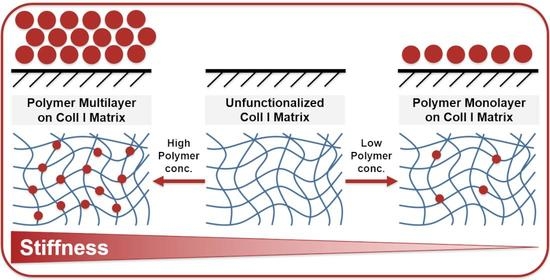
Stiffness Variation of 3D Collagen Networks by Surface Functionalization of Network Fibrils with Sulfonated Polymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Defined Fibrillar 3D Collagen Matrices
2.2. Non-Monotonic Influence of Sulfonated Polymer Adsorption on Coll I Network Stiffness
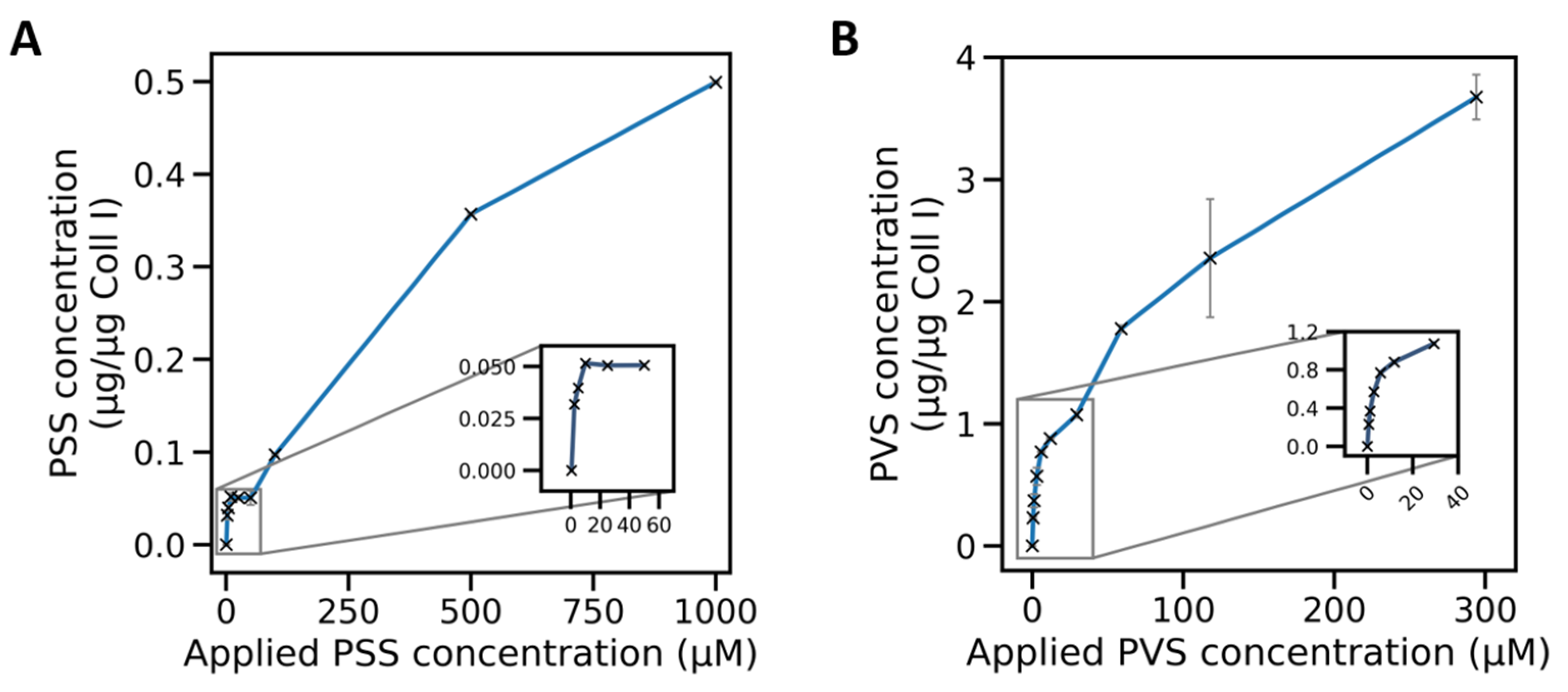
2.3. Adsorption Isotherms of Sulfonate Polymers for Coll I Networks Exhibited Mono- and Multilayer Characteristics
2.4. Desorption Kinetics of PSS and PVS from Coll I Networks Indicated High Monolayer Stability
2.5. Correlating Sulfonated Polymer Adsorption to Coll I Fibril Stiffness
3. Conclusions
4. Materials and Methods
4.1. Reconstitution of Collagen I Networks
4.2. Polymer Functionalization of Collagen I Networks
4.3. Desorption Measurements
4.4. Adsorption Measurements
4.5. Toluidine Blue Assay
4.6. Colloidal Probe Force Spectroscopy
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen Type I: A Versatile Biomaterial. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018; pp. 389–414. ISBN 978-981-13-0947-2. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Sapudom, J.; Pompe, T. Biomimetic tumor microenvironments based on collagen matrices. Biomater. Sci. 2018, 6, 2009–2024. [Google Scholar] [CrossRef]
- Scott, R.A.; Panitch, A. Glycosaminoglycans in biomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Freudenberg, U.; Liang, Y.; Kiick, K.L.; Werner, C. Glycosaminoglycan-Based Biohybrid Hydrogels: A Sweet and Smart Choice for Multifunctional Biomaterials. Adv. Mater. 2016, 28, 8861–8891. [Google Scholar] [CrossRef] [PubMed]
- Pourhanifeh, M.H.; Mohammadi, R.; Noruzi, S.; Hosseini, S.A.; Fanoudi, S.; Mohamadi, Y.; Hashemzehi, M.; Asemi, Z.; Mirzaei, H.R.; Salarinia, R.; et al. The role of fibromodulin in cancer pathogenesis: Implications for diagnosis and therapy. Cancer Cell Int. 2019, 19, 157. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T.; Lee, E.J.; Choi, I. Fibromodulin: A regulatory molecule maintaining cellular architecture for normal cellular function. Int. J. Biochem. Cell Biol. 2016, 80, 66–70. [Google Scholar] [CrossRef]
- Parry, D.A.D.; Flint, M.H.; Gillard, G.C.; Craig, A.S. A role for glycosaminoglycans in the development of collagen fibrils. FEBS Lett. 1982, 149, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hempel, U.; Matthäus, C.; Preissler, C.; Möller, S.; Hintze, V.; Dieter, P. Artificial Matrices With High-Sulfated Glycosaminoglycans and Collagen Are Anti-Inflammatory and Pro-Osteogenic for Human Mesenchymal Stromal Cells. J. Cell. Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Salbach-Hirsch, J.; Ziegler, N.; Thiele, S.; Moeller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; Rauner, M.; Hofbauer, L.C. Sulfated Glycosaminoglycans Support Osteoblast Functions and Concurrently Suppress Osteoclasts. J. Cell. Biochem. 