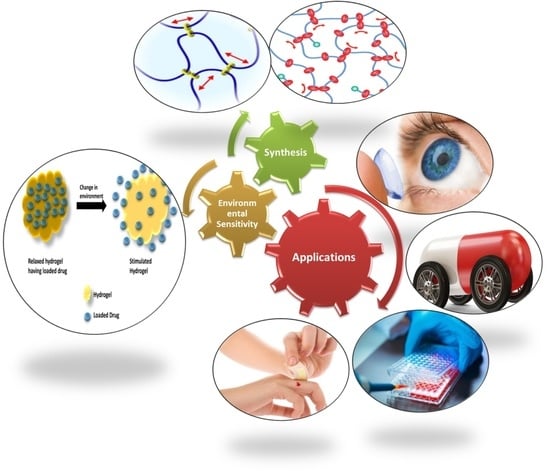
Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications
Abstract
:1. Introduction
1.1. Classification of Hydrogel
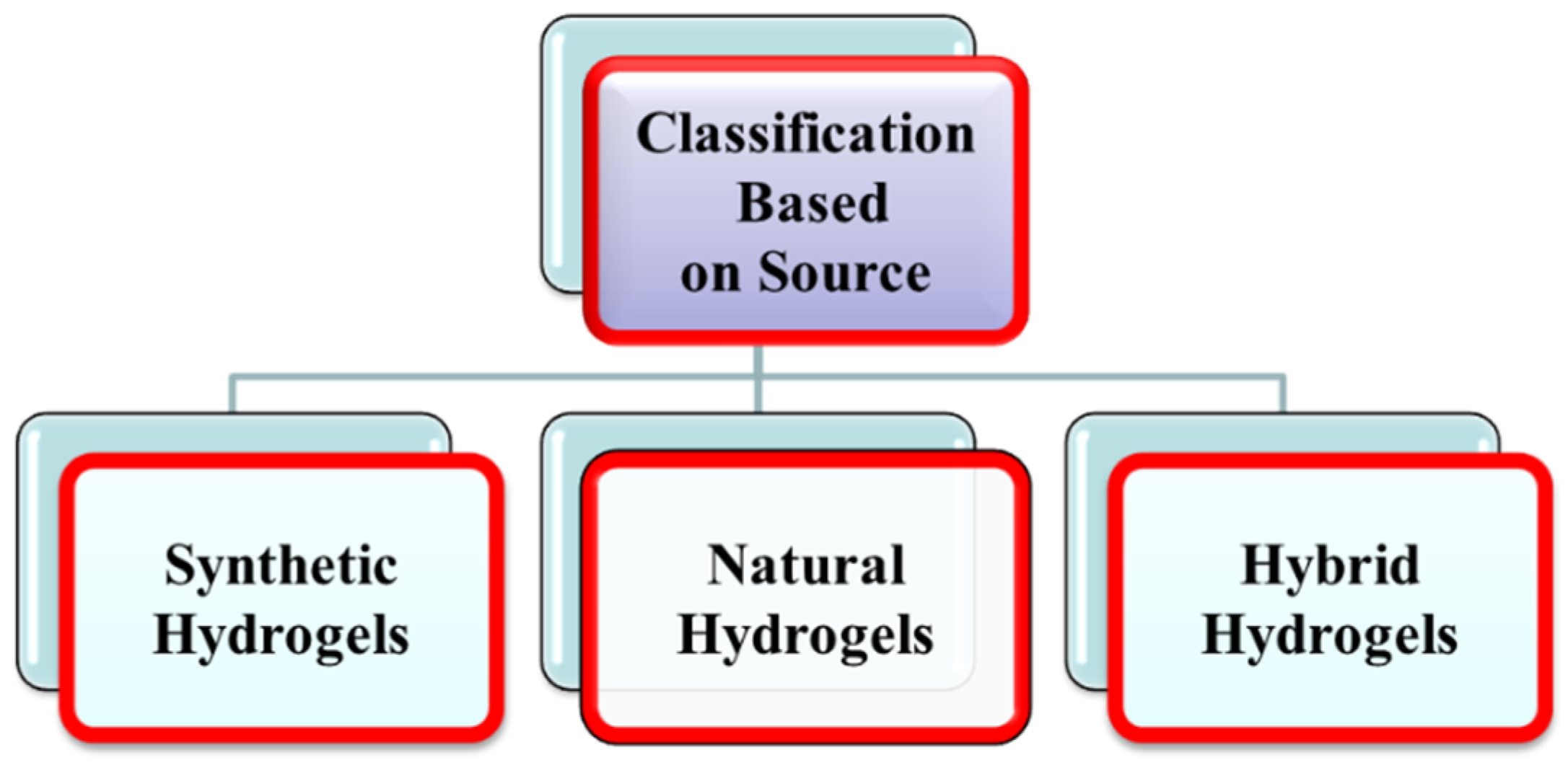
1.1.1. Classification of Hydrogels Based on Source
Synthetic Hydrogels
Natural Hydrogels
Hybrid Hydrogels
1.1.2. Classification Based on Polymeric Composition
Homopolymeric Hydrogels
Copolymeric Hydrogels
Multi-Polymer Integrating Polymer Network (IPN) Hydrogels
1.1.3. Classification Based on Cross-Linking
1.1.4. Classification Based on Network Electrical Charge
Neutral Hydrogel/Non-Ionic Hydrogels
Ionic Hydrogels
1.1.5. Classification Based on Durability
1.1.6. Classification Based on Response to Stimuli
2. Methods of Hydrogels Synthesis
2.1. Physical Cross-Linking
2.1.1. Ionic-Interaction
2.1.2. Complex Coacervation
2.1.3. Hydrogen bonding
2.1.4. Maturation (Heat-Induced Aggregation)
2.1.5. Hydrophobic Interactions
2.2. Chemical Cross-Linking
2.2.1. Schiff Base Reactions: Cross-Linking between Amino- and Aldehyde Groups
2.2.2. Polymer-Polymer Cross-Linking or Hybrid Polymer Networks (HPN)
2.2.3. Photo Cross-Linking and Free Radical Polymerization
2.2.4. Cross-Linking by Chemical Reactions of Complementary Groups
2.3. Enzymatic Cross-Linking
2.4. Grafting
2.4.1. Chemical Grafting
2.4.2. Radiation Grafting
2.5. Radiation Cross-Linking
3. Properties of Hydrogels
3.1. Swelling Properties
3.2. Mechanical Properties
3.3. Biocompatible Properties
3.4. Biodegradability
4. Characterization of Hydrogels
4.1. Solubility
4.1.1. Method A
4.1.2. Method B
4.2. Swelling Measurement
4.3. FTIR
4.4. Scanning Electron Microscopy (SEM)
4.5. Rheology
4.6. X-ray Scattering Techniques
4.7. SANS
5. In Vitro Drug Release Study
Spreadability Study
6. Biomedical Applications of Hydrogels
6.1. Hydrogel for Drug Delivery
6.2. Tissue Engineering (TE)
6.3. Bone Regeneration
6.4. Applications in Spinal Cord Regeneration
6.5. Biosensors
6.6. Applications in Wound Healing
6.7. Hygiene Products
6.8. Applications in the Treatment of Cancer
6.9. Anti-Fungal Applications
6.10. Anti-Bacterial Applications of Hydrogels
