.K.; Gedanken, A.; Linse, S.; Åkerfeldt, K.S.; Dobson, C.M.; Weitz, D.A.; et al. Protein microgels from amyloid fibril networks. ACS Nano 2015, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Freemont, T.J.; Saunders, B.R. pH-Responsive microgel dispersions for repairing damaged load-bearing soft tissue. Soft Matter 2008, 4, 919–924. [Google Scholar] [CrossRef]
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic encapsulation of cells in polymer microgels. Small 2012, 8, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Takeuchi, S. Three-dimensional cell culture based on microfluidic techniques to mimic living tissues. Biomater. Sci. 2013, 1, 257–264. [Google Scholar] [CrossRef]
- Thomas, D.; Fontana, G.; Chen, X.; Sanz-Nogués, C.; Zeugolis, D.I.; Dockery, P.; O’Brien, T.; Pandit, A. A shape-controlled tuneable microgel platform to modulate angiogenic paracrine responses in stem cells. Biomaterials 2014, 35, 8757–8766. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Liu, Y.; Narkar, A.; Lee, B.P. Gelatin Microgel Incorporated Poly(ethylene glycol)-Based Bioadhesive with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces 2016, 8, 11980–11989. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, E.; Kaneda, I. Rheological properties of agar microgel suspensions prepared using water-in-oil emulsions. J. Biorheol. 2010, 24, 70–76. [Google Scholar] [CrossRef]
- Lyon, L.A.; Fernandez-Nieves, A. The polymer/colloid duality of microgel suspensions. Annu. Rev. Phys. Chem. 2012, 63, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, C.; Fujiwara, K.; Morita, M.; Kawamata, I.; Kawagishi, Y.; Sakai, A.; Murayama, Y.; Shin-ichiro, M.N.; Murata, S.; Takinoue, M.; et al. DNA cytoskeleton for stabilizing artificial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 7228–7233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Yu, X.; Patil, A.J.; Li, M.; Mann, S. Cytoskeletal-like supramolecular assembly and nanoparticle-based motors in a model protocell. Angew. Chem. Int. Ed. Engl. 2011, 50, 9343–9347. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Wöll, D.; Flors, C. Super-resolution Fluorescence Imaging for Materials Science. Small Methods 2017, 1, 1700191. [Google Scholar] [CrossRef]
- Sacanna, S.; Pine, D.J. Shape-anisotropic colloids: Building blocks for complex assemblies. Curr. Opin. Colloid Interface Sci. 2011, 16, 96–105. [Google Scholar] [CrossRef]
- Sacanna, S.; Irvine, W.T.M.; Chaikin, P.M.; Pine, D.J. Lock and key colloids. Nature 2010, 464, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Sacanna, S.; Korpics, M.; Rodriguez, K.; Colón-Meléndez, L.; Kim, S.H.; Pine, D.J.; Yi, G.R. Shaping colloids for self-assembly. Nat. Commun. 2013, 4, 1688. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Du, X.; Ren, X.; Xie, Y.; Sheng, X.; Zhang, X. Morphology control of anisotropic nonspherical functional polymeric particles by one-pot dispersion polymerization. React. Funct. Polym. 2017, 112, 53–59. [Google Scholar] [CrossRef]
- Glotzer, S.C.; Solomon, M.J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 2007, 6, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Yamashita, Y.; Mukai, S.-A.; Annaka, M.; Tokita, M. Phase separation in binary polymer solution: Gelatin/poly (ethylene glycol) system. J. Mol. Liq. 2014, 200, 2–6. [Google Scholar] [CrossRef]
- Kanamori, K.; Yonezawa, H.; Nakanishi, K.; Hirao, K.; Jinnai, H. Structural formation of hybrid siloxane-based polymer monolith in confined spaces. J. Sep. Sci. 2004, 27, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Weitz, D.A.; Lee, C.S. One step formation of controllable complex emulsions: From functional particles to simultaneous encapsulation of hydrophilic and hydrophobic agents into desired position. Adv. Mater. 2013, 25, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Abate, A.R.; Lee, D.; Studart, A.R.; Wang, B.; Chen, C.H.; Thiele, J.; Shah, R.K.; Krummel, A.; Weitz, D.A. Droplet microfluidics for fabrication of non-spherical particles. Macromol. Rapid Commun. 2010, 31, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Muller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Wang, Y.C.; Liu, J. Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines 2017, 8, 255. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Abbaspourrad, A.; Ardekani, A.M. Fabrication of shape controllable Janus alginate/pNIPAAm microgels via microfluidics technique and off-chip ionic cross-linking. Langmuir 2015, 31, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Thiele, J.; Liu, X.; Bai, Y.; Abell, C.; Huck, W.T. Fabrication of microgel particles with complex shape via selective polymerization of aqueous two-phase systems. Small 2012, 8, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Nigorikawa, S.; Sakaue, T.; Fujiwara, K.; Tokita, M. Multiple patterns of polymer gels in microspheres due to the interplay among phase separation, wetting and gelation. Proc. Natl. Acad. Sci. USA 2014, 111, 15894–15899. [Google Scholar] [CrossRef] [PubMed]