2014, 115, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, M.; Kalbitzer, L.; Franz, S.; Moeller, S.; Schnabelrauch, M.; Simon, J.-C.; Pompe, T.; Franke, K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv. Healthc. Mater. 2017, 6, 1600967. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/glycosaminoglycan-based matrices for controlling skin cell responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.A.; Calabro, A.; Darr, A.B.; Dours-Zimmermann, M.T.; Sandy, J.D.; Zimmermann, D.R.; Sorrell, J.M.; Hascall, V.C.; Caplan, A.I. Age-related differences in human skin proteoglycans. Glycobiology 2011, 21, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1227–1246. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Kim, Y.K.; Jung, J.-Y.; Shin, J.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Oh, J.-H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underhill, C.B.; Hahn, P.J.; Brown, D.B.; Uitto, J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br. J. Dermatol. 1996, 135, 255–262. [Google Scholar] [CrossRef]
- Oh, J.-H.; Shin, M.K.; Lee, H.; Lim, J.; Choi, M.; Cho, S.; Chung, J.H. Analysis of sulfated glycosaminoglycan composition change in intrinsically aged and photoaged human skin using an enzymatic degradation method. J. Dermatol. Sci. 2018, 92, 281–283. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kalbitzer, L.; Pompe, T. Fibril growth kinetics link buffer conditions and topology of 3D collagen I networks. Acta Biomater. 2018, 67, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalbitzer, L.; Franke, K.; Möller, S.; Schnabelrauch, M.; Pompe, T. Glycosaminoglycan functionalization of mechanically and topologically defined collagen I matrices. J. Mater. Chem. B 2015, 3, 8902–8910. [Google Scholar] [CrossRef] [Green Version]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Franke, K.; Sapudom, J.; Kalbitzer, L.; Anderegg, U.; Pompe, T. Topologically defined composites of collagen types I and V as in vitro cell culture scaffolds. Acta Biomater. 2014, 10, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Hattori, Y.; Kaneko, K.; Ohba, T. Adsorption Properties. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 25–44. [Google Scholar]
- Skalny, J.; Hearn, N. Surface Area Measurements. In Handbook of Analytical Techniques in Concrete Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 505–527. [Google Scholar]
- Qu, D.; Baigl, D.; Williams, C.E.; Möhwald, H.; Fery, A. Dependence of Structural Forces in Polyelectrolyte Solutions on Charge Density: A Combined AFM/SAXS Study. Macromolecules 2003, 36, 6878–6883. [Google Scholar] [CrossRef]
