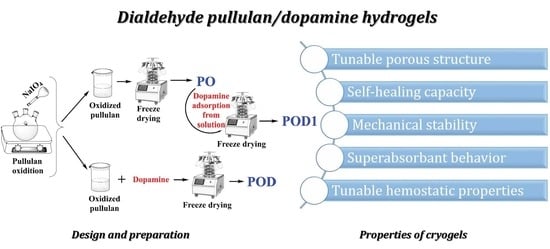
Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of the Pullulan–Dopamine Cryogels
2.1.1. NMR Spectra Analysis
2.1.2. FTIR Spectra Analysis
2.2. Internal Morphology and Network Parameters
2.2.1. Environmental Scanning Microscopy (ESEM) Studies
2.2.2. Porosity and Density of the Cryogels
2.3. Dynamic Water Vapor Sorption Efficiency
2.4. Swelling Behavior—Analysis of the Kinetics and Mechanism
2.5. Rheological Behavior
2.6. Mechanical Stability—Behavior under Compression Stress
2.7. Hemocompatibility of the Pullulan–Dopamine Cryogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cryogel Preparation
4.2.1. Synthesis of Oxidized Pullulan
4.2.2. Preparation of Pullulan–Dopamine Cryogels
4.3. Methods
4.3.1. Nuclear Magnetic Resonance Spectroscopy
4.3.2. Fourier-Transform Infrared Spectroscopy
4.3.3. Environmental Scanning Microscopy (ESEM)
4.3.4. Determination of the Apparent Density and Porosity of the Cryogel
4.3.5. Dynamic Water Vapor Sorption Studies
4.3.6. Swelling Measurements
4.3.7. Mechanical Tests
4.3.8. Rheological Measurements
4.3.9. In Vitro Hemolysis Assays
4.3.10. In Vitro Blood-Clotting Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Lynne Taylor, D.; In het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Wang, Y.; Maitz, P.K.M. Advances and new technologies in the treatment of burn injury. Adv. Drug Deliv. Rev. 2018, 123, 1–2. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Yu, H.; Liu, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive Sol-Gel transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Ren, J.; Xuan, H.; Dai, W.; Zhu, Y.; Ge, L. Double network self-healing film based on metal chelation and Schiff-base interaction and its biological activities. Appl. Surf. Sci. 2018, 448, 609–617. [Google Scholar] [CrossRef]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Q.; Liu, J.; Pei, Y.; Tang, K. A unique high mechanical strength dialdehyde microfibrillated cellulose/gelatin composite hydrogel with a giant network structure. RSC Adv. 2016, 6, 71999–72007. [Google Scholar] [CrossRef]
- Draye, J.P.; Delaey, B.; Van de Voorde, A.; Van Den Bulcke, A.; Bogdanov, B.; Schacht, E. In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films. In Proceedings of the 8th International Symposium on Recent Advances in Drug Delivery Systems, Salt Lake City, UT, USA, 24–27 February 1997; Volume 19, pp. 169–170. [Google Scholar]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, characterization and feasibility study of dialdehyde carboxymethyl cellulose as a novel crosslinking reagent. Carbohydr. Polym. 2016, 137, 632–641. [Google Scholar] [CrossRef]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef]
- Li, X.; Xue, W.; Liu, Y.; Li, W.; Fan, D.; Zhu, C.; Wang, Y. HLC/pullulan and pullulan hydrogels: Their microstructure, engineering process and biocompatibility. Mater. Sci. Eng. C 2016, 58, 1046–1057. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Biliuta, G.; Avadanei, M.; Baron, R.I.; Butnaru, M.; Coseri, S. Self-healing hydrogels of oxidized pullulan and poly(vinyl alcohol). Carbohydr. Polym. 2019, 206, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide hydrogels for multiscale 3D printing of pullulan scaffolds. Mater. Des. 2019, 165, 107566. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Bruneel, D.; Schacht, E. Chemical modification of pullulan: 1. Periodate oxidation. Polymer (Guildf.) 1993, 34, 2628–2632. [Google Scholar] [CrossRef]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar] [CrossRef]
- De Nooy, A.E.J.; Besemer, A.C.; Van Bekkum, H.; Van Dijk, J.A.P.P.; Smit, J.A.M. TEMPO-mediated oxidation of pullulan and influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 1996, 29, 6541–6547. [Google Scholar] [CrossRef]
- Duceac, I.A.; Vereștiuc, L.; Coroaba, A.; Arotăriței, D.; Coseri, S. All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan. Int. J. Biol. Macromol. 2021, 181, 1047–1062. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical hydrogels of oxidized polysaccharides and poly(vinyl alcohol) forwound dressing applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef] [Green Version]
- Biliuta, G.; Baron, R.I.; Coseri, S. Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide. Materials 2022, 15, 6086. [Google Scholar] [CrossRef]
