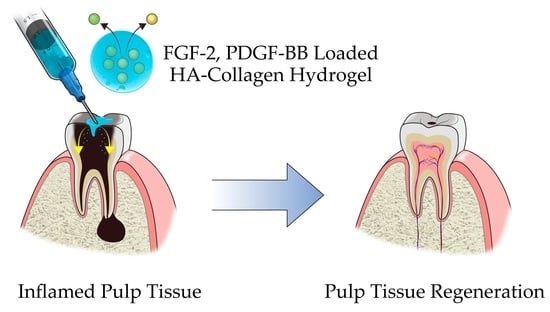
Development of Growth Factor Releasing Hyaluronic Acid-Based Hydrogel for Pulp Regeneration: A Preliminary Study
Abstract
:1. Introduction
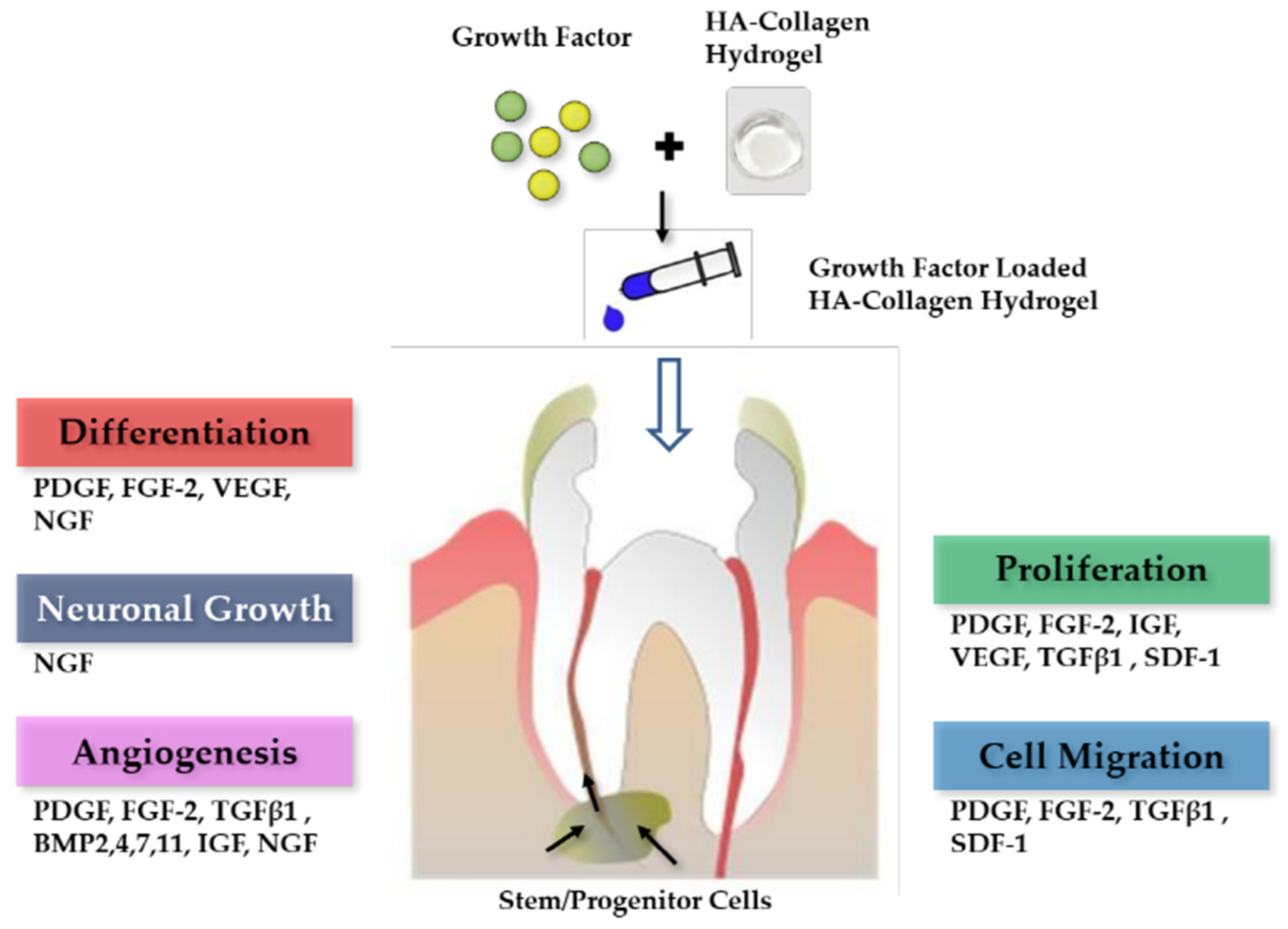
2. Results and Discussion
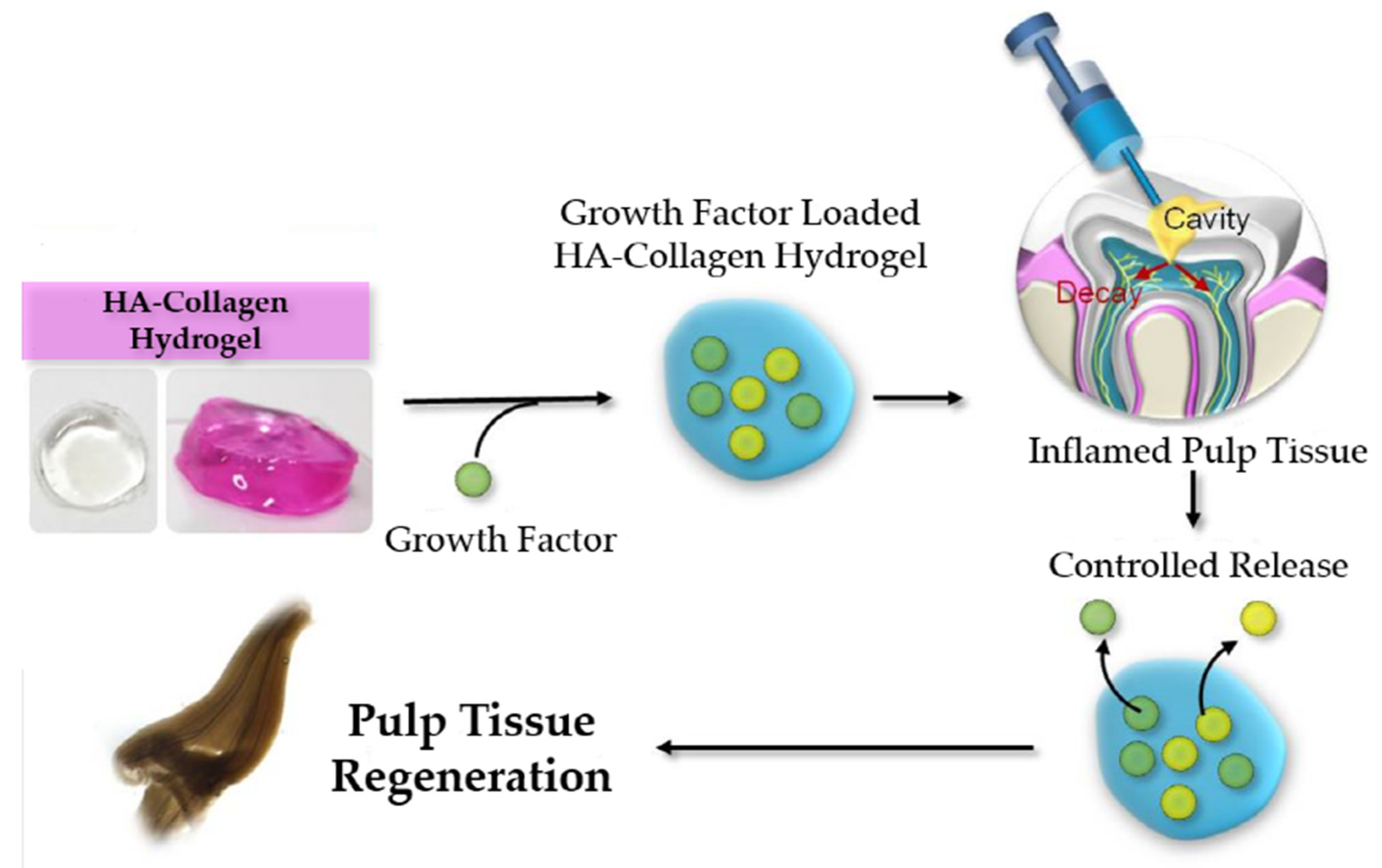
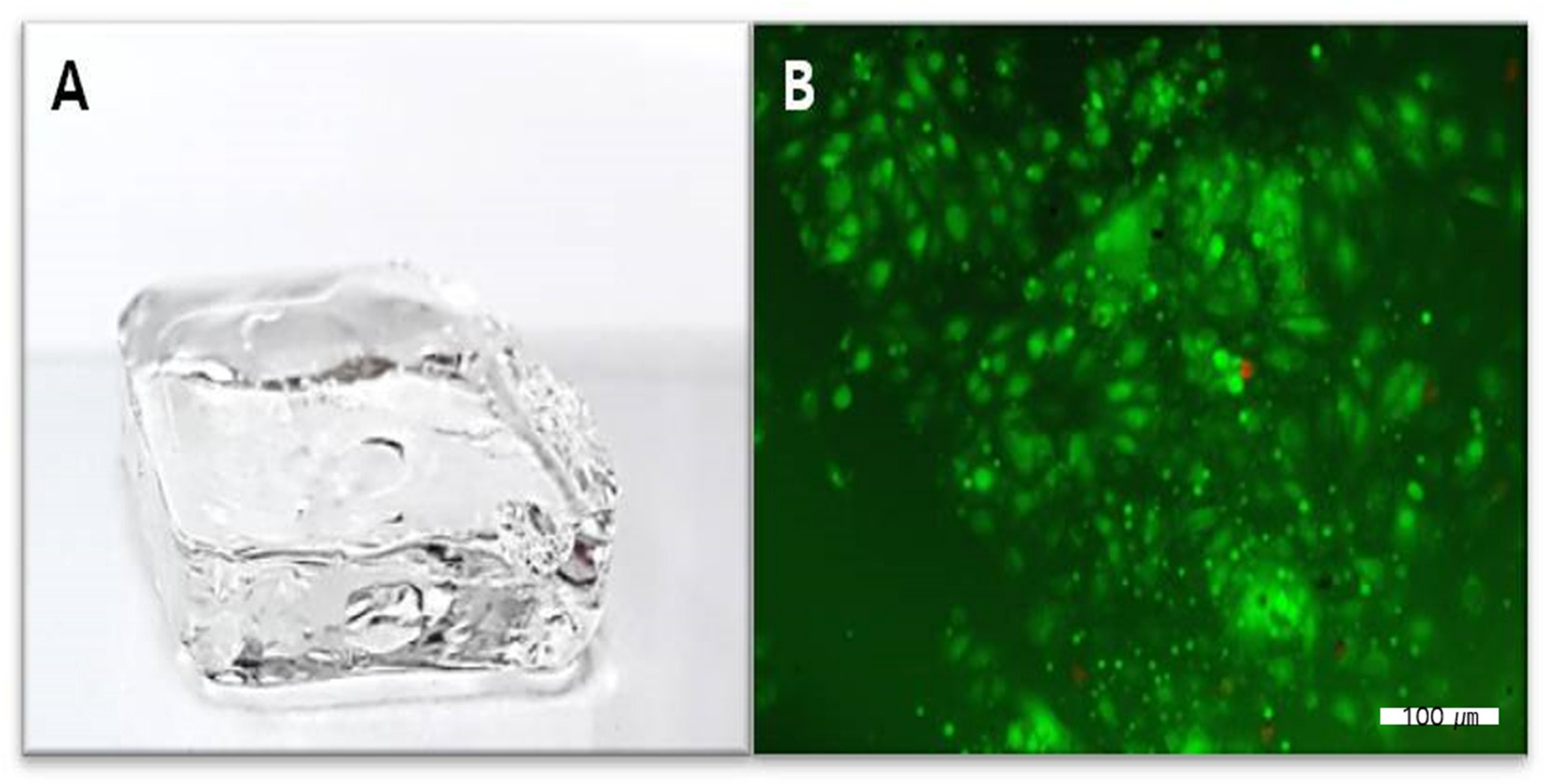
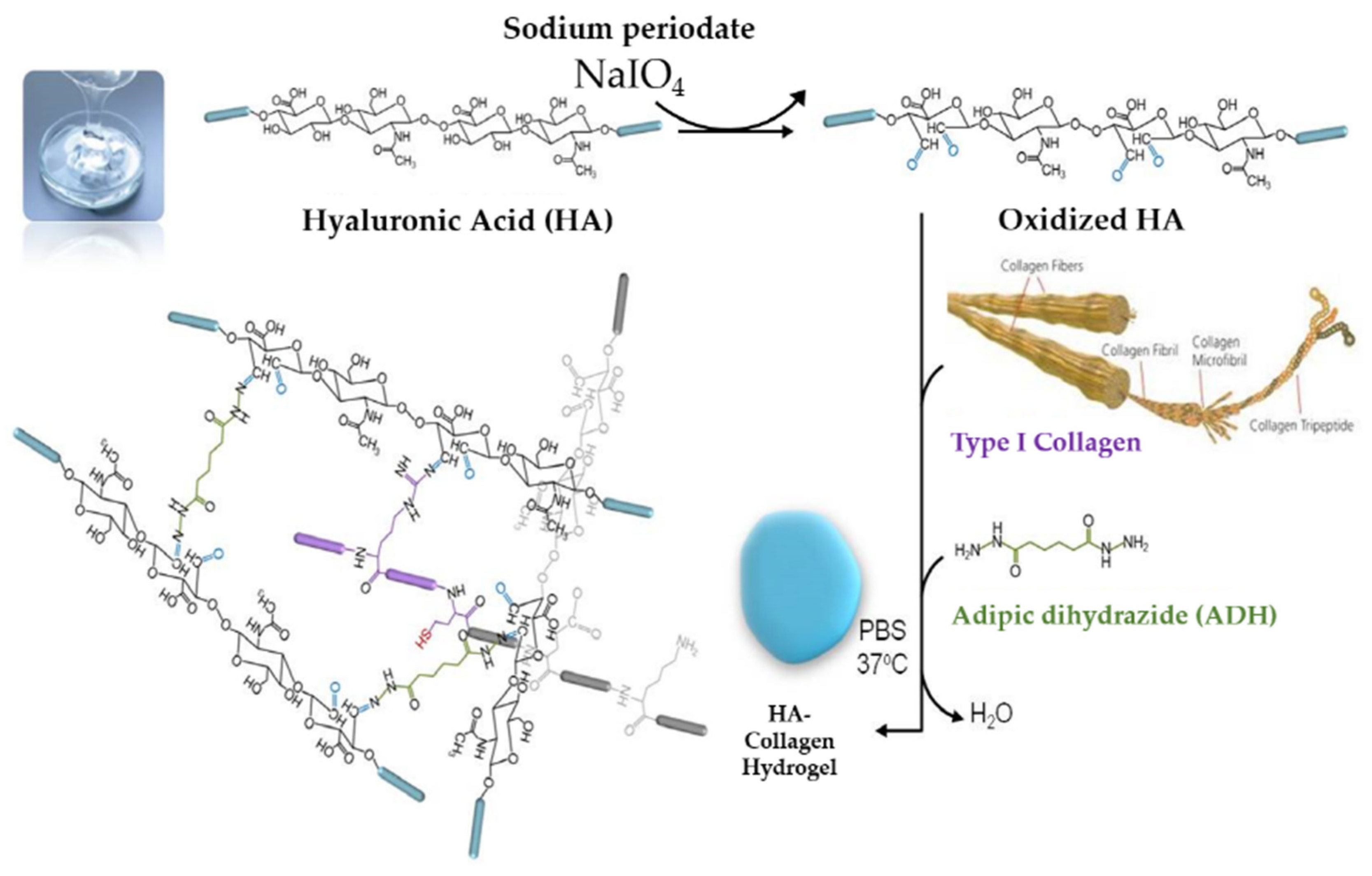
2.1. Characterization of the HA + Collagen Hybrid Hydrogel
2.2. Release Kinetics
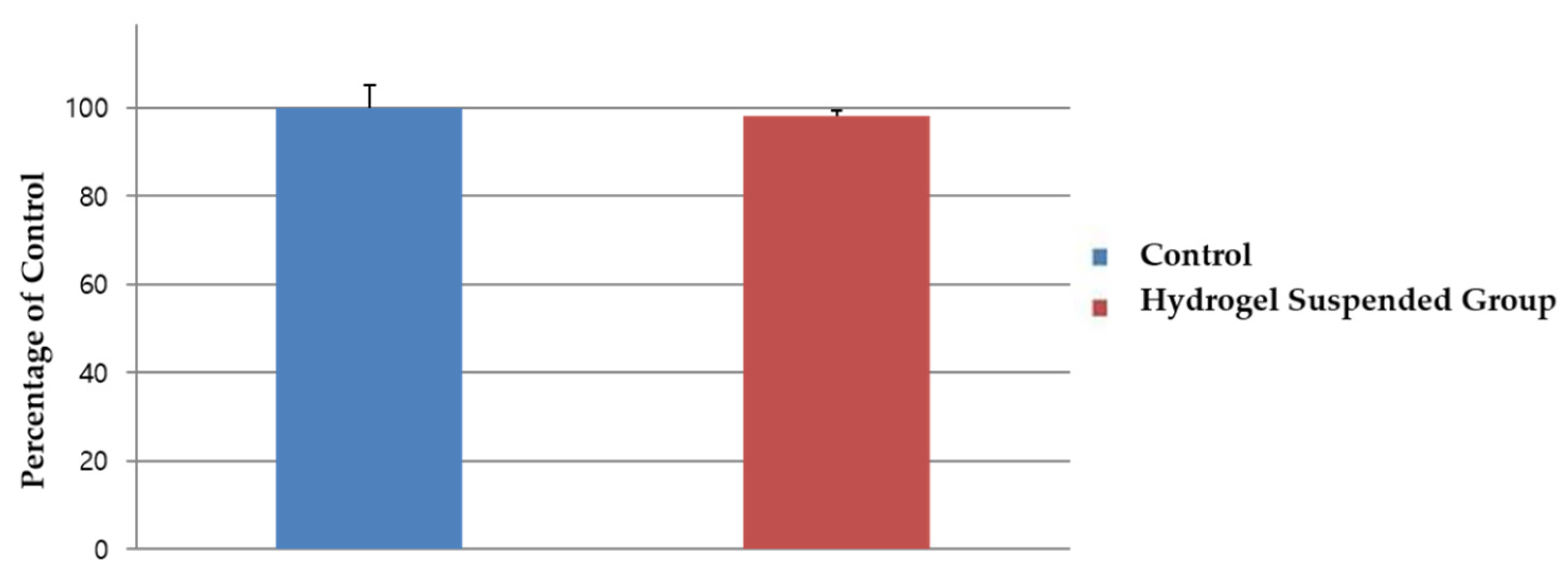
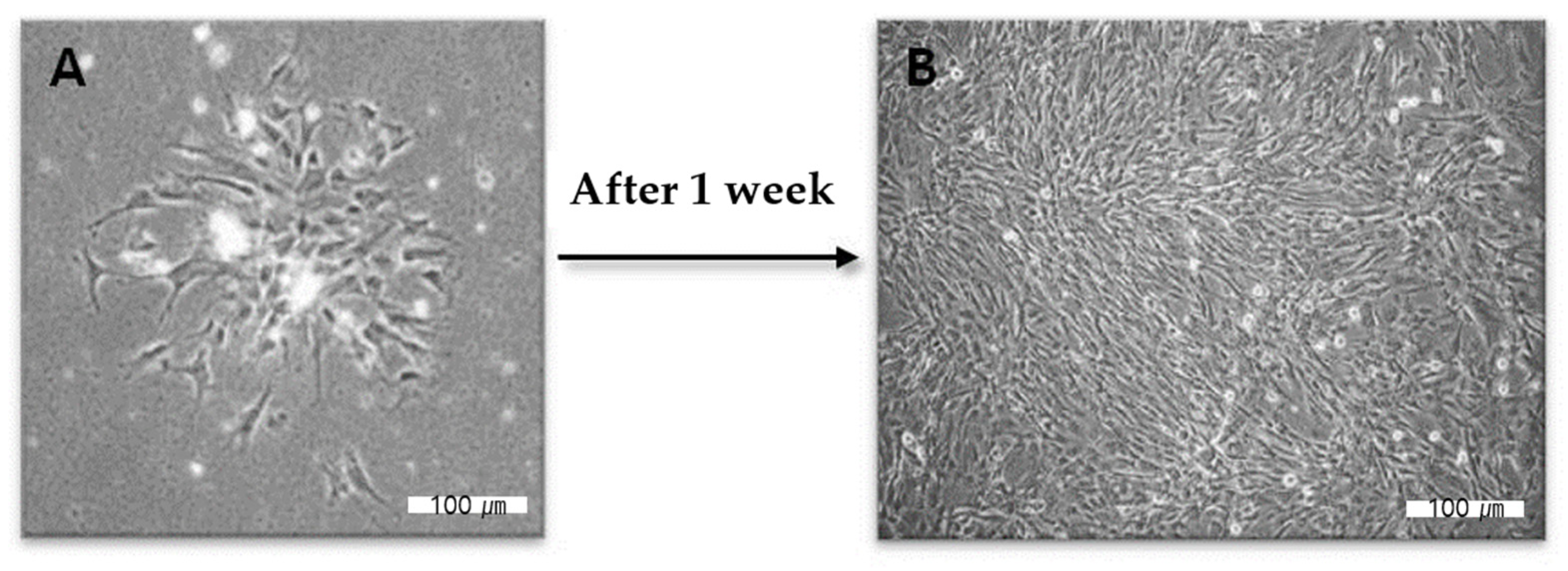
2.3. Cellular Interaction and Cytotoxicity Test
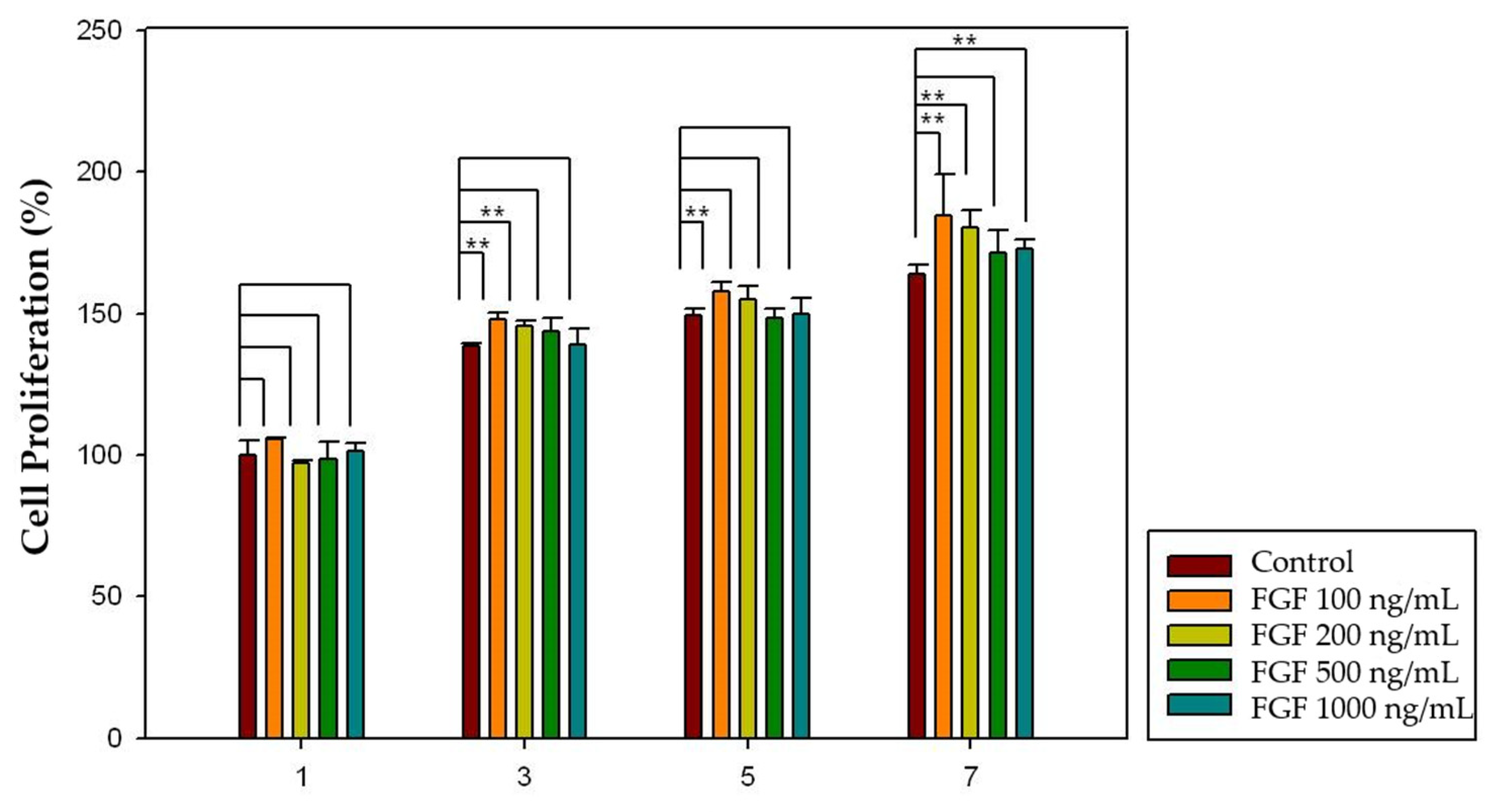
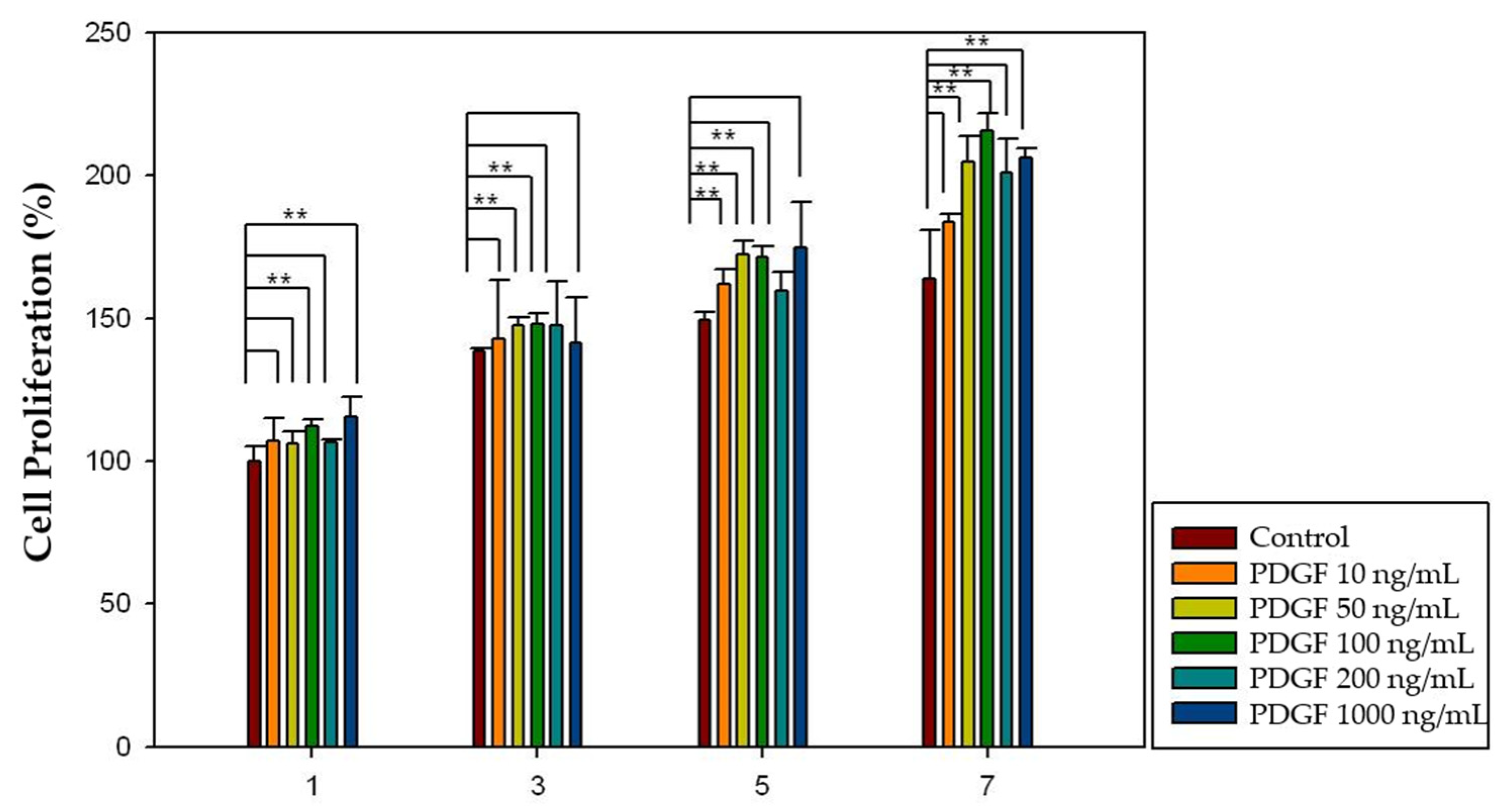
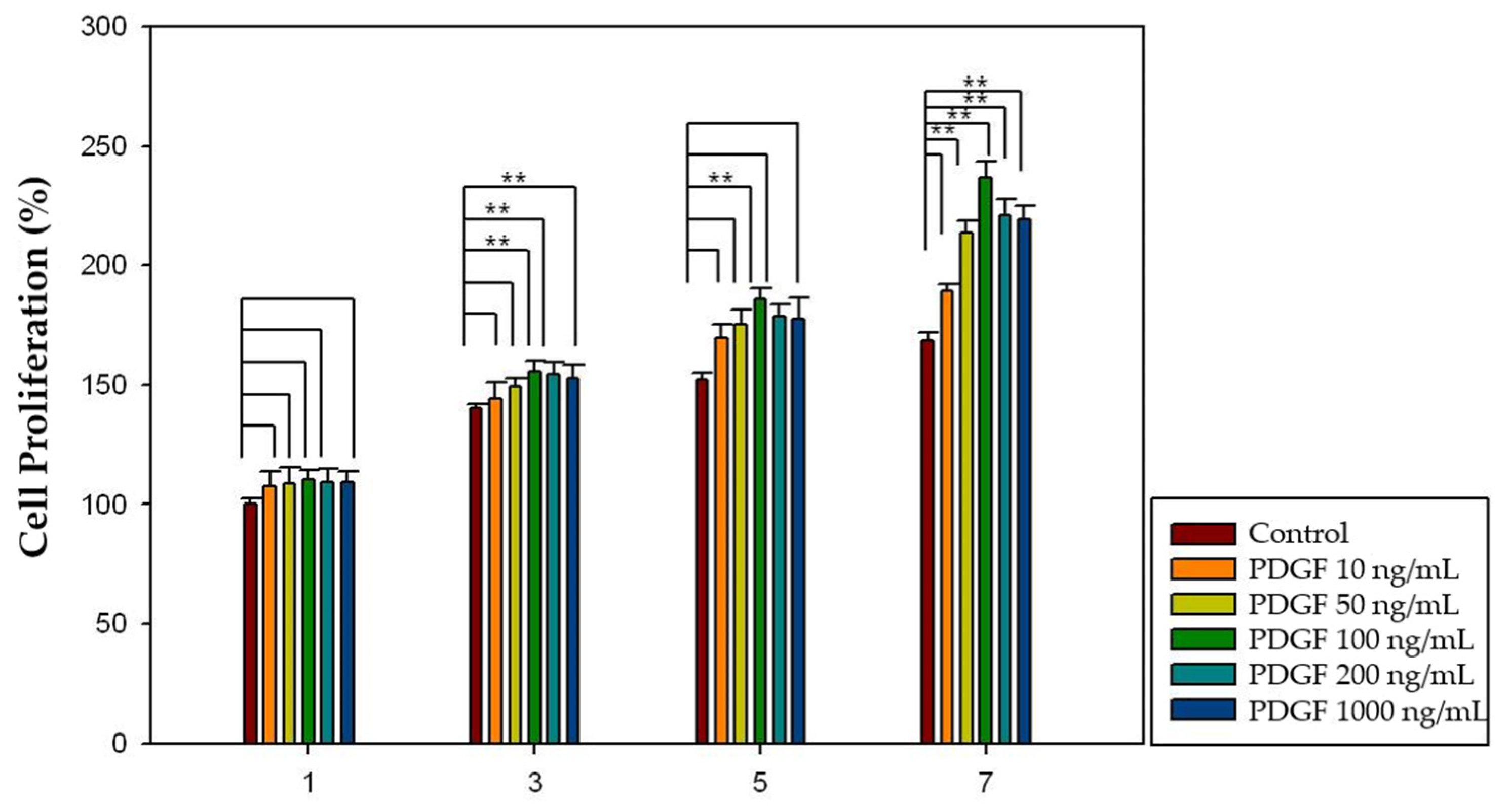
2.4. Effect of Growth Factors on Cell Proliferation
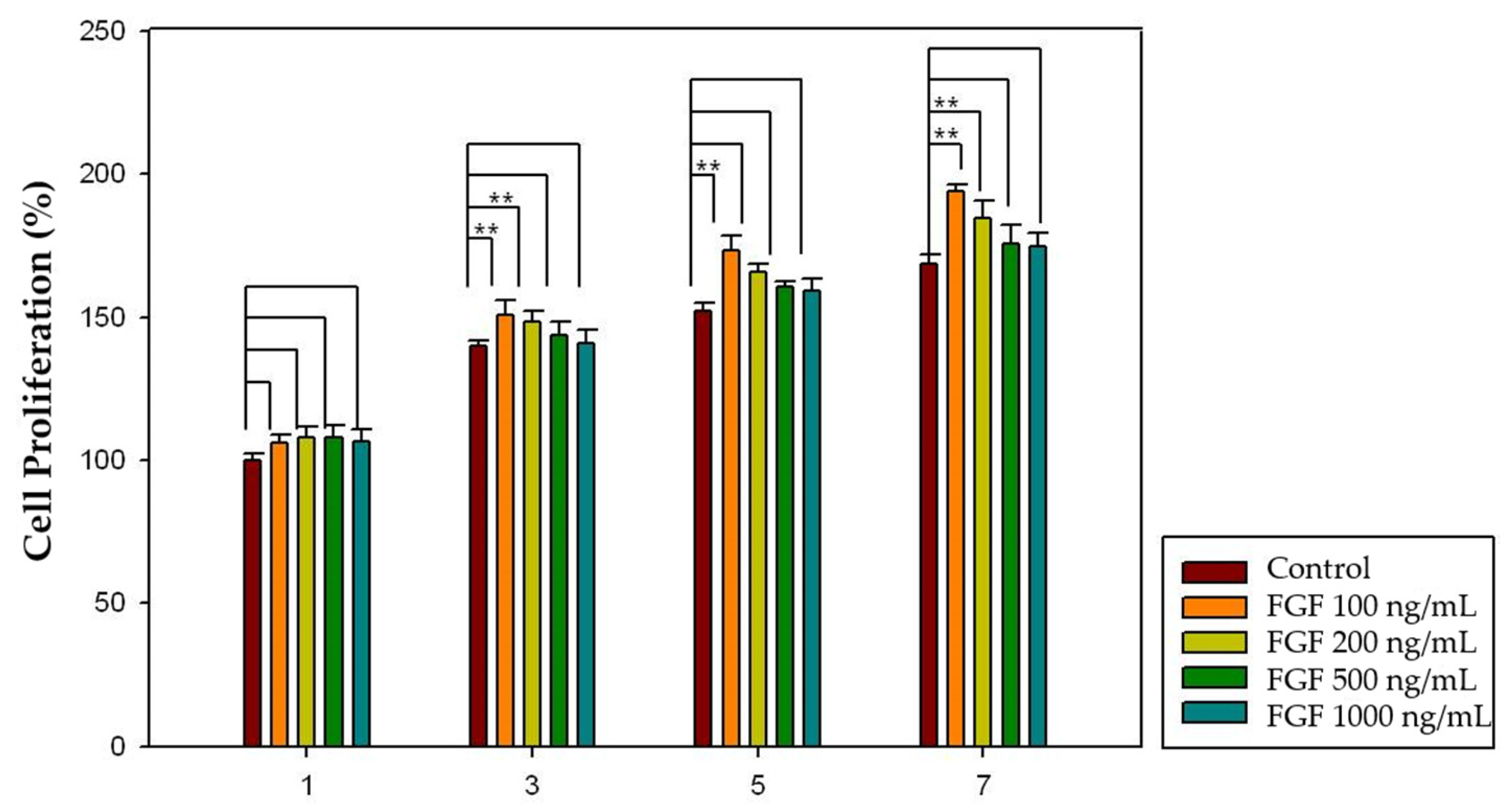
2.5. Effect of Growth Factors Released from HA-Collagen Hydrogel on Cell Proliferation
2.6. Discussion
3. Conclusions
4. Materials and Methods
4.1. Human Pulp Cells Preparation
4.2. HA + Collagen Hybrid-Hydrogel Preparation and Characterization
4.3. Release Kinetics Study
4.4. Cellular Interaction and Cytotoxicity Test
4.5. Effect of Growth Factors on Cell Proliferation
4.6. Effect of Growth Factors Released from HA-Collagen Hydrogel on Cell Proliferation
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of growth factors on dental stem/progenitor cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, Y. Tissue regeneration based on tissue engineering technology. Congenit. Anom. 2004, 44, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lind, M. Growth factors: Possible new clinical tools: A review. Acta. Orthop. Scand. 1996, 67, 407–417. [Google Scholar] [CrossRef]
- Nugent, M.A.; Iozzo, R.V. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 2000, 32, 115–120. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Ichikawa, T.; Takayama, S.; Yamada, S.; Takedachi, M.; Terakura, M.; Hashikawa, T.; Murakami, S. Fibroblast growth factor-2 regulates the synthesis of hyaluronan by human periodontal ligament cells. J. Cell. Physiol. 2005, 203, 557–563. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor–2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef]
- Rutherford, R.B.; TrailSmith, M.D.; Ryan, M.E.; Charette, M.F. Synergistic effects of dexamethasone on platelet-derived growth factor mitogenesis in vitro. Arch. Oral. Biol. 1992, 37, 139–145. [Google Scholar] [CrossRef]
- Nakashima, M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005, 16, 369–376. [Google Scholar] [CrossRef]
- Denholm, I.A.; Moule, A.J.; Bartold, P.M. The behaviour and proliferation of human dental pulp cell strains in vitro, and their response to the application of platelet-derived growth factor-BB and insulin-like growth factor-1. Int. Endod. J. 1998, 31, 251–258. [Google Scholar] [CrossRef]
- Yokose, S.; Kadokura, H.; Tajima, N.; Hasegawa, A.; Sakagami, H.; Fujieda, K.; Katayama, T. Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res. 2004, 315, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yu, J.; Liu, Y.; Lu, S.; Liu, H.; Shi, J.; Jin, Y. Effects of FGF2 and TGF-β1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol. Int. 2008, 32, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Washio, A. Local regeneration of dentin-pulp complex using controlled release of FGF-2 and naturally derived sponge-like scaffolds. Int. J. Dent. 2012, 2012, 190561. [Google Scholar] [CrossRef] [PubMed]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Steinbrech, D.S.; Mehrara, B.J.; Longaker, M.T. Factors in the fracture microenvironment induce primary osteoblast angiogenic cytokine production. Plast. Reconstr. Surg. 2002, 110, 139–148. [Google Scholar] [CrossRef]
- Ishimatsu, H.; Kitamura, C.; Morotomi, T.; Tabata, Y.; Nishihara, T.; Chen, K.K.; Terashita, M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor–2 from gelatin hydrogels. J. Endod. 2009, 35, 858–865. [Google Scholar] [CrossRef]
- Kikuchi, N.; Kitamura, C.; Morotomi, T.; Inuyama, Y.; Ishimatsu, H.; Tabata, Y.; Nishihara, T.; Terashita, M. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J. Endod. 2007, 33, 1198–1202. [Google Scholar] [CrossRef]
- Ross, R.; Raines, E.W.; Bowen-Pope, D.F. The biology of platelet-derived growth factor. Cell 1986, 46, 155–169. [Google Scholar] [CrossRef]
- Westermark, B.; Claesson-Welsh, L.; Heldin, C.H. Structural and functional aspects of the receptors for platelet-derived growth factor. Prog. Growth Factor Res. 1989, 1, 253–266. [Google Scholar] [CrossRef]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Seppä, H.E.; Kleinman, H.K.; Martin, G.R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 1981, 78, 3669–3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppä, H.; Grotendorst, G.; Seppä, S.; Schiffmann, E.; Martin, G.R. Platelet-derived growth factor in chemotactic for fibroblasts. J. Cell Biol. 1982, 92, 584–588. [Google Scholar] [CrossRef]
- Deuel, T.F.; Senior, R.M.; Huang, J.S.; Griffin, G.L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J. Clin. Investig. 1982, 69, 1046–1049. [Google Scholar] [CrossRef] [Green Version]
- Mark Saltzman, W.; Baldwin, S.P. Materials for protein delivery in tissue engineering. Adv. Drug Deliv. Rev. 1998, 33, 71–86. [Google Scholar] [CrossRef]
- Jen, A.C.; Wake, M.C.; Mikos, A.G. Review: Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996, 50, 357–364. [Google Scholar] [CrossRef]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 30, S51–S56. [Google Scholar] [CrossRef]
- Sloan, A.J.; Smith, A.J. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral. Dis. 2007, 13, 151–157. [Google Scholar] [CrossRef]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef]
- Tziafas, D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004, 38, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Ochi, M.; Uchio, Y.; Adachi, N.; Matsusaki, M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J. Cell. Physiol. 1999, 179, 142–148. [Google Scholar] [CrossRef]
- Lu, L.; Yaszemski, M.J.; Mikos, A.G. TGF-β1 release from biodegradable polymer microparticles: Its effects on marrow stromal osteoblast function. J. Bone Jt. Surg. 2001, 83 (Suppl. 1), S82–S91. [Google Scholar] [CrossRef]





| Growth Factors | Target Cells | Primary Effects |
|---|---|---|
| PDGF | dental pulp cells | cell proliferation [14,16,17] |
| dentin matrix synthesis [14,16,17] | ||
| odontogenic differentiation | ||
| dentinogenesis [18] | ||
| FGF-2 | dental pulp stem cells | chemotaxis [19] |
| cell proliferation [20] | ||
| dental pulp cells | cell proliferation [21,22,23] | |
| dentinogenesis [21,22,23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Hwang, Y.-S.; Lee, H.-S.; Nam, O.H.; Choi, S.C. Development of Growth Factor Releasing Hyaluronic Acid-Based Hydrogel for Pulp Regeneration: A Preliminary Study. Gels 2022, 8, 825. https://doi.org/10.3390/gels8120825
Kim MS, Hwang Y-S, Lee H-S, Nam OH, Choi SC. Development of Growth Factor Releasing Hyaluronic Acid-Based Hydrogel for Pulp Regeneration: A Preliminary Study. Gels. 2022; 8(12):825. https://doi.org/10.3390/gels8120825
Chicago/Turabian StyleKim, Mi Sun, Yu-Shik Hwang, Hyo-Seol Lee, Ok Hyung Nam, and Sung Chul Choi. 2022. "Development of Growth Factor Releasing Hyaluronic Acid-Based Hydrogel for Pulp Regeneration: A Preliminary Study" Gels 8, no. 12: 825. https://doi.org/10.3390/gels8120825