6.11. Hydrogels in Contact Lens
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, W.; Drahoslav, L. Hydrophilic gels in biologic use. Nature 1960, 185, 117–118. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-regulated healable polymeric hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef]
- Rehman, W.U.; Asim, M.; Hussain, S.; Khan, S.A.; Khan, S.B. Hydrogel: A Promising Material in Pharmaceutics. Curr. Pharm. Des. 2020, 26, 5892–5908. [Google Scholar] [CrossRef]
- Breedveld, V.; Nowak, A.P.; Sato, J.; Deming, T.J.; Pine, D.J. Rheology of block copolypeptide solutions: Hydrogels with tunable properties. Macromolecules 2004, 37, 3943–3953. [Google Scholar] [CrossRef] [Green Version]
- Guilherme, M.; Silva, R.; Girotto, E.; Rubira, A.; Muniz, E. Hydrogels based on PAAm network with PNIPAAm included: Hydrophilic–hydrophobic transition measured by the partition of Orange II and Methylene Blue in water. Polymer 2003, 44, 4213–4219. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Uto, K.; Aoyagi, T.; Kim, Y.-J.; Narain, R.; Idota, N.; Hoffman, J.M. Smart hydrogels. In Smart Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–65. [Google Scholar]
- Garg, T.; Singh, S.; Goyal, A.K. Stimuli-sensitive hydrogels: An excellent carrier for drug and cell delivery. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 369–409. [Google Scholar] [CrossRef]
- Sri, B.; Ashok, V.; Arkendu, C. As a review on hydrogels as drug delivery in the pharmaceutical field. Int. J. Pharm. Chem. Sci. 2012, 1, 642–661. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Oyen, M. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25. [Google Scholar]
- Zaman, M.; Siddique, W.; Waheed, S.; Sarfraz, R.; Mahmood, A.; Qureshi, J.; Iqbal, J.; Chughtai, F.S.; Rahman, M.S.U.; Khalid, U. Hydrogels, their applications and polymers used for hydrogels: A review. Int. J. Biol. Pharm. Allied Sci 2015, 4, 6581–6603. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Desai, P. Synthesis and Characterization of Polyionic Hydrogels. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2008. [Google Scholar]