- Veelaert, S.; De Wit, D.; Gotlieb, K.F.; Verhé, R. Chemical and physical transitions of periodate oxidized potato starch in water. Carbohydr. Polym. 1997, 33, 153–162. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Z.; Li, Y.; Wang, L.; Fu, F.; Diao, H.; Liu, X. A novel pullulan oxidation approach to preparing a shape memory sponge with rapid reaction capability for massive hemorrhage. Chem. Eng. J. 2022, 447, 137482. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Liu, C.; Wang, N.; Chen, H.; Yao, W.; Sun, G.; Song, Q.; Qiao, W. A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 2019, 205, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, G.; Dan, N.; Huang, Y.; Bai, Z.; Yang, C.; Dan, W. Preparation and characterization of dopamine–sodium carboxymethyl cellulose hydrogel. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int. J. Biol. Macromol. 2020, 143, 714–723. [Google Scholar] [CrossRef]
- Serrero, A.; Trombotto, S.; Cassagnau, P.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide gels based on chitosan and modified starch: Structural characterization and linear viscoelastic behavior. Biomacromolecules 2010, 11, 1534–1543. [Google Scholar] [CrossRef]
- Gutiérrez-Tauste, D.; Domènech, X.; Domingo, C.; Ayllón, J.A. Dopamine/TiO2 hybrid thin films prepared by the liquid phase deposition method. Thin Solid Films 2008, 516, 3831–3835. [Google Scholar] [CrossRef]
- Huang, W.M.; Jiang, P.; Wei, C.Y.; Zhuang, D.K.; Shi, J. Low-temperature one-step synthesis of covalently chelated ZnO/dopamine hybrid nanoparticles and their optical properties. J. Mater. Res. 2008, 23, 1946–1952. [Google Scholar] [CrossRef]
- Wu, L.; Shi, M.; Guo, R.; Dong, W. Development of a novel pullulan/polydopamine composite hydrogel adsorbent for dye removal. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 652, 129632. [Google Scholar] [CrossRef]
- Gao, B.; Chen, L.; Zhao, Y.; Yan, X.; Wang, X.; Zhou, C.; Shi, Y.; Xue, W. Methods to prepare dopamine/polydopamine modified alginate hydrogels and their special improved properties for drug delivery. Eur. Polym. J. 2019, 110, 192–201. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sollich, P. Rheological constitutive equation for a model of soft glassy materials. Phys. Rev. E 1998, 58, 738. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Wu, L.; Zuo, G.; Pan, X.; Shi, M.; Zhang, C.; Qi, X.; Dong, W. Incorporation of dumbbell-shaped and Y-shaped cross-linkers in adjustable pullulan/polydopamine hydrogels for selective adsorption of cationic dyes. Environ. Res. 2020, 182, 109010. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Wang, X.; Yang, M. Biocompatible materials of pulsatile and rotary blood pumps: A brief review. Rev. Adv. Mater. Sci. 2020, 59, 322–339. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Pontara, E.; Cheng, C.; Gerosa, G.; Pengo, V.; Bagno, A. Preliminary hemocompatibility assessment of an innovative material for blood contacting surfaces. J. Mater. Sci. Mater. Med. 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Duan, G.; Bagheri, A.R.; Jiang, S.; Golenser, J.; Agarwal, S.; Greiner, A. Exploration of Macroporous Polymeric Sponges As Drug Carriers. Biomacromolecules 2017, 18, 3215–3221. [Google Scholar] [CrossRef]
- Su, C.; Yang, H.; Song, S.; Lu, B.; Chen, R. A magnetic superhydrophilic/oleophobic sponge for continuous oil-water separation. Chem. Eng. J. 2017, 309, 366–373. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, Y.; Qian, C.; Yang, D.; Nie, J.; Ma, G. A natural polymer-based porous sponge with capillary-mimicking microchannels for rapid hemostasis. Acta Biomater. 2020, 114, 193–205. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Controll. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhu, Y.; Zheng, L.; Zhang, L.; Hu, Y.; Yu, B.; Wang, Y.; Xu, F.J. Biofilm-Sensitive Photodynamic Nanoparticles for Enhanced Penetration and Antibacterial Efficiency. Adv. Funct. Mater. 2021, 31, 2103591. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, W.; Yang, Z.; Zhang, H.; Ma, J.; Li, T.; Niu, H.; Zhou, Y.; Yao, Q.; Chang, J.; et al. An ultralong hydroxyapatite nanowire aerogel for rapid hemostasis and wound healing. Chem. Eng. J. 2022, 430, 132912. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable Gelatin-Based IPN Cryogel Hemostat for Rapidly Stopping Deep Noncompressible Hemorrhage and Simultaneously Improving Wound Healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]













| Sample Code | PO | POD | POD1 |
|---|---|---|---|
| Simulation plot |  |  |  |
| R2 | 0.9959 | 0.9581 | 0.9495 |
| n | 0.3223 | 0.0285 | 0.0445 |
| k | 0.2511 | 0.5101 | 1.1852 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostănaru-Iliescu, A.-C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726. https://doi.org/10.3390/gels8110726
Baron RI, Duceac IA, Morariu S, Bostănaru-Iliescu A-C, Coseri S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels. 2022; 8(11):726. https://doi.org/10.3390/gels8110726
Chicago/Turabian StyleBaron, Raluca Ioana, Ioana A. Duceac, Simona Morariu, Andra-Cristina Bostănaru-Iliescu, and Sergiu Coseri. 2022. "Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings" Gels 8, no. 11: 726. https://doi.org/10.3390/gels8110726