Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties
Abstract
:1. Introduction
2. Results
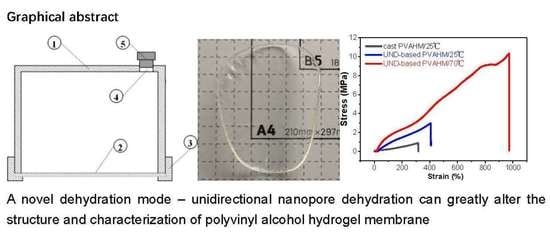
2.1. UND Preparation of PVAHM
2.2. Thickness
2.3. PVA Molecular Weight
2.4. Temperature Influence on Physicomechanical Properties
2.5. Stress and Strain Curves
2.6. Thermal Properties
2.7. Fourier Transform Infrared Spectrometer (FTIR)
2.8. X-Ray Diffraction (XRD) and Small-Angle X-Ray Scattering (SAXS)
2.9. Scanning Electron Microscopy (SEM)
2.10. Cell Culture
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. PVAHM Preparation
5.2.1. Aqueous 10% PVA Solution
5.2.2. Mold Installation and Preparation
5.2.3. PVAHM Preparation
5.3. Analytical Methods
5.3.1. Mechanical Properties
5.3.2. Swelling Ratio Test
5.3.3. Scanning Electron Microscopy Investigation
5.3.4. Thermal Analysis
5.3.5. FTIR Spectroscopy Analysis
5.3.6. Powder X-Ray Diffraction Study
5.3.7. Small-Angle X-Ray Scattering (SAXS)
5.4. Cell Culture
5.4.1. Cell Culture Procedure
5.4.2. Cell Pavement
5.4.3. Cell Morphology Observations
5.4.4. Cell Viability Assay
5.5. Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UND | unidirectional nanopore dehydration |
| PBS | phosphate-buffered saline |
| TG | thermogravimetric |
| DTG | differential thermogravimetric |
| DSC | differential scanning calorimetry |
| DMEM | Dulbecco’s modified Eagle’s medium |
| calcein AM | calcein acetoxymethyl ester |
| OD | optical density |
| PVAHM | PVA hydrogel membrane |
References
- Tubbs, R.K. Sequence distribution of partially hydrolyzed poly(vinyl acetate). J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 623–629. [Google Scholar] [CrossRef]
- Hueper, W.C. Carcinogenic studies on water-soluble and insoluble macromolecules. Arch. Pathol. 1959, 67, 589–617. [Google Scholar]
- Tamada, Y.; Ikada, Y. Cell Attachment to Various Polymer Surfaces. In Polymers in Medicine II; Springer: Boston, MA, USA, 1986; Volume 34, pp. 101–115. [Google Scholar]
- Jiang, T.; Wang, G.X.; Qiu, J.H.; Luo, L.L.; Zhang, G.Q. Preparation and Biocompatibility of Polyvinyl Alcohol-Small Intestinal Submucosa Hydrogel Membranes. J. Med. Biol. Eng. 2009, 29, 102–107. [Google Scholar]
- Oliveira, M.; Amato, V.S.; Lugao, A.B.; Parra, D.F. Hybrid hydrogels produced by ionizing radiation technique. Radiat. Phys. Chem. 2012, 81, 1471–1474. [Google Scholar] [CrossRef]
- Hameed, N.; Glattauer, V.; Ramshaw, J. Evaluation of polyvinyl alcohol composite membranes containing collagen and bone particles. J. Mech. Behav. Biomed. Mater. 2015, 48, 38–45. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Klopp, L.S.; Toth, J.M.; Welch, W.C.; Rao, S.; Tai, J.W.; Thomas, K.A.; Turner, S. Bioresorbable film for the prevention of adhesion to the anterior spine after anterolateral discectomy. Spine J. 2009, 9, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.A.; Brodke, D.S.; Pimenta, L.; Bono, C.M.; Hilibrand, A.S.; Harrop, J.S.; Riew, K.D.; Youssef, J.A.; Vaccaro, A.R. Revision strategies in lumbar total disc arthroplasty. Spine 2008, 33, 1276. [Google Scholar] [CrossRef]
- Jeffords, P.; Li, J.; Panchal, D.; Denoziere, G.; Fetterolf, D. Safety and effectiveness of a polyvinyl alcohol barrier in reducing risks of vascular tissue damage during anterior spinal revision surgery. J. Spinal Disord. Tech. 2012, 25, 150–156. [Google Scholar] [CrossRef]
- Freytag, C.; Odermatt, E.K. Standard Biocompatibility Studies Do Not Predict All Effects of PVA/CMC Anti-Adhesive Gel in vivo. Eur. Surg. Res. 2016, 56, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Fu, J.; Wu, J.; Jia, X.; Zhou, Y.; Li, C.; Dong, M.; Wang, S.; Zhang, J.; Chen, F. Bioinformatics analysis and characterization of highly efficient polyvinyl alcohol (PVA)-degrading enzymes from the novel PVA degrader Stenotrophomonas rhizophila QL-P4. Appl. Environ. Microbiol. 2017, 84, 01898–17. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Cao, M.; Wen, H.; Tan, Z.; Jia, S.; Cui, J. Biodegradation of polyvinyl alcohol using cross-linked enzyme aggregates of degrading enzymes from Bacillus niacini. Int. J. Biol. Macromol. 2019, 124, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, L.; Song, Z.; Hua, Z.; Zhu, Y.; Chen, J. Production of polyvinyl alcohol-degrading enzyme with Janthinobacterium sp. and its application in cotton fabric desizing. Biotechnol. J. 2007, 2, 752–758. [Google Scholar] [CrossRef]