- Allcock, H.R.; Lampe, F.W.; Mark, J.E.; Allcock, H. Contemporary Polymer Chemistry; Prentice-Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Sperling, L.H. Interpenetrating Polymer Networks and Related Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Marsano, E.; Bianchi, E.; Viscardi, A. Stimuli responsive gels based on interpenetrating network of hydroxy propylcellulose and poly (N-isopropylacrylamide). Polymer 2004, 45, 157–163. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Gupta, K. A review: Tailor-made hydrogel structures (classifications and synthesis parameters). Polym.-Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Palacio, D.A.; Urbano, B.F.; Palencia, M.; Rivas, B.L. Preparation of alkylated chitosan-based polyelectrolyte hydrogels: The effect of monomer charge on polymerization. Eur. Polym. J. 2019, 118, 551–560. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Lee, W.-F.; Chiu, R.-J. Investigation of charge effects on drug release behavior for ionic thermosensitive hydrogels. Mater. Sci. Eng. C 2002, 20, 161–166. [Google Scholar] [CrossRef]
- Guo, G.; Chen, Y.; Liu, X.; Zhu, D.Y.; Zhang, B.; Lin, N.; Gao, L. Tough and durable hydrogels with robust skin layers formed via soaking treatment. J. Mater. Chem. B 2018, 6, 8043–8054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Chu, C.-C. Synthesis and characterization of partially biodegradable, temperature and pH sensitive Dex–MA/PNIPAAm hydrogels. Biomaterials 2004, 25, 4719–4730. [Google Scholar] [CrossRef]
- Boral, S.; Gupta, A.N.; Bohidar, H. Swelling and de-swelling kinetics of gelatin hydrogels in ethanol–water marginal solvent. Int. J. Biol. Macromol. 2006, 39, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Elsayed, M.M. Hydrogel preparation technologies: Relevance kinetics, thermodynamics and scaling up aspects. J. Polym. Environ. 2019, 27, 871–891. [Google Scholar] [CrossRef]
- Esteban, C.; Severian, D. Polyionic Hydrogels Based on Xanthan and Chitosan for Stabilising and Controlled Release of Vitamins, Vol. WO0004086 (A1) 2000. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2000004086 (accessed on 25 February 2022).
