Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions
Abstract
:1. Introduction
2. Results and Discussion
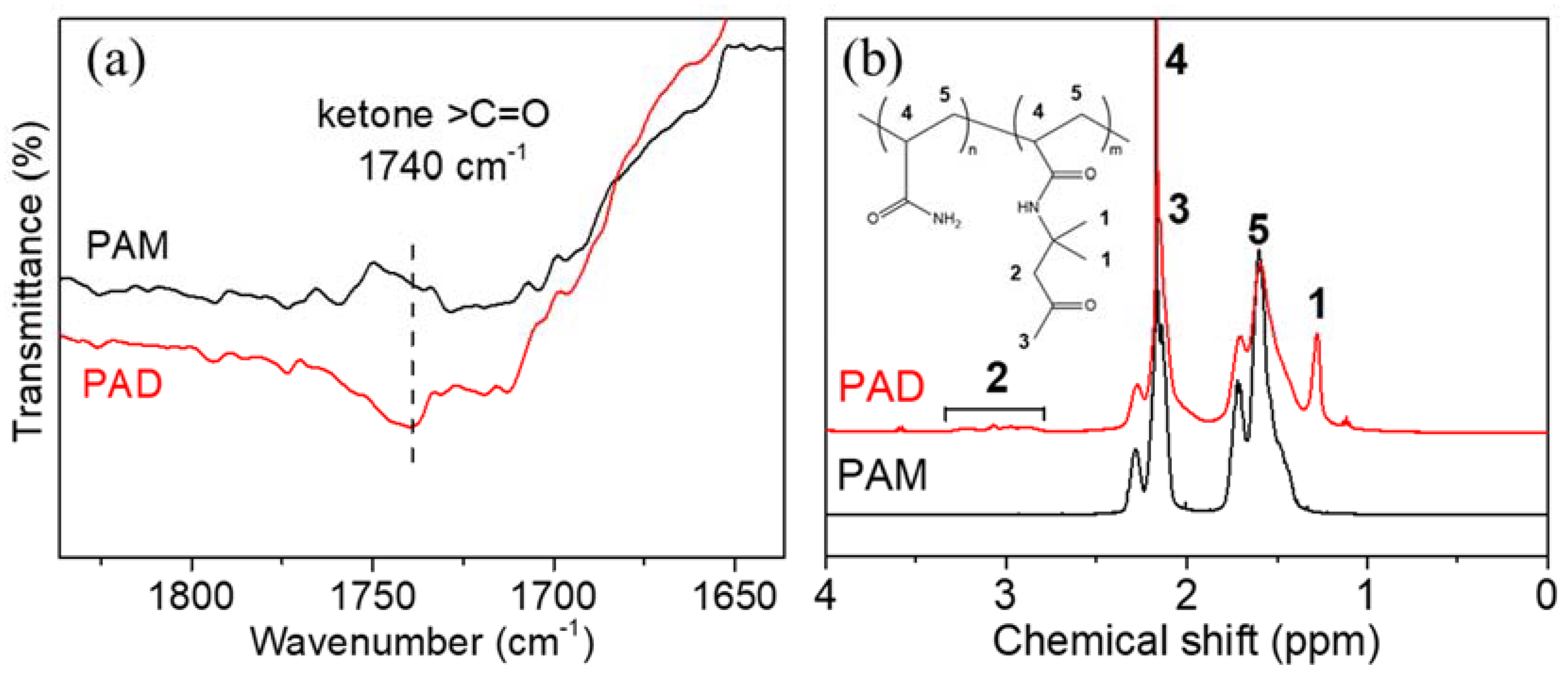
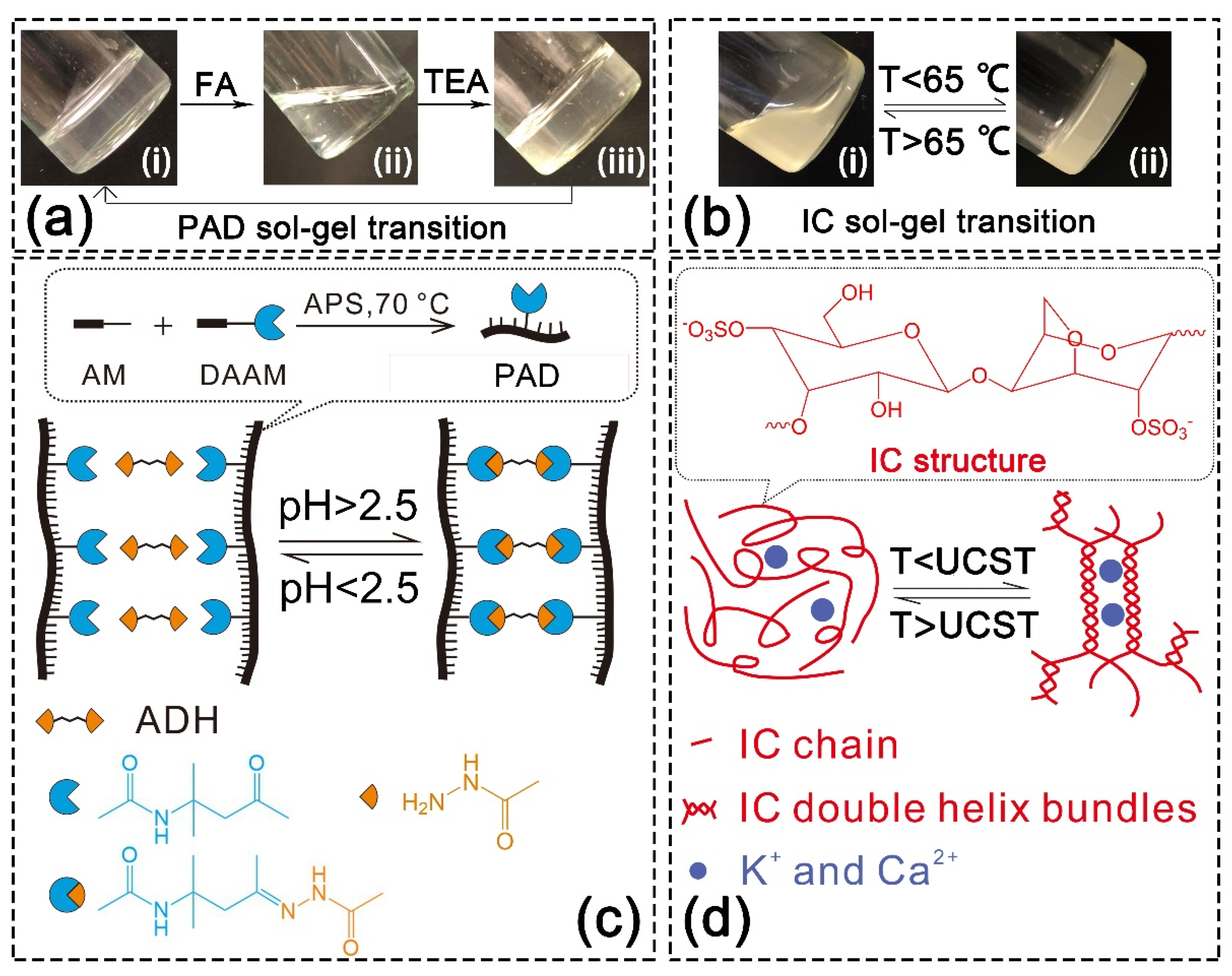
2.1. SN Hydrogels from Dynamic Covalent and Supramolecular Networks
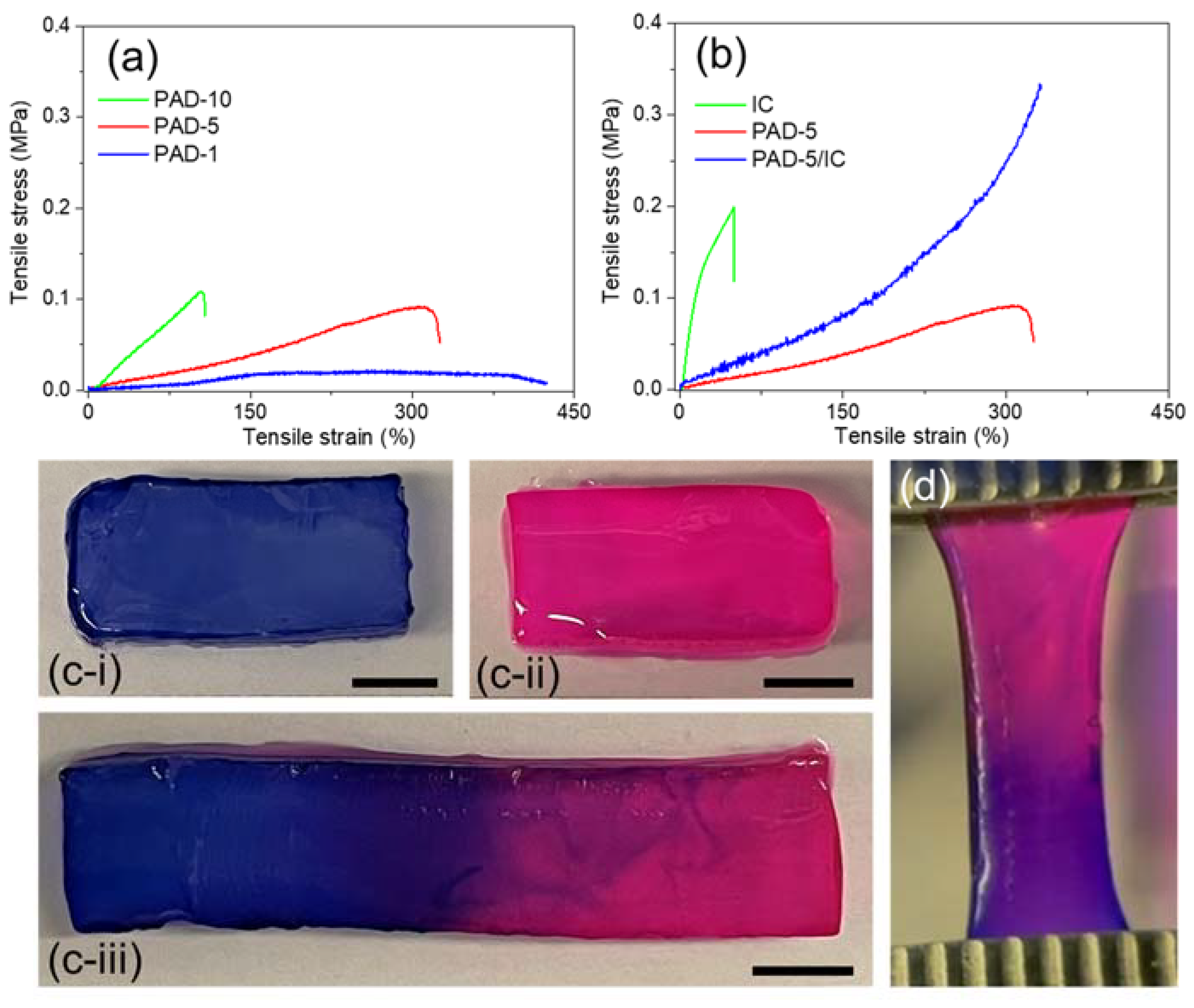
2.2. Tensile Properties and Self-Healing Performances of DN Hydrogels
2.3. Spinning, Tensile Properties, Self-Healing Efficiencies and Shape Memory of DN Hydrogel Fibers
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PAM and PAD
4.3. Preparation of PAD, IC and PAD/IC Hydrogels
4.4. Spinning of PAD/IC Hydrogel Fibers
4.5. Fabrication and Shape Recovery Study of PAD/IC SMFs
4.6. Characterizations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.R.; Wang, J.F.; Chen, Z.; Fang, S.L.; Zhu, Y.; Baughman, R.H.; Jiang, L. Tunable, Fast, Robust Hydrogel Actuators Based on Evaporation-Programmed Heterogeneous Structures. Chem. Mater. 2017, 29, 9793–9801. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, H.W.; Wang, W.Y.; Lee, K.I.; Gao, C.; He, L.; Wang, Y.F.; Lai, C.L.; Fei, B.; Xin, J.H. Antibacterial modification of an injectable, biodegradable, non-cytotoxic block copolymer-based physical gel with body temperature-stimulated sol-gel transition and controlled drug release. Colloids Surf. B Biointerfaces 2016, 143, 342–351. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Liu, H.; Wang, Z.; Ling, H.; Wang, C.; Ni, J.; Çelebi-Saltik, B.; Wang, X.; Meng, X.; et al. Room-Temperature-Formed PEDOT:PSS Hydrogels Enable Injectable, Soft, and Healable Organic Bioelectronics. Adv. Mater. 2020, 32, 1904752. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.-H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Jiang, N.; Fallahi, A.; Montelongo, Y.; Ruiz-Esparza, G.U.; Tamayol, A.; Zhang, Y.S.; Mahmood, I.; Yang, S.A.; Kim, K.S.; et al. Glucose-Sensitive Hydrogel Optical Fibers Functionalized with Phenylboronic Acid. Adv. Mater. 2017, 29, 1606380. [Google Scholar] [CrossRef]