- Yang, Y.; Ko, T.P.; Liu, L.; Li, J.; Huang, C.H.; Chan, H.C.; Ren, F.; Jia, D.; Wang, A.H.; Guo, R.T.; et al. Structural Insights into Enzymatic Degradation of Oxidized Polyvinyl Alcohol. ChemBioChem 2014, 15, 1882–1886. [Google Scholar] [CrossRef]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef]
- Wu, H.F.; Yue, L.Z.; Jiang, S.L.; Lu, Y.Q.; Wu, Y.X.; Wan, Z.Y. Biodegradation of polyvinyl alcohol by different dominant degrading bacterial strains in a baffled anaerobic bioreactor. Water Sci. Technol. 2019, 79, 2005–2012. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, G.; Gao, G.; Liu, F. Influences of Intramolecular Cyclization on Structure and Cross-Linking Reaction Processes of PVA Hydrogels. Macromolecules 2006, 39, 1160–1164. [Google Scholar] [CrossRef]
- Ying, A.; Koyama, T.; Hanabusa, K.; Shirai, H.; Ikeda, J.; Yoneno, H. Preparation and properties of highly phosphorylated poly(vinyl alcohol) hydrogels chemically crosslinked by glutaraldehyde. Polymer 1995, 36, 2297–2301. [Google Scholar]
- Suzuki, A.; Sasaki, S. Swelling and mechanical properties of physically crosslinked poly(vinyl alcohol) hydrogels. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Cheaburu Yilmaz, C.N.; Pamfil, D.; Vasile, C.; Bibire, N.; Lupuşoru, R.V.; Zamfir, C.L.; Lupușoru, C.E. Toxicity, Biocompatibility, pH-Responsiveness and Methotrexate Release from PVA/Hyaluronic Acid Cryogels for Psoriasis Therapy. Polymers 2017, 9, 123. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M.; Ogino, K.; Nambu, M. Simple extension of poly (vinyl alcohol) gels. Polym. Commun. 1983, 24, 345–347. [Google Scholar]
- Yokoyama, E.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 601, 595–601. [Google Scholar] [CrossRef]
- Nagura, M.; Hamano, T.; Ishikawa, H. Structure of poly(vinyl alcohol) hydrogel prepared by repeated freezing and melting. Polymer 1989, 30, 762–765. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Peppast, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly (vinyl alcohol) membranes prepared by freezing/thawing techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Fukumori, T.; Nakaoki, T. High-Tensile-Strength Polyvinyl Alcohol Films Prepared from Freeze/Thaw Cycled Gels. J. Appl. Polym. Sci. 2014, 131, 40578. [Google Scholar] [CrossRef]
- Millon, L.E.; Mohammadi, H.; Wan, W.K. Anisotropic Polyvinyl Alcohol Hydrogel for Cardiovascular Applications. J. Biomed. Mater. Res. B 2006, 79, 305–311. [Google Scholar] [CrossRef]
- Gouws, G.; Kivell, D.; Ryan, B.; Thomson, M.; Dawson, A. Properties of PVA tissue phantoms produced by directional solidification. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 1341–1344. [Google Scholar]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef]
- Zhang, H.; Cooper, A.I. Aligned porous structures by directional freezing. Adv. Mater. 2007, 19, 1529–1533. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zhu, J.; He, C.; Wang, H. Anisotropic tough poly(vinyl alcohol) hydrogels. Soft Matter 2012, 8, 10439–10447. [Google Scholar] [CrossRef]
- Pramanik, R.; Narayanan, A.; Rajan, A.; Konar, S.; Arockiarajan, A. Transversely isotropic freeze-dried PVA hydrogels: Theoretical modelling and experimental characterization. Int. J. Eng. Sci. 2019, 144, 103144. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matsumura, K.; Suong Hyu, H. A preliminary in vivo study of artificial meniscus using the uniaxial oriented reinforced compressive polyvinyl alcohol hydrogel. Trends Biomater. Artif. Organs 2011, 25, 107–111. [Google Scholar]
- Honciuc, A.; Solonaru, A.M.; Teodorescu, M. Flexible Composites with Variable Conductivity and Memory of Deformation Obtained by Polymerization of Polyaniline in PVA Hydrogel. Polymers 2022, 14, 4638. [Google Scholar] [CrossRef]
- Watanabe, K.; Shimura, T.; Nagasawa, A.; Nagao, D. Multipoint Lock-and-Key Assembly of Particles with Anisotropic Dents toward Modeling Rigid Macromolecules in a Colloidal Scale. Langmuir 2021, 37, 9451–9456. [Google Scholar] [CrossRef]
- Yang, C.C. Synthesis and characterization of the cross-linked PVA/TiO2 composite polymer membrane for alkaline DMFC. J. Membr. Sci. 2007, 288, 51–60. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and mechanical properties of poly(vinyl alcohol) plasticized with glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Nooeaid, P.; Ummartyotin, S. Development of a gallic acid-loaded chitosan and polyvinyl alcohol hydrogel composite: Release characteristics and antioxidant activity. Int. J. Biol. Macromol. 2018, 107, 363–370. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [PubMed]
- Yu, C.; Tang, X.; Liu, S.; Yang, Y.; Shen, X.; Gao, C. Crystallinity and stability of poly(vinyl alcohol)-fumed silica mixed matrix membranes. J. Macromol. Sci. Phys. 2008, 47, 39–51. [Google Scholar]
- Yu, C.; Tang, X.; Liu, S.; Yang, Y.; Shen, X.; Gao, C. Laponite crosslinked starch/polyvinyl alcohol hydrogels by freezing/thawing process and studying their cadmium ion absorption. Int. J. Biol. Macromol. 2018, 117, 1–6. [Google Scholar] [PubMed]
- Peppas, N.A. Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Macromol. Chem. Phys. 1975, 176, 3433–3440. [Google Scholar] [CrossRef]
- Birman, T.; Seliktar, D. Injectability of Biosynthetic Hydrogels: Consideration for Minimally Invasive Surgical Procedures and 3D Bioprinting. Adv. Funct. Mater. 2021, 31, 2100628. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854. [Google Scholar] [CrossRef]
- Mao, Y.; Mohanty, P.; Ghosh, G. Morphology and properties of poly vinyl alcohol (PVA) scaffolds: Impact of process variables. Mater. Sci. Eng. C 2014, 42, 289–294. [Google Scholar]
- Chen, Y.; Jiao, C.; Peng, X.; Liu, T.; Shi, Y.; Liang, M.; Wang, H. Biomimetic anisotropic poly(vinyl alcohol) hydrogels with significantly enhanced mechanical properties by freezing-thawing under drawing. J. Mater. Chem. B 2019, 7, 3243–3249. [Google Scholar] [CrossRef]
- Ray, R.S.; Agrawal, N.; Sharma, A.; Hans, R.K. Use of L-929 cell line for phototoxicity assessment. Toxicol. Vitr. 2008, 22, 1775–1781. [Google Scholar] [CrossRef]






| Mowiol® | Alcoholysis Degree (%) | Mw (kDa) | Water Stability | σ (MPa) | ±SD | EB (%) | ±SD | E (MPa) | ±SD | Note |
|---|---|---|---|---|---|---|---|---|---|---|
| PVA-105 | 98~99 | ~47 | √ | 0 | 0 | 0 | 0 | 0 | 0 | |
| PVA-117 | 98~99 | ~145 | √ | 1.19 | 0.25 | 333 | 73 | 0.43 | 0.07 | toxicity |
| PVA-124 | 98~99 | ~190 | √ | 2.57 | 0.46 | 409 | 36 | 0.59 | 0.02 | in use |
| PVA-203 | 87~89 | ~31 | × | — | — | — | — | — | — | — |
| PVA-210 | 87~89 | ~67 | × | — | — | — | — | — | — | — |
| PVA-224 | 87~89 | ~205 | × | — | — | — | — | — | — | — |
| No. | Hydrogels | Mean Size (µm) | ±SD | Max | Mini | Pore Number | ±SD | Porosity (%) | ±SD |
|---|---|---|---|---|---|---|---|---|---|
| a | PVAEM | 0.40 | 0.30 | 1.40 | 0.10 | 664 | 3.20 | 13.22 | 5.51 |
| b | PVAFT | 3.43 | 1.05 | 4.29 | 0.54 | 180 | 5.03 | 80.45 | 10.94 |
| c | PVAHM/25 °C | 1.05 | 0.27 | 1.50 | 0.25 | 1263 | 1.72 | 56.96 | 0.71 |
| d | PVAHM/70 °C | 0.27 | 0.08 | 0.37 | 0.02 | 1484 | 1.03 | 46.54 | 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, F.-Y.; Zhang, Y.-Q. Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties. Gels 2022, 8, 803. https://doi.org/10.3390/gels8120803
Jing F-Y, Zhang Y-Q. Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties. Gels. 2022; 8(12):803. https://doi.org/10.3390/gels8120803
Chicago/Turabian StyleJing, Feng-Ya, and Yu-Qing Zhang. 2022. "Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties" Gels 8, no. 12: 803. https://doi.org/10.3390/gels8120803