- Zarzar, L.D.; Sresht, V.; Sletten, E.M.; Kalow, J.A.; Blankschtein, D.; Swager, T.M. Dynamically reconfigurable complex emulsions via tunable interfacial tensions. Nature 2015, 518, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Mora, T.; Boudaoud, A. Buckling of swelling gels. Eur. Phys. J. E Soft Matter 2006, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kumachev, A.; Tumarkin, E.; Walker, G.C.; Kumacheva, E. Characterization of the mechanical properties of microgels acting as cellular microenvironments. Soft Matter 2013, 9, 2959–2965. [Google Scholar] [CrossRef]
- Mohapatra, H.; Kruger, T.M.; Lansakara, T.I.; Tivanski, A.V.; Stevens, L.L. Core and surface microgel mechanics are differentially sensitive to alternative crosslinking concentrations. Soft Matter 2017, 13, 5684–5695. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, A.; Richter, M.; Uzum, C.; von Klitzing, R. Effect of cross-linker density of P(NIPAM-co-AAc) microgels at solid surfaces on the swelling/shrinking behaviour and the Young’s modulus. Colloid Polym. Sci. 2011, 289, 613–624. [Google Scholar] [CrossRef]
- Guo, M.Y.; Wyss, H.M. Micromechanics of Soft Particles. Macromol. Mater. Eng. 2011, 296, 223–229. [Google Scholar] [CrossRef]
- Evans, E.A. Structure and Deformation Properties of Red Blood-Cells—Concepts and Quantitative Methods. Method Enzymol. 1989, 173, 3–35. [Google Scholar]
- Guilak, F.; Tedrow, J.R.; Burgkart, R. Viscoelastic properties of the cell nucleus. Biochem. Biophys. Res. Commun. 2000, 269, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Sakai, A.; Murayama, Y.; Fujiwara, K.; Fujisawa, T.; Sasaki, S.; Kidoaki, S.; Yanagisawa, M. Increasing elasticity through changes in the secondary structure of gelatin by gelation in a microsized lipid space. ACS Cent. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Wyss, H.M.; Franke, T.; Mele, E.; Weitz, D.A. Capillary micromechanics: Measuring the elasticity of microscopic soft objects. Soft Matter 2010, 6, 4550–4555. [Google Scholar] [CrossRef]
- Kong, T.T.; Wang, L.Q.; Wyss, H.M.; Shum, H.C. Capillary micromechanics for core-shell particles. Soft Matter 2014, 10, 3271–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, G.; Boltyanskiy, R.; Nejati, S.; Thiam, A.R.; Loewenberg, M.; Dufresne, E.R.; Osuji, C.O. Single-step microfluidic fabrication of soft monodisperse polyelectrolyte microcapsules by interfacial complexation. Lab Chip 2014, 14, 3494–3497. [Google Scholar] [CrossRef] [PubMed]
- Mercadé-Prieto, R.; Nguyen, B.; Allen, R.; York, D.; Preece, J.A.; Goodwin, T.E.; Zhang, Z. Determination of the elastic properties of single microcapsules using micromanipulation and finite element modeling. Chem. Eng. Sci. 2011, 66, 2042–2049. [Google Scholar] [CrossRef]
- Tagit, O.; Tomczak, N.; Vancso, G.J. Probing the morphology and nanoscale mechanics of single poly(N-isopropylacrylamide) microgels across the lower-critical-solution temperature by atomic force microscopy. Small 2008, 4, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Xu, G.; Kang, W.; Meng, L.; Gong, H.; Zhou, T. Surface rheological behavior of gelatin/ionic liquid-type imidazolium gemini surfactant mixed systems. Soft Matter 2011, 7, 1199–1206. [Google Scholar] [CrossRef]






| Microgel Sample | Bulk Modulus, K (GPa) | BLS Young’s Modulus, E (GPa) | AFM Young’s Modulus, E (GPa) |
|---|---|---|---|
| pS-co-NIPAM-1 | 4.92 ± 0.2 | 3.59 ± 0.14 | 0.24 ± 0.03 |
| pS-co-NIPAM-2 | 4.86 ± 0.19 | 3.70 ± 0.15 | 0.54 ± 0.03 |
| pS-co-NIPAM-3 | 4.86 ± 0.19 | 4.07 ± 0.16 | 0.61 ± 0.05 |
| pS-co-NIPAM-4 | 4.92 ± 0.2 | 4.25 ± 0.16 | 0.91 ± 0.06 |
| pS-co-NIPAM-5 | 5.02 ± 0.2 | 4.65 ± 0.19 | 0.99 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagisawa, M.; Watanabe, C.; Fujiwara, K. Single Micrometer-Sized Gels: Unique Mechanics and Characters for Applications. Gels 2018, 4, 29. https://doi.org/10.3390/gels4020029
Yanagisawa M, Watanabe C, Fujiwara K. Single Micrometer-Sized Gels: Unique Mechanics and Characters for Applications. Gels. 2018; 4(2):29. https://doi.org/10.3390/gels4020029
Chicago/Turabian StyleYanagisawa, Miho, Chiho Watanabe, and Kei Fujiwara. 2018. "Single Micrometer-Sized Gels: Unique Mechanics and Characters for Applications" Gels 4, no. 2: 29. https://doi.org/10.3390/gels4020029
APA StyleYanagisawa, M., Watanabe, C., & Fujiwara, K. (2018). Single Micrometer-Sized Gels: Unique Mechanics and Characters for Applications. Gels, 4(2), 29. https://doi.org/10.3390/gels4020029