- Magnin, D.; Lefebvre, J.; Chornet, E.; Dumitriu, S. Physicochemical and structural characterization of a polyionic matrix of interest in biotechnology, in the pharmaceutical and biomedical fields. Carbohydr. Polym. 2004, 55, 437–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole–dipole and H-bonding interactions significantly enhance the multifaceted mechanical properties of thermoresponsive shape memory hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. Prog. Mol. Environ. Bioeng. Anal. Modeling Technol. Appl. 2011, 117–150. [Google Scholar]
- Gurny, R.; Doelker, E.; Peppas, N. Modelling of sustained release of water-soluble drugs from porous, hydrophobic polymers. Biomaterials 1982, 3, 27–32. [Google Scholar] [CrossRef]
- Huh, K.M.; Cho, Y.W.; Park, K. PLGA-PEG block copolymers for drug formulations. Drug Deliv. Technol. 2003, 3, 42–44. [Google Scholar]
- Napoli, A.; Tirelli, N.; Kilcher, G.; Hubbell, A. New synthetic methodologies for amphiphilic multiblock copolymers of ethylene glycol and propylene sulfide. Macromolecules 2001, 34, 8913–8917. [Google Scholar] [CrossRef]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Shim, W.S.; Kim, J.-H.; Kim, K.; Kim, Y.-S.; Park, R.-W.; Kim, I.-S.; Kwon, I.C.; Lee, D.S. pH-and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int. J. Pharm. 2007, 331, 11–18. [Google Scholar] [CrossRef]
- Jin, J.; Song, M.; Hourston, D. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Hiemstra, C.; van der Aa, L.J.; Zhong, Z.; Dijkstra, P.J.; Feijen, J. Novel in situ forming, degradable dextran hydrogels by Michael addition chemistry: Synthesis, rheology, and degradation. Macromolecules 2007, 40, 1165–1173. [Google Scholar] [CrossRef]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Akaike, T.; Ishihara, M. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Jiao, T.; Xing, R.; Hong, W.; Zhang, L.; Zhang, Q.; Peng, Q. Preparation of graphene oxide-polymer composite hydrogels via thiol-ene photopolymerization as efficient dye adsorbents for wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 668–676. [Google Scholar] [CrossRef]
- Piluso, S.; Hiebl, B.; Gorb, S.N.; Kovalev, A.; Lendlein, A.; Neffe, A.T. Hyaluronic acid-based hydrogels crosslinked by copper-catalyzed azide-alkyne cycloaddition with tailorable mechanical properties. Int. J. Artif. Organs 2011, 34, 192–197. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, Z.; Zhu, X.L.; Huang, G.Y.; Xu, F.; Yang, J.H.; Osada, Y.; Zrínyi, M.; Li, J.H.; Chen, Y.M. Dextran-based hydrogel formed by thiol-Michael addition reaction for 3D cell encapsulation. Colloids Surf. B Biointerfaces 2015, 128, 140–148. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Liu, Y.; Chan-Park, M.B. Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials 2009, 30, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels–Alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Kotsuchibashi, Y.; Vshyvenko, S.; Liu, Y.; Hall, D.; Zeng, H.; Narain, R. Self-healing and injectable shear thinning hydrogels based on dynamic oxaborole-diol covalent cross-linking. ACS Biomater. Sci. Eng. 2016, 2, 2315–2323. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.E.; Ding, S.; Forster, R.E.; Pinkas, D.M.; Barron, A.E. Modular enzymatically crosslinked protein polymer hydrogels for in situ gelation. Biomaterials 2010, 31, 7288–7297. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Hiemstra, C.; Zhong, Z.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.; Wu, L.; Tullman, J.; Payne, G.; Bentley, W.; Barbari, T. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; Yuen, H.P.; Berger, G.; Francey, S.; Hung, T.-C.; Nelson, B.; Phillips, L.; McGorry, P. Declining transition rate in ultra high risk (prodromal) services: Dilution or reduction of risk? Schizophr. Bull. 2007, 33, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lim, S.Y.; Nam, D.H.; Ryu, J.; Ku, S.H.; Park, C.B. Self-assembled, photoluminescent peptide hydrogel as a versatile platform for enzyme-based optical biosensors. Biosens. Bioelectron. 2011, 26, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Sartori, S.; Ciardelli, G. Biomimetic materials for medical application through enzymatic modification. In Biofunctionalization of Polymers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 181–205. [Google Scholar]
- Chen, K.; Manga, P.; Orlow, S.J. Pink-eyed dilution protein controls the processing of tyrosinase. Mol. Biol. Cell 2002, 13, 1953–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, M. In vitro release studies of vitamin B12 from poly N-vinyl pyrrolidone/starch hydrogels grafted with acrylic acid synthesized by gamma radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 5020–5026. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Kumar Giri, T.; Nakhate, K.T.; Kashyap, P.; Krishna Tripathi, D. Xanthan gum and its derivatives as a potential bio-polymeric carrier for drug delivery system. Curr. Drug Deliv. 2013, 10, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Schmer, G.; Harris, C.; Kraft, W. Covalent binding of biomolecules to radiation-grafted hydrogels on inert polymer surfaces. ASAIO J. 1972, 18, 10–16. [Google Scholar] [CrossRef]
- Said, H.M.; Abd Alla, S.G.; El-Naggar, A.W.M. Synthesis and characterization of novel gels based on carboxymethyl cellulose/acrylic acid prepared by electron beam irradiation. React. Funct. Polym. 2004, 61, 397–404. [Google Scholar] [CrossRef]
- Lugao, A.B.; Malmonge, S.M. Use of radiation in the production of hydrogels. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 37–42. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, H.S.; Lee, Y.M.; Jeong, C.N. Properties of electroresponsive poly (vinyl alcohol)/poly (acrylic acid) IPN hydrogels under an electric stimulus. J. Appl. Polym. Sci. 1999, 73, 1675–1683. [Google Scholar] [CrossRef]
- Shih, H.; Lin, C.-C. Cross-linking and degradation of step-growth hydrogels formed by thiol–ene photoclick chemistry. Biomacromolecules 2012, 13, 2003–2012. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Wirsén, A.; Albertsson, A.C. Structural change and swelling mechanism of pH-sensitive hydrogels based on chitosan and D, L-lactic acid. J. Appl. Polym. Sci. 1999, 74, 3186–3192. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, M.; Bowyer, A.; Eisenthal, R.; Hubble, J. A novel pH-and ionic-strength-sensitive carboxy methyl dextran hydrogel. Biomaterials 2005, 26, 4677–4683. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Dolbow, J.; Fried, E.; Ji, H. Chemically induced swelling of hydrogels. J. Mech. Phys. Solids 2004, 52, 51–84. [Google Scholar] [CrossRef] [Green Version]
- Ehrenhofer, A.; Binder, S.; Gerlach, G.; Wallmersperger, T. Multisensitive swelling of hydrogels for sensor and actuator design. Adv. Eng. Mater. 2020, 22, 2000004. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Li, D.; Zhang, G.; Long, S.; Wang, H. Hybrid dual crosslinked polyacrylic acid hydrogels with ultrahigh mechanical strength, toughness and self-healing properties via soaking salt solution. Polymer 2017, 121, 55–63. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, P.; Hao, J.; Wang, X. Bioinspired self-healing of kinetically inert hydrogels mediated by chemical nutrient supply. ACS Appl. Mater. Interfaces 2020, 12, 6471–6478. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Colombo, I.; Lapasin, R. Experimental determination of the theophylline diffusion coefficient in swollen sodium-alginate membranes. J. Control. Release 2001, 76, 93–105. [Google Scholar] [CrossRef]
- Hoshino, K.-I.; Nakajima, T.; Matsuda, T.; Sakai, T.; Gong, J.P. Network elasticity of a model hydrogel as a function of swelling ratio: From shrinking to extreme swelling states. Soft Matter 2018, 14, 9693–9701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.; Lin, P.; Chung, C.; Wang, S. Biocompatibility and surface modification of NiTi shape memory alloy. In Proceedings of the Medical Device Materials: Proceedings from the Materials & Processes for Medical Devices Conference 2003, Anaheim, CA, USA, 8–10 September 2003; p. 120. [Google Scholar]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kamath, K.R.; Park, K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Officer, E.; Ferreira, M.M. Leonardo da Vinci Program. Available online: https://biodeg.net/fichiers/Training%20course%20(Eng).pdf (accessed on 25 February 2022).