- Pial, T.H.; Sachar, H.S.; Das, S. Quantification of Mono- and Multivalent Counterion-Mediated Bridging in Polyelectrolyte Brushes. Macromolecules 2021, 54, 4154–4163. [Google Scholar] [CrossRef]
- Yu, J.; Jackson, N.E.; Xu, X.; Brettmann, B.K.; Ruths, M.; de Pablo, J.J.; Tirrell, M. Multivalent ions induce lateral structural inhomogeneities in polyelectrolyte brushes. Sci. Adv. 2017, 3, eaao1497. [Google Scholar] [CrossRef] [PubMed]
- Broedersz, C.P.; MacKintosh, F.C. Modeling semiflexible polymer networks. Rev. Mod. Phys. 2014, 86, 995–1036. [Google Scholar] [CrossRef] [Green Version]
- Sapudom, J.; Kalbitzer, L.; Wu, X.; Martin, S.; Kroy, K.; Pompe, T. Fibril bending stiffness of 3D collagen matrices instructs spreading and clustering of invasive and non-invasive breast cancer cells. Biomaterials 2019, 193, 47–57. [Google Scholar] [CrossRef]
- Freudenberg, U.; Behrens, S.H.; Welzel, P.B.; Müller, M.; Grimmer, M.; Salchert, K.; Taeger, T.; Schmidt, K.; Pompe, W.; Werner, C. Electrostatic Interactions Modulate the Conformation of Collagen I. Biophys. J. 2007, 92, 2108–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertinetti, L.; Masic, A.; Schuetz, R.; Barbetta, A.; Seidt, B.; Wagermaier, W.; Fratzl, P. Osmotically driven tensile stress in collagen-based mineralized tissues. J. Mech. Behav. Biomed. Mater. 2015, 52, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Katchalsky, A.; Oplatka, A. Dynamic-elastic investigation of the chemical denaturation of collagen fibers. Biopolymers 1968, 6, 1159–1168. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masic, A.; Bertinetti, L.; Schuetz, R.; Chang, S.-W.; Metzger, T.H.; Buehler, M.J.; Fratzl, P. Osmotic pressure induced tensile forces in tendon collagen. Nat. Commun. 2015, 6, 5942. [Google Scholar] [CrossRef] [Green Version]
- Chialvo, A.A.; Simonson, J.M. Solvation Behavior of Short-Chain Polystyrene Sulfonate in Aqueous Electrolyte Solutions: A Molecular Dynamics Study. J. Phys. Chem. B 2005, 109, 23031–23042. [Google Scholar] [CrossRef] [PubMed]
- Stamov, D.R.; Pompe, T. Structure and function of ECM-inspired composite collagen type I scaffolds. Soft Matter 2012, 8, 10200. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, P.; Schricker, M.; Pompe, T. Stiffness Variation of 3D Collagen Networks by Surface Functionalization of Network Fibrils with Sulfonated Polymers. Gels 2021, 7, 266. https://doi.org/10.3390/gels7040266
Riedl P, Schricker M, Pompe T. Stiffness Variation of 3D Collagen Networks by Surface Functionalization of Network Fibrils with Sulfonated Polymers. Gels. 2021; 7(4):266. https://doi.org/10.3390/gels7040266
Chicago/Turabian StyleRiedl, Philipp, Maria Schricker, and Tilo Pompe. 2021. "Stiffness Variation of 3D Collagen Networks by Surface Functionalization of Network Fibrils with Sulfonated Polymers" Gels 7, no. 4: 266. https://doi.org/10.3390/gels7040266