- Hua, J.C.; Li, Z.; Xia, W.; Yang, N.; Gong, J.X.; Zhang, J.F.; Qiao, C.S. Preparation and properties of EDC/NHS mediated crosslinking poly (gamma-glutamic acid)/epsilon-polylysine hydrogels. Mater. Sci. Eng. C 2016, 61, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, L.; Li, Y.L.; Lee, K.I.; Fei, B. Super-tough hydrogels from shape-memory polyurethane with wide-adjustable mechanical properties. J. Mater. Sci. 2017, 52, 4421–4434. [Google Scholar] [CrossRef]
- Lu, X.K.; Chan, C.Y.; Lee, K.I.; Ng, P.F.; Fei, B.; Xin, J.H.; Fu, J. Super-tough and thermo-healable hydrogel—Promising for shape-memory absorbent fiber. J. Mater. Chem. B 2014, 2, 7631–7638. [Google Scholar] [CrossRef]
- Hua, J.; Liu, C.; Ng, P.F.; Fei, B. Bacterial cellulose reinforced double-network hydrogels for shape memory strand. Carbohydr. Polym. 2021, 259, 117737. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, H.W.; Yang, Z.Y.; He, L.; Kong, Y.Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough hydrogels with rapid self-reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, X.; Ouchi, T.; Kouznetsova, T.B.; Beech, H.K.; Av-Ron, S.; Matsuda, T.; Bowser, B.H.; Wang, S.; Johnson, J.A.; et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 2021, 374, 193–196. [Google Scholar] [CrossRef]
- Hua, J.C.; Fei, B. Super-Tough Polyacrylamide/iota-Carrageenan Double-Network Hydrogels Strengthened By Bacterial Cellulose Microclusters. Mater. Today Proc. 2019, 16, 1497–1501. [Google Scholar] [CrossRef]
- Bilici, C.; Ide, S.; Okay, O. Yielding Behavior of Tough Semicrystalline Hydrogels. Macromolecules 2017, 50, 3647–3654. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, X.H.; Dong, C.M. Dual Stimuli-Responsive Supramolecular Polypeptide-Based Hydrogel and Reverse Micellar Hydrogel Mediated by Host-Guest Chemistry. Adv. Funct. Mater. 2010, 20, 579–586. [Google Scholar] [CrossRef]
- Wang, J.F.; Lin, L.; Cheng, Q.F.; Jiang, L. A Strong Bio-Inspired Layered PNIPAM-Clay Nanocomposite Hydrogel. Angew. Chem. Int. Ed. 2012, 51, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Hai, Z.B.; Cui, C.H.; Li, H.H.; Chen, J.F.; Yu, S.H. In Situ Controlled Synthesis of Thermosensitive Poly(N-isopropylacrylamide)/Au Nanocomposite Hydrogels by Gamma Radiation for Catalytic Application. Small 2012, 8, 930–936. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Xavier, J.R.; Carrow, J.K.; Desai, P.; Alge, D.; Gaharwar, A.K. Mechanically Stiff Nanocomposite Hydrogels at Ultralow Nanoparticle Content. ACS Nano 2016, 10, 246–256. [Google Scholar] [CrossRef]
- Huang, T.; Xu, H.G.; Jiao, K.X.; Zhu, L.P.; Brown, H.R.; Wang, H.L. A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater. 2007, 19, 1622–1626. [Google Scholar] [CrossRef]
- Zhao, J.; Jiao, K.; Yang, J.; He, C.; Wang, H. Mechanically strong and thermosensitive macromolecular microsphere composite poly(N-isopropylacrylamide) hydrogels. Polymer 2013, 54, 1596–1602. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, D.; Chen, H.; Zhu, L.; Jiao, C.; Liu, G.; Huang, L.; Yang, J.; Wang, L.; Zheng, J. Simultaneous Enhancement of Stiffness and Toughness in Hybrid Double-Network Hydrogels via the First, Physically Linked Network. Macromolecules 2015, 48, 8003–8010. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Q.; Hu, R.D.; Wang, H.; Newby, B.M.Z.; Chang, Y.; Zheng, J. Mechanically strong hybrid double network hydrogels with antifouling properties. J. Mater. Chem. B 2015, 3, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wei, K.C.; Zhang, K.Y.; Yang, B.G.; Tian, F.; Wang, G.X.; Bian, L.M. One-pot solvent exchange preparation of non-swellable, thermoplastic, stretchable and adhesive supramolecular hydrogels based on dual synergistic physical crosslinking. NPG Asia Mater. 2018, 10, e455. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.H.; Yao, C.; Kou, X.H.; Tang, J.P.; Luo, D.; Yang, D.Y. A Fluorescent Biofunctional DNA Hydrogel Prepared by Enzymatic Polymerization. Adv. Healthc. Mater. 2018, 7, 7. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, J.; Liu, Z.; Sun, Z.; Wu, Y.; Liu, L.; Yan, F. Ionic liquid-based click-ionogels. Sci. Adv. 2019, 5, eaax0648. [Google Scholar] [CrossRef] [Green Version]
- He, Q.Y.; Huang, Y.; Wang, S.Y. Hofmeister Effect-Assisted One Step Fabrication of Ductile and Strong Gelatin Hydrogels. Adv. Funct. Mater. 2018, 28, 1705069. [Google Scholar] [CrossRef]
- Taylor, D.L.; Panhuis, M.I.H. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Mredha, M.T.I.; Na, J.Y.; Seon, J.-K.; Cui, J.; Jeon, I. Multifunctional poly (disulfide) hydrogels with extremely fast self-healing ability and degradability. Chem. Eng. J. 2020, 394, 124941. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Wang, Q.; Xu, C.; Tang, Q.; Yang, H. An injectable, dual responsive, and self-healing hydrogel based on oxidized sodium alginate and hydrazide-modified poly (ethyleneglycol). Molecules 2018, 23, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathan, M.; Kovaříček, P.; Jurissek, C.; Senf, A.; Dallmann, A.; Thünemann, A.F.; Hecht, S. Control of imine exchange kinetics with photoswitches to modulate self-healing in polysiloxane networks by light illumination. Angew. Chem. Int. Ed. 2016, 55, 13882–13886. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Rapid diels-alder cross-linking of cell encapsulating hydrogels. Chem. Mater. 2019, 31, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Liao, W.; Yang, Z. Liquid Crystal Elastomer Twist Fibers towards Rotating Microengines. Adv. Mater. 2022, 34, 2107840. [Google Scholar] [CrossRef]
- Wei, P.; Hou, K.; Chen, T.; Chen, G.; Mugaanire, I.T.; Zhu, M. Reactive spinning to achieve nanocomposite gel fibers: From monomer to fiber dynamically with enhanced anisotropy. Mater. Horiz. 2020, 7, 811–819. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Sun, J.; He, H.; Li, B.; Ma, C.; Liu, K.; Zhang, H. Bioinspired and Mechanically Strong Fibers Based on Engineered Non-Spider Chimeric Proteins. Angew. Chem. 2020, 132, 8225–8229. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Ahmed, R.; Mataji-Kojouri, A.; Soto, F.; Wang, J.; Liu, S.; Stoyanova, T.; Marques, A.P.; Reis, R.L.; Demirci, U. Engineering Polysaccharide-Based Hydrogel Photonic Constructs: From Multiscale Detection to the Biofabrication of Living Optical Fibers. Adv. Mater. 2021, 33, 2105361. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, J.; Liu, J.; Wang, Y.; Guo, J.; Zhong, Z.; Liu, X. High mechanical performance based on the alignment of cellulose nanocrystal/chitosan composite filaments through continuous coaxial wet spinning. Cellulose 2021, 28, 7995–8008. [Google Scholar] [CrossRef]
- Bao, X.; Hayashi, K.; Li, Y.; Teramoto, A.; Abe, K. Novel agarose and agar fibers: Fabrication and characterization. Mater. Lett. 2010, 64, 2435–2437. [Google Scholar] [CrossRef]
- Koeck, K.S.; Salehi, S.; Humenik, M.; Scheibel, T. Processing of Continuous Non-Crosslinked Collagen Fibers for Microtissue Formation at the Muscle-Tendon Interface. Adv. Funct. Mater. 2021, 2112238. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, A.; He, X.; Li, Q.; Sun, J.; Lei, Z.; Liu, Z.H. Full-Temperature All-Solid-State Ti3C2Tx/Aramid Fiber Supercapacitor with Optimal Balance of Capacitive Performance and Flexibility. Adv. Funct. Mater. 2021, 31, 2010944. [Google Scholar]
- Ng, P.F.; Hua, J.; Liu, C.; Wang, Y.; Yin, R.; Fei, B. Solar Energy Storage Silks via Coaxial Wet Spinning. ACS Mater. Lett. 2020, 2, 801–807. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Z.; Zhang, W.; Yan, M.; Xia, Y. Preparation and properties of wet-spun agar fibers. Carbohydr. Polym. 2018, 181, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Song, J.C.; Chen, S.; Sun, L.J.; Guo, Y.F.; Zhang, L.Z.; Wang, S.L.; Xuan, H.X.; Guan, Q.B.; You, Z.W. Mechanically and Electronically Robust Transparent Organohydrogel Fibers. Adv. Mater. 2020, 32, 1906994. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Maçon, A.L.; Iguchi, M.; Ozeki, Y.; Koeda, S.; Obata, A.; Kasuga, T.; Mizuno, T. Construction of enzyme-encapsulated fibermats from the cross-linkable copolymers poly(acrylamide)-co-poly(diacetone acrylamide) with the bi-functional cross-linker, adipic acid dihydrazide. Polymer 2017, 132, 342–352. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, W.; Gu, H.; Feng, Y.; He, Z.; Chen, Q.; Mao, X.; Zhang, J.; Zheng, L. pH-Switchable and self-healable hydrogels based on ketone type acylhydrazone dynamic covalent bonds. Soft Matter 2017, 13, 7371–7380. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly efficient self-healable and dual responsive cellulose-based hydrogels for controlled release and 3D cell culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Dirksen, A.; Dirksen, S.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of hydrazone formation and transimination: Implications for dynamic covalent chemistry. J. Am. Chem. Soc. 2006, 128, 15602–15603. [Google Scholar] [CrossRef]
- Liu, S.J.; Li, L. Recoverable and Self-Healing Double Network Hydrogel Based on kappa-Carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, J.; Wang, Y.; Shao, C.; Chi, J.; Li, Z.; Wang, X.; Zhao, Y. Photocontrolled healable structural color hydrogels. Small 2019, 15, 1903104. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Wang, B. Preparation of carrageenan fibers with extraction of Chondrus via wet spinning process. Carbohydr. Polym. 2018, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Mredha, M.T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A Facile Method to Fabricate Anisotropic Hydrogels with Perfectly Aligned Hierarchical Fibrous Structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.F.; Hua, J.C.; Jia, S.R.; Zhang, J.F.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Ming, X.; Yao, L.; Zhu, H.; Zhang, Q.; Zhu, S. Dramatic and Reversible Water-Induced Stiffening Driven by Phase Separation within Polymer Gels. Adv. Funct. Mater. 2022, 32, 2109850. [Google Scholar] [CrossRef]

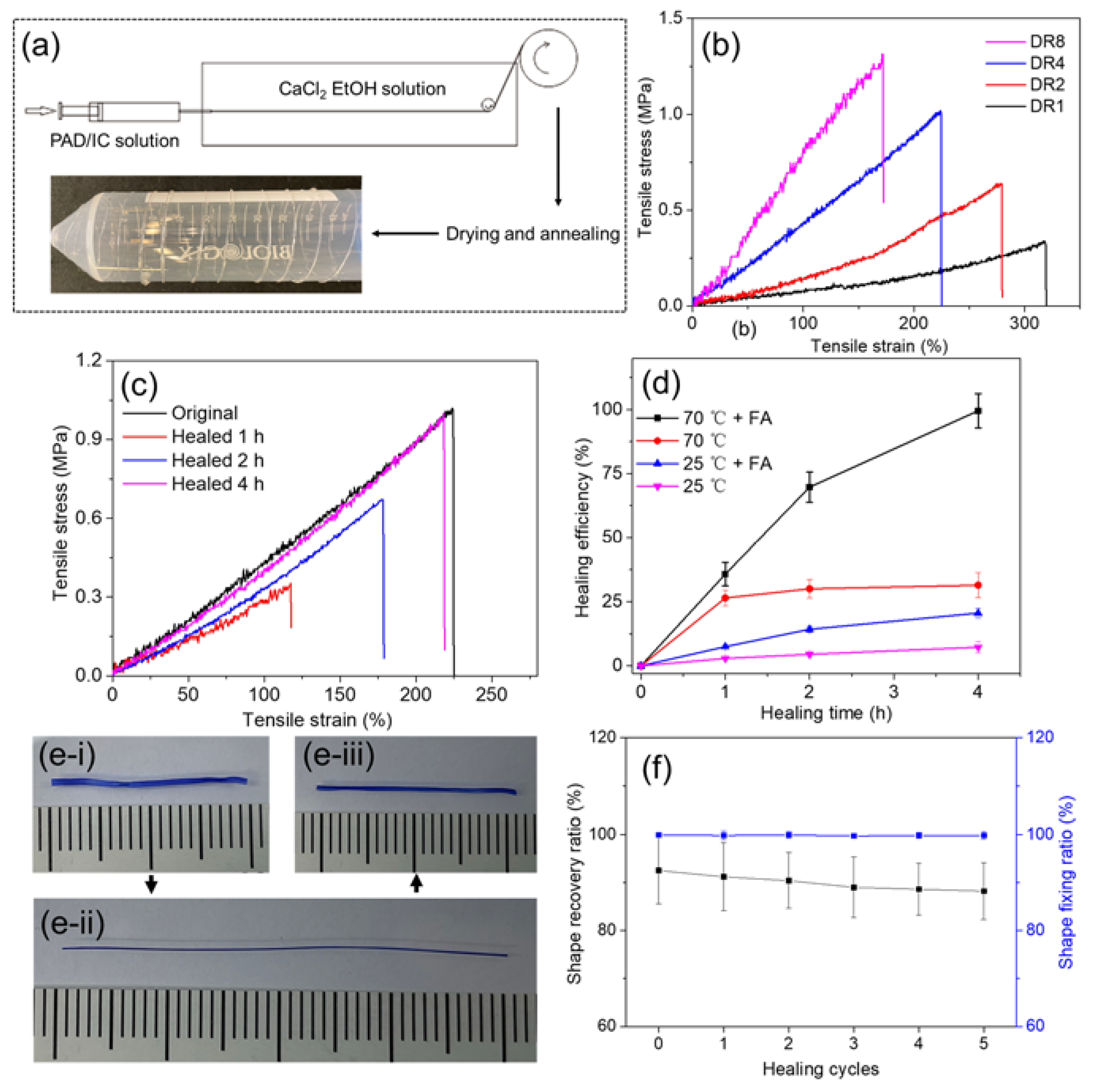
| PAD-5/IC Fibers | Tensile Properties | Swelling Capacity (%) | |||
|---|---|---|---|---|---|
| σ (MPa) | ε (%) | E (MPa) | Γ (MJ/m3) | ||
| DR1 | 0.32 (0.02) | 332.7 (23.99) | 0.11 (0.01) | 0.56 (0.08) | 1235 (11) |
| DR2 | 0.66 (0.09) | 281.6 (16.9) | 0.39 (0.02) | 0.82 (0.09) | 1121 (7) |
| DR4 | 1.07 (0.05) | 225.4 (15.8) | 0.58 (0.04) | 1.22 (0.10) | 1087 (10) |
| DR8 | 1.35 (0.12) | 168.4 (12.5) | 0.81 (0.06) | 1.09 (0.09) | 1030 (9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, J.; Liu, C.; Fei, B.; Liu, Z. Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions. Gels 2022, 8, 101. https://doi.org/10.3390/gels8020101
Hua J, Liu C, Fei B, Liu Z. Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions. Gels. 2022; 8(2):101. https://doi.org/10.3390/gels8020101
Chicago/Turabian StyleHua, Jiachuan, Chang Liu, Bin Fei, and Zunfeng Liu. 2022. "Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions" Gels 8, no. 2: 101. https://doi.org/10.3390/gels8020101