- Kim, M.; Cha, C. Integrative control of mechanical and degradation properties of in situ crosslinkable polyamine-based hydrogels for dual-mode drug release kinetics. Polymer 2018, 145, 272–280. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Obst, M.; Steinbüchel, A. Microbial degradation of poly (amino acid) s. Biomacromolecules 2004, 5, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Matica, A.; Menghiu, G.; Ostafe, V. Toxicity of chitosan based products. New Front. Chem. 2017, 26, 75–86. [Google Scholar]
- Tabata, Y.; Nagano, A.; Ikada, Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999, 5, 127–138. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Nagasawa, N.; Yagi, T.; Kume, T.; Yoshii, F. Radiation crosslinking of carboxymethyl starch. Carbohydr. Polym. 2004, 58, 109–113. [Google Scholar] [CrossRef]
- Yoshii, F.; Kume, T. Process for Producing Grosslinked Starch Derivatives and Crosslinked Starch Derivatives Produced by the Same. U.S. Patent 6,617,448, 9 September 2003. [Google Scholar]
- Valles, E.; Durando, D.; Katime, I.; Mendizabal, E.; Puig, J. Equilibrium swelling and mechanical properties of hydrogels of acrylamide and itaconic acid or its esters. Polym. Bull. 2000, 44, 109–114. [Google Scholar] [CrossRef]
- Liu, P.; Zhai, M.; Li, J.; Peng, J.; Wu, J. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Katayama, T.; Nakauma, M.; Todoriki, S.; Phillips, G.O.; Tada, M. Radiation-induced polymerization of gum arabic (Acacia senegal) in aqueous solution. Food Hydrocoll. 2006, 20, 983–989. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Khan, L.U.; Farooq, A.; Akhtar, K.; Asiri, A.M. Fourier Transform Infrared Spectroscopy: Fundamentals and Application in Functional Groups and Nanomaterials Characterization. In Handbook of Materials Characterization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–344. [Google Scholar]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning electron microscopy: Principle and applications in nanomaterials characterization. In Handbook of Materials Characterization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–145. [Google Scholar]
- Ganesh, T.A.; Manohar, S.D.; Bhanudas, S.R. Hydrogel-a novel technique for prepartion of topical gel. World J. Pharm. Pharm. Sci. 2013, 2, 4520–4541. [Google Scholar]
- Aikawa, K.; Matsumoto, K.; Uda, H.; Tanaka, S.; Shimamura, H.; Aramaki, Y.; Tsuchiya, S. Hydrogel formation of the pH response polymer polyvinylacetal diethylaminoacetate (AEA). Int. J. Pharm. 1998, 167, 97–104. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R.; Fernandes, P.R.; Rubira, A.F.; Muniz, E.C. Optical and morphological characterization of polyacrylamide hydrogel and liquid crystal systems. Eur. Polym. J. 2005, 41, 2134–2141. [Google Scholar] [CrossRef]
- Sahiner, N.; Singh, M.; De Kee, D.; John, V.T.; McPherson, G.L. Rheological characterization of a charged cationic hydrogel network across the gelation boundary. Polymer 2006, 47, 1124–1131. [Google Scholar] [CrossRef]
- Coviello, T.; Coluzzi, G.; Palleschi, A.; Grassi, M.; Santucci, E.; Alhaique, F. Structural and rheological characterization of Scleroglucan/borax hydrogel for drug delivery. Int. J. Biol. Macromol. 2003, 32, 83–92. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Bastrop, M.; