Structurally Stable, High-Strength Graphene Oxide/Carbon Nanotube/Epoxy Resin Aerogels as Three-Dimensional Skeletal Precursors for Wave-Absorbing Materials
Abstract
:1. Introduction
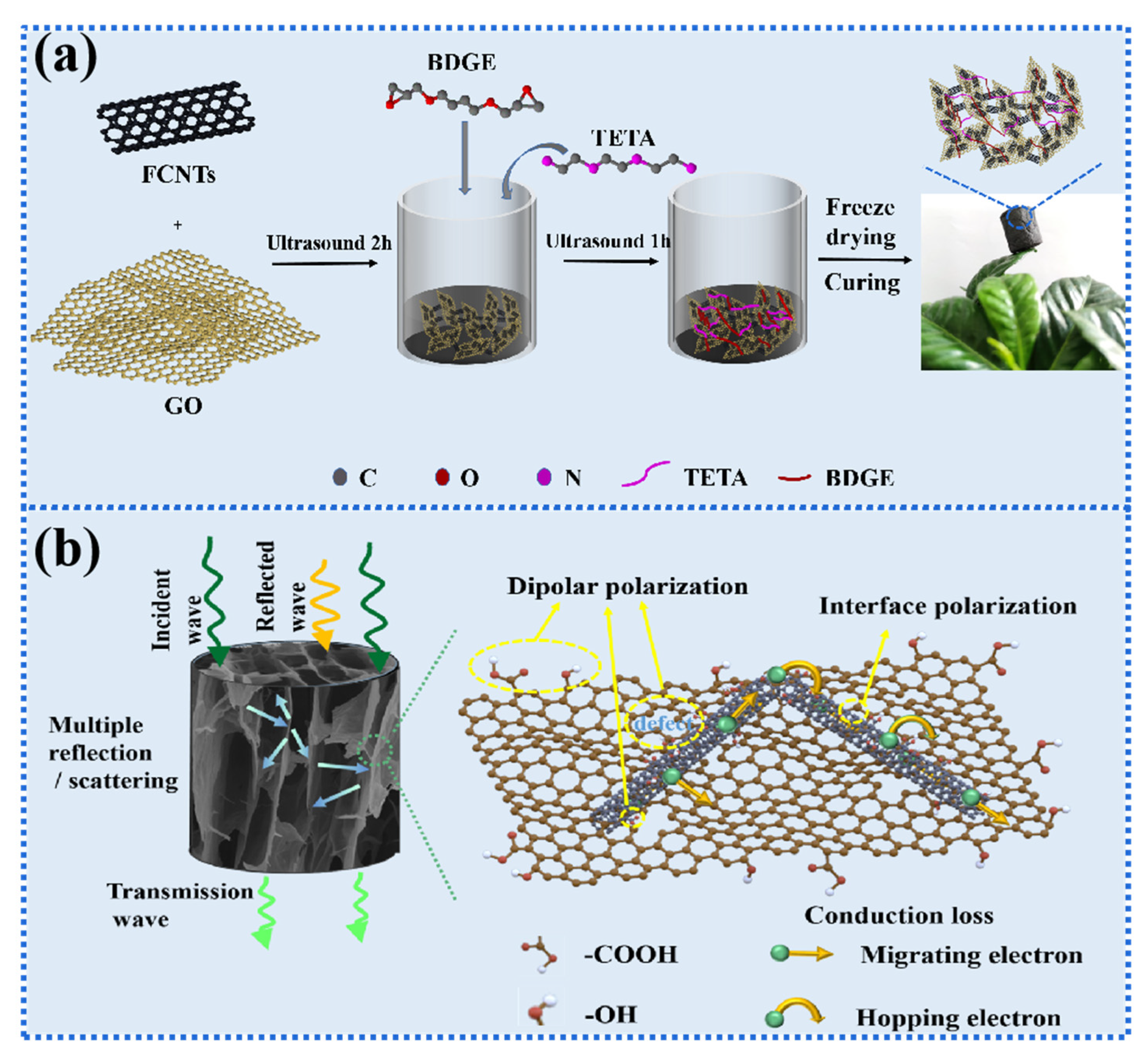
2. Results and Discussion
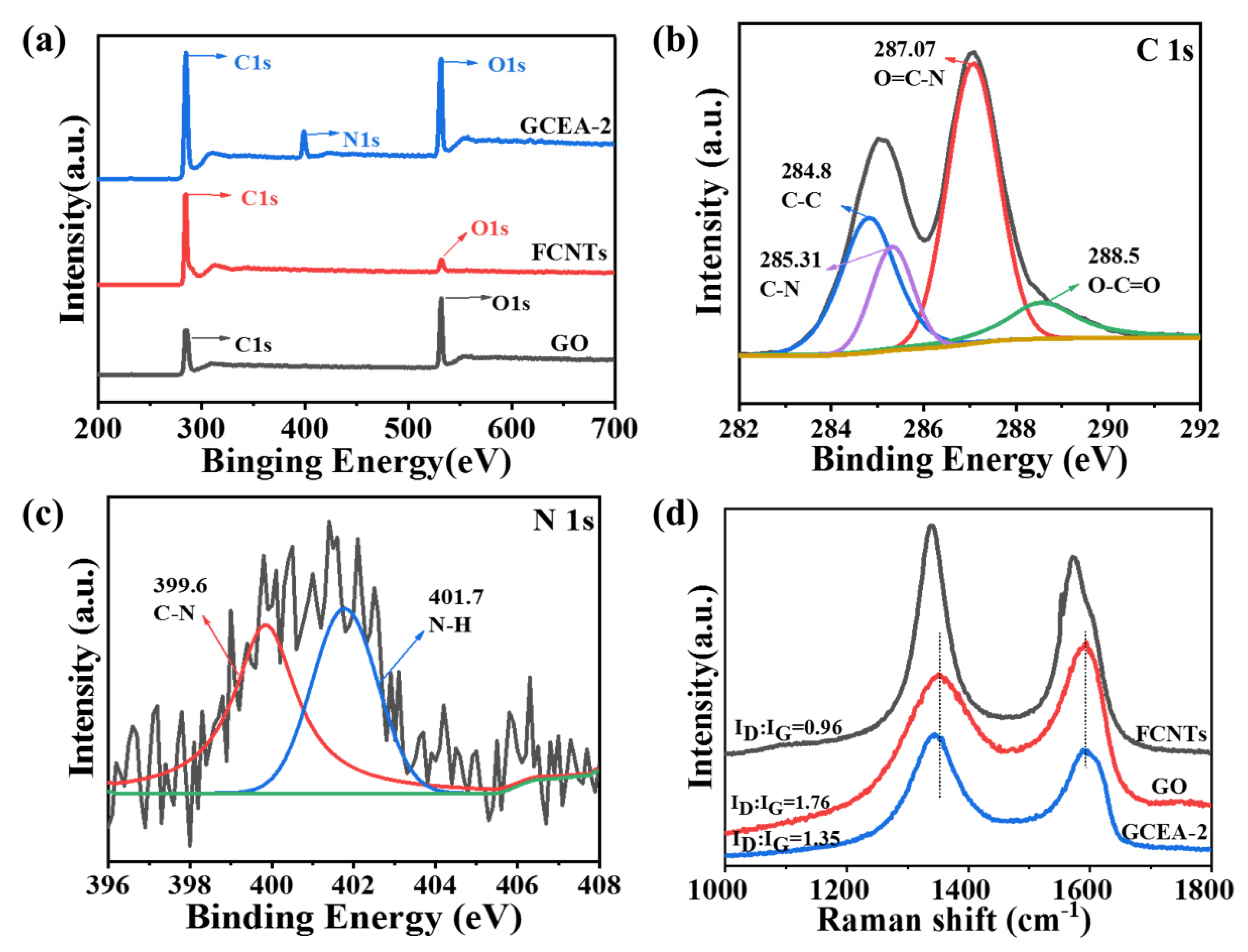
2.1. Elemental Analysis
2.2. Morphological Characterization
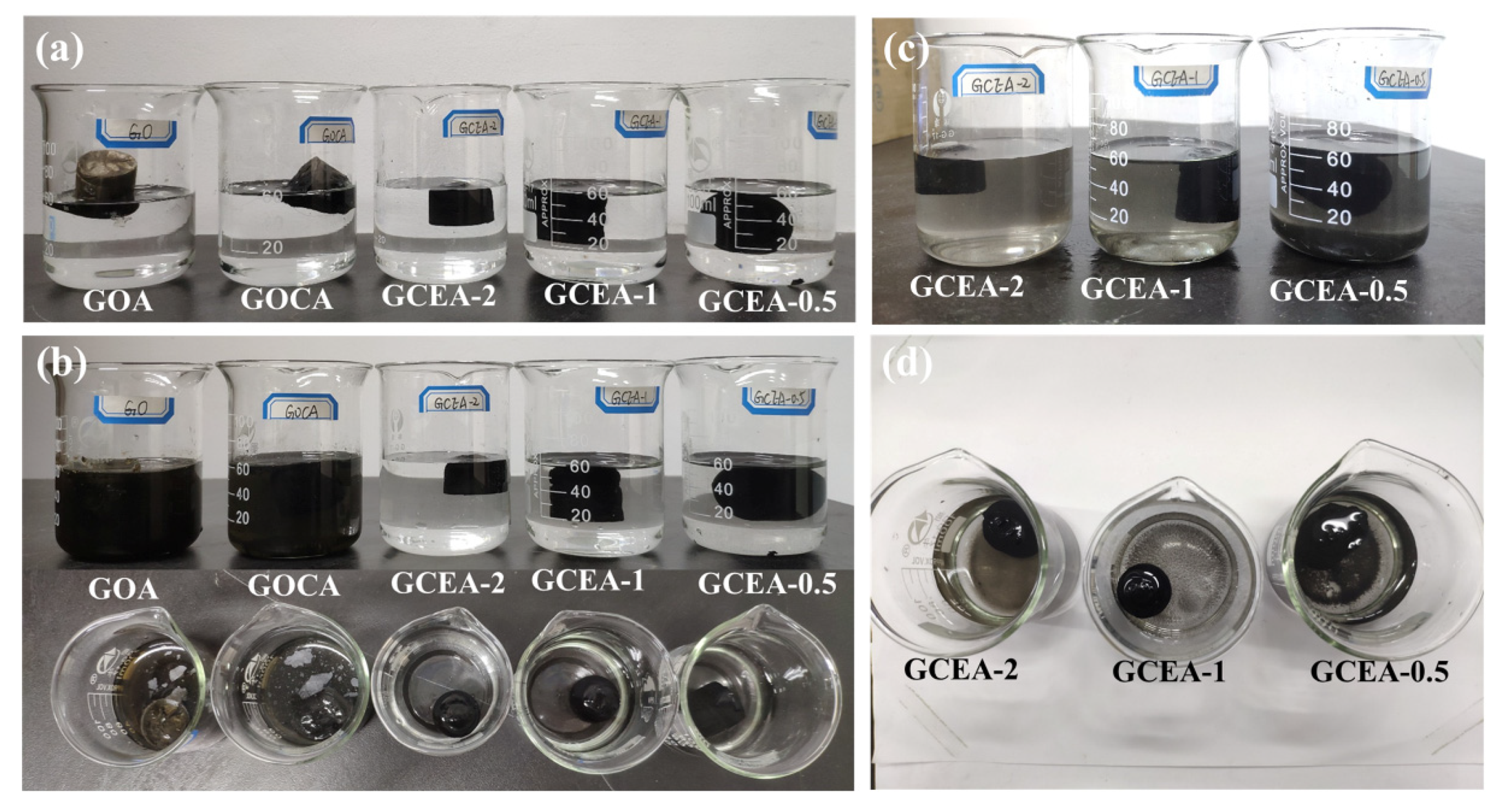
2.3. Hydrophilic and Structural Stability
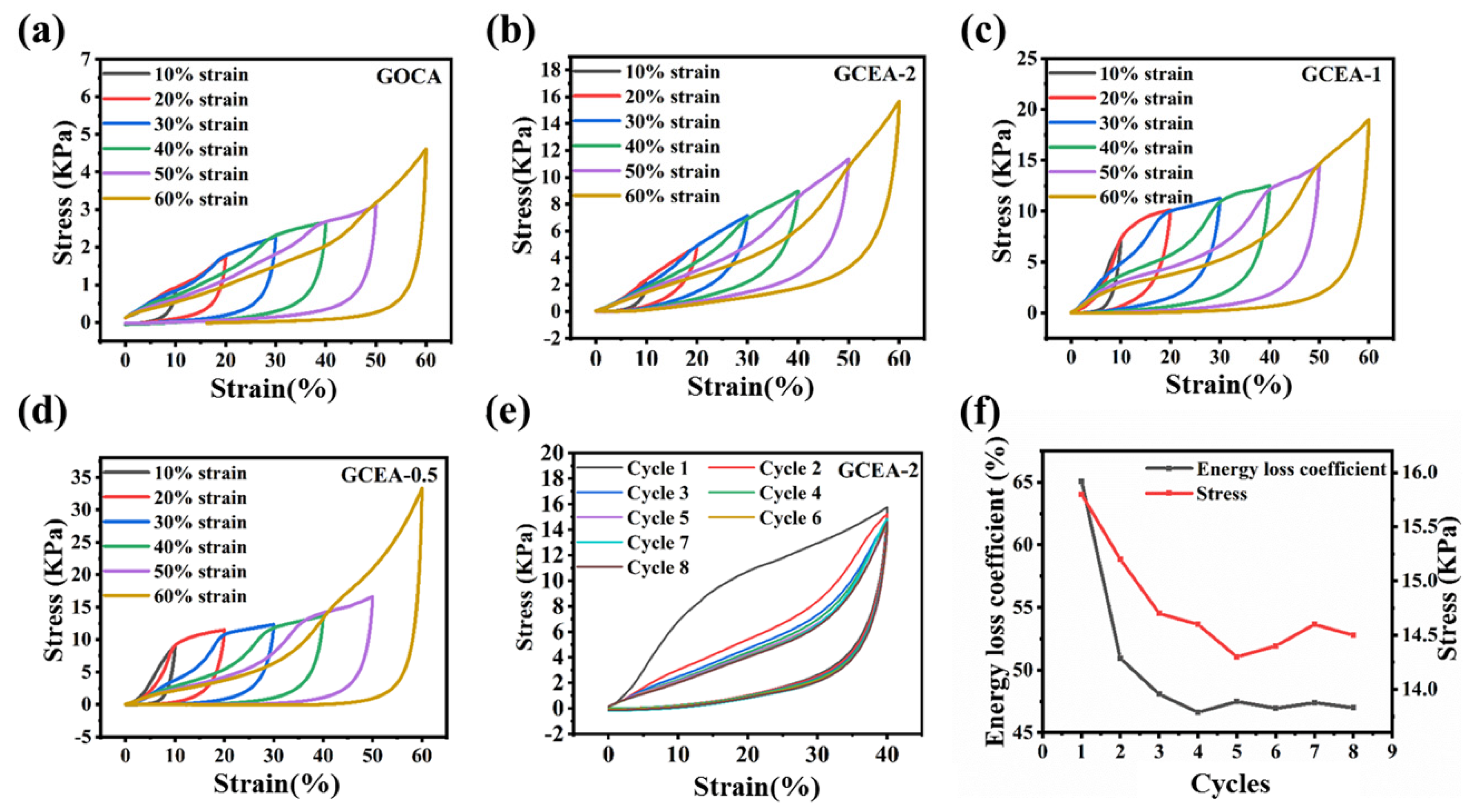
2.4. Mechanical Properties
2.5. Thermal Stability Analysis
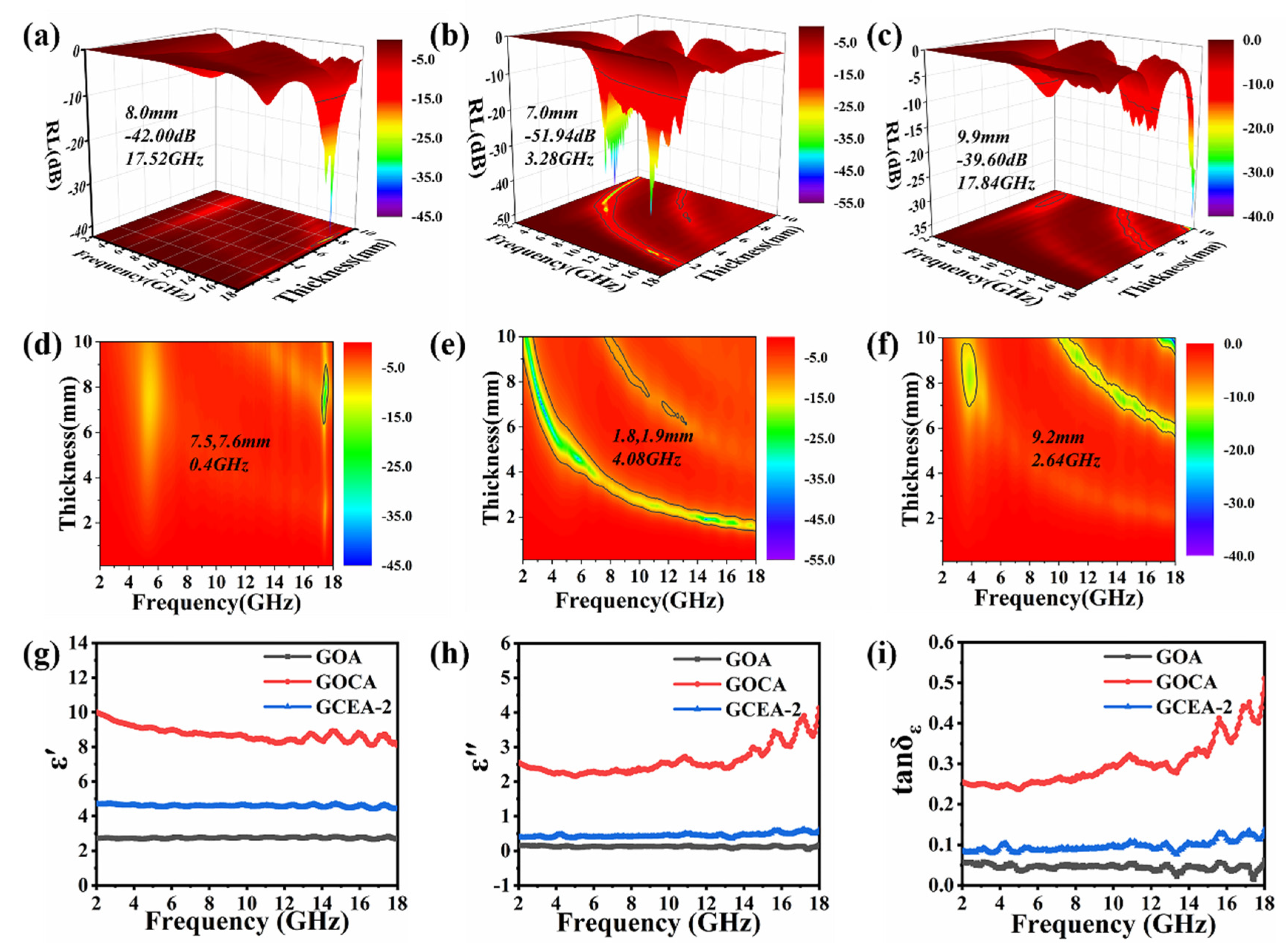
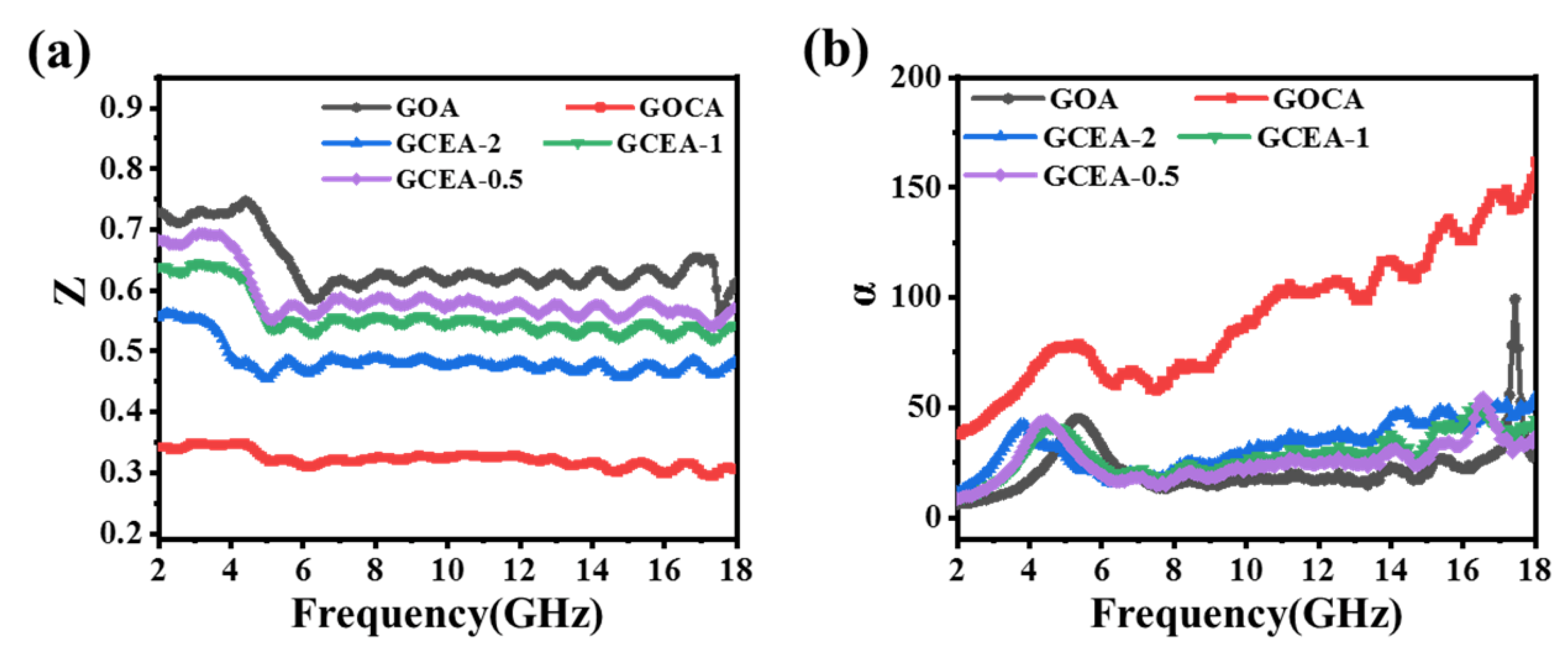
2.6. Microwave Absorption Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of GO
4.3. Preparation of FCNTs
4.4. Preparation of GCEA
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, S.; Datar, S. Wideband (8–18 GHz) microwave absorption dominated electromagnetic interference (EMI) shielding composite using copper aluminum ferrite and reduced graphene oxide in polymer matrix. J. Appl. Phys. 2020, 128, 104902. [Google Scholar] [CrossRef]
- Shu, R.W.; Zhang, G.Y.; Zhang, C.; Wu, Y.; Zhang, J.B. Nitrogen-Doping-Regulated Electromagnetic Wave Absorption Properties of Ultralight Three-Dimensional Porous Reduced Graphene Oxide Aerogels. Adv. Electron. Mater. 2021, 7, 2001001. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Malfait, W.J.; Sadeghpour, A.; Girão, A.V.; Silva, R.F.; Durães, L. Influence of 1D and 2D carbon nanostructures in silica-based aerogels. Carbon 2021, 180, 146–162. [Google Scholar] [CrossRef]
- Puthiyedath Narayanan, A.; Narayanan Unni, K.N.; Peethambharan Surendran, K. Aerogels of V2O5 nanowires reinforced by polyaniline for electromagnetic interference shielding. Chem. Eng. J. 2021, 408, 127239. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional Magnetic Ti3C2Tx MXene/Graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Yuan, H.; Li, K.; Ouyang, Q.; Zhu, C.; Zhang, S.; Chen, Y. CoNi nanoparticles encapsulated by nitrogen-doped carbon nanotube arrays on reduced graphene oxide sheets for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 123208. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Zeng, Q.; Duan, H.; Guo, Y.; Liu, X.; Sun, C.; Liu, H. Fabrication of ultralight three-dimensional graphene networks with strong electromagnetic wave absorption properties. J. Mater. Chem. A 2015, 3, 3739–3747. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and tunable high-performance microwave absorption of an ultralight and highly compressible graphene foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.W.; Xu, J.; Wan, Z.L.; Cao, X. Synthesis of hierarchical porous nitrogen-doped reduced graphene oxide/zinc ferrite composite foams as ultrathin and broadband microwave absorbers. J. Colloid Interface Sci. 2022, 608, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Liang, C.; Wang, L.; Qiu, H.; Gu, H.; Kong, J.; Gu, J. Obviously improved electromagnetic interference shielding performances for epoxy composites via constructing honeycomb structural reduced graphene oxide. Compos. Sci. Technol. 2019, 181, 107698. [Google Scholar] [CrossRef]
- Guo, T.; Chen, X.; Zeng, G.; Yang, J.; Huang, X.; Li, C.; Tang, X.-Z. Impregnating epoxy into N-doped-CNTs@carbon aerogel to prepare high-performance microwave-absorbing composites with extra-low filler content. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106159. [Google Scholar] [CrossRef]
- Shen, B.; Li, Y.; Zhai, W.; Zheng, W. Compressible Graphene-Coated Polymer Foams with Ultralow Density for Adjustable Electromagnetic Interference (EMI) Shielding. ACS Appl. Mater. Interfaces 2016, 8, 8050–8057. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Li, P.; Ma, L.; Li, H.; Shi, L.; Wang, M.; Chen, H.; Song, G. Enhancing mechanical performances of polystyrene composites via constructing carbon nanotube/graphene oxide aerogel and hot pressing. Compos. Sci. Technol. 2020, 195, 108191. [Google Scholar] [CrossRef]
- Afroze, J.D.; Abden, M.J.; Yuan, Z.; Wang, C.; Wei, L.; Chen, Y.; Tong, L. Core-shell structured graphene aerogels with multifunctional mechanical, thermal and electromechanical properties. Carbon 2020, 162, 365–374. [Google Scholar] [CrossRef]
- Barg, S.; Perez, F.M.; Ni, N.; do Vale Pereira, P.; Maher, R.C.; Garcia-Tunon, E.; Eslava, S.; Agnoli, S.; Mattevi, C.; Saiz, E. Mesoscale assembly of chemically modified graphene into complex cellular networks. Nat. Commun. 2014, 5, 4328. [Google Scholar] [CrossRef]
- Xu, H.; Song, G.; Ma, L.; Feng, P.; Xu, L.; Zhang, L.; Zhao, Z.; Jin, J.; Chen, Z.; Li, X. Evolution of properties and enhancement mechanism of large-scale three-dimensional graphene oxide-carbon nanotube aerogel/polystyrene nanocomposites. Polym. Test. 2021, 97, 107158. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yue, K.; Li, A.; Qian, L. Multi-scale structural nitrogen-doped rGO@CNTs composites with ultra-low loading towards microwave absorption. Appl. Surf. Sci. 2021, 538, 147943. [Google Scholar] [CrossRef]
- Lv, P.; Tan, X.-W.; Yu, K.-H.; Zheng, R.-L.; Zheng, J.-J.; Wei, W. Super-elastic graphene/carbon nanotube aerogel: A novel thermal interface material with highly thermal transport properties. Carbon 2016, 99, 222–228. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Sha, P.; Wang, B.; Ding, Y.; Du, S. High-efficiency electromagnetic wave absorption of epoxy composites filled with ultralow content of reduced graphene/carbon nanotube oxides. Compos. Sci. Technol. 2020, 189, 108020. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Xu, J.; Ouyang, Q.; Zhu, C.; Zhang, X.; Zhang, X.; Chen, Y. N-doped carbon nanotube arrays on reduced graphene oxide as multifunctional materials for energy devices and absorption of electromagnetic wave. Carbon 2021, 177, 216–225. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.-B.; Wang, M.; Qian, X.; Dasari, A.; Yu, Z.-Z. Phenolic resin-enhanced three-dimensional graphene aerogels and their epoxy nanocomposites with high mechanical and electromagnetic interference shielding performances. Compos. Sci. Technol. 2017, 152, 254–262. [Google Scholar] [CrossRef]
- Huo, J.B.; Yu, G.; Wang, J. Adsorptive removal of Sr(II) from aqueous solution by polyvinyl alcohol/graphene oxide aerogel. Chemosphere 2021, 278, 130492. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, J.; Ge, S.; Jiang, C.; Guo, T.; Peng, T.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient Oil-Water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Mo, S.; Zhang, Y.; Li, Z.; Ou, H. Prominent adsorption of Cr(VI) with graphene oxide aerogel twined with creeper-like polymer based on chitosan oligosaccharide. Carbohydr. Polym. 2020, 247, 116733. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-J.; Zhu, P.-L.; Yu, S.-H.; Sun, R.; Wong, C.-P.; Liao, W.-H. Ultralight, super-elastic and volume-preserving cellulose fiber/graphene aerogel for high-performance electromagnetic interference shielding. Carbon 2017, 115, 629–639. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Li, X.; Zhang, Y.; Qu, J.; Yu, Z.-Z. Cellulose/graphene aerogel supported phase change composites with high thermal conductivity and good shape stability for thermal energy storage. Carbon 2016, 98, 50–57. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Sun, X.; Su, Z.B.; Huang, Y.H.; Weng, W.; Hu, S.R.; Guo, H.X.; Wu, W.B.; He, Y.S. Highly effective removal of Cu(II) by triethylenetetramine-magnetic reduced graphene oxide composite. Appl. Surf. Sci. 2015, 356, 355–363. [Google Scholar] [CrossRef]
- Huo, J.; Liu, X.; Li, X.; Qin, L.; Kang, S.-Z. An efficient photocatalytic system containing Eosin Y, 3D mesoporous graphene assembly and CuO for visible-light-driven H2 evolution from water. Int. J. Hydrogen Energy 2017, 42, 15540–15550. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Zhao, J.; Qu, W.; Li, X.; Li, S.; Xing, B.; Fu, Y. In-situ grafted graphene oxide-based waterborne epoxy curing agent for reinforcement corrosion protection of waterborne epoxy coating. Surf. Coat. Technol. 2021, 412, 127043. [Google Scholar] [CrossRef]
- Song, B.; Sizemore, C.; Li, L.; Huang, X.; Lin, Z.; Moon, K.-S.; Wong, C.-P. Triethanolamine functionalized graphene-based composites for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 21789–21796. [Google Scholar] [CrossRef]
- Liu, T.; Huang, M.; Li, X.; Wang, C.; Gui, C.-X.; Yu, Z.-Z. Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 2016, 100, 456–464. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Chen, Z.; Zhong, L.; Lai, H.; Liu, L.; Jing, S.; Liu, Q.; Liu, C.; et al. A supercompressible, elastic, and bendable carbon aerogel with ultrasensitive detection limits for compression strain, pressure, and bending angle. Adv. Mater. 2018, 30, 1706705. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Jia, Z.; Dong, Y.; Liu, X.; Wu, G. Layered 3D structure derived from MXene/magnetic carbon nanotubes for ultra-broadband electromagnetic wave absorption. Chem. Eng. J. 2022, 431, 133919. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Luo, H.; Deng, L.; Wei, S.; Zheng, Y.; Jia, Q.; Liu, J. Rapid and direct growth of bipyramid TiO2 from Ti3C2Tx MXene to prepare Ni/TiO2/C heterogeneous composites for high-performance microwave absorption. Chem. Eng. J. 2020, 383, 123095. [Google Scholar] [CrossRef]
- Li, N.; Shu, R.; Zhang, J.; Wu, Y. Synthesis of ultralight three-dimensional nitrogen-doped reduced graphene oxide/multi-walled carbon nanotubes/zinc ferrite composite aerogel for highly efficient electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 596, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Kang, J.; Zhang, S.; Li, C.; Zhu, C.; Zhang, X.; Chen, Y. Enhanced electromagnetic wave absorption induced by void spaces in hollow nanoparticles. Nanoscale 2018, 10, 18742–18748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.; Du, Y.; Bai, J.; Fan, H.; Zhang, H.; Peng, Y.; Li, F. One-pot synthesis of CoFe2O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Qu, B.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Coupling hollow Fe3O4-Fe nanoparticles with graphene sheets for high-performance electromagnetic wave Absorbing Material. ACS Appl. Mater. Interfaces 2016, 8, 3730–3735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Liu, X.; Zhang, S.; Yuan, H.; Zhu, C.; Zhang, X.; Chen, Y. Metal organic framework-derived three-dimensional graphene-supported nitrogen-doped carbon nanotube spheres for electromagnetic wave absorption with ultralow filler mass loading. Carbon 2019, 155, 233–242. [Google Scholar] [CrossRef]
- Song, S.; Zhang, A.; Chen, L.; Jia, Q.; Zhou, C.; Liu, J.; Wang, X. A novel multi-cavity structured MOF derivative/porous graphene hybrid for high performance microwave absorption. Carbon 2021, 176, 279–289. [Google Scholar] [CrossRef]
- Deng, L.; Shu, R.; Zhang, J. Fabrication of ultralight nitrogen-doped reduced graphene oxide/nickel ferrite composite foams with three-dimensional porous network structure as ultrathin and high-performance microwave absorbers. J. Colloid Interface Sci. 2022, 614, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Huang, Y.; Cheng, Y.; Wang, S.; Yin, W. Achieving Super Broadband Electromagnetic Absorption by Optimizing Impedance Match of rGO Sponge Metamaterials. Adv. Funct. Mater. 2022, 32, 2107508. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Afroze, J.D.; Tong, L.; Abden, M.J.; Yuan, Z.; Chen, Y. Hierarchical honeycomb graphene aerogels reinforced by carbon nanotubes with multifunctional mechanical and electrical properties. Carbon 2021, 175, 312–321. [Google Scholar] [CrossRef]
- Qazi, R.A.; Khattak, R.; Ali Shah, L.; Ullah, R.; Khan, M.S.; Sadiq, M.; Hessien, M.M.; El-Bahy, Z.M. Effect of MWCNTs Functionalization on Thermal, Electrical, and Ammonia-Sensing Properties of MWCNTs/PMMA and PHB/MWCNTs/PMMA Thin Films Nanocomposites. Nanomaterials 2021, 11, 2625. [Google Scholar] [CrossRef]
- Song, G.; Gai, L.; Yang, K.; Wang, X.; An, Q.; Xiao, Z.; Zhai, S. A versatile N-doped honeycomb-like carbonaceous aerogels loaded with bimetallic sulfide and oxide for superior electromagnetic wave absorption and supercapacitor applications. Carbon 2021, 181, 335–347. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Wan, G.; Shi, S.; Hao, C.; Tang, Y.; Wang, G. Magnetic Ni/graphene connected with conductive carbon nano-onions or nanotubes by atomic layer deposition for lightweight and low-frequency microwave absorption. Chem. Eng. J. 2020, 382, 122980. [Google Scholar] [CrossRef]




| Sample | GO/(12.63 mg/mL)/mL | FCNTs/mg | BDGE/mg | TETA/mg | H2O/mL | Ethanol |
|---|---|---|---|---|---|---|
| GOCA | 16 | 84 | 0 | 0 | 12 | 2.2 |
| GCEA-3 | 16 | 84 | 75.39 | 18.12 | 12 | 2.2 |
| GCEA-2 | 16 | 84 | 112.82 | 27.18 | 12 | 2.2 |
| GCEA-1 | 16 | 84 | 225.64 | 54.36 | 12 | 2.2 |
| GCEA-0.5 | 16 | 84 | 451.26 | 108.74 | 12 | 2.2 |
| GCEA-0.33 | 16 | 84 | 676.89 | 163.11 | 12 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Song, G.; Zhao, Z.; Ma, L.; Xu, H.; Wu, G.; Song, Y.; Liu, Y.; Qiu, L.; Li, X. Structurally Stable, High-Strength Graphene Oxide/Carbon Nanotube/Epoxy Resin Aerogels as Three-Dimensional Skeletal Precursors for Wave-Absorbing Materials. Gels 2022, 8, 618. https://doi.org/10.3390/gels8100618
Zhang L, Song G, Zhao Z, Ma L, Xu H, Wu G, Song Y, Liu Y, Qiu L, Li X. Structurally Stable, High-Strength Graphene Oxide/Carbon Nanotube/Epoxy Resin Aerogels as Three-Dimensional Skeletal Precursors for Wave-Absorbing Materials. Gels. 2022; 8(10):618. https://doi.org/10.3390/gels8100618
Chicago/Turabian StyleZhang, Lina, Guojun Song, Zetian Zhao, Lichun Ma, Hui Xu, Guanglei Wu, Yinghu Song, Yinuo Liu, Lihan Qiu, and Xiaoru Li. 2022. "Structurally Stable, High-Strength Graphene Oxide/Carbon Nanotube/Epoxy Resin Aerogels as Three-Dimensional Skeletal Precursors for Wave-Absorbing Materials" Gels 8, no. 10: 618. https://doi.org/10.3390/gels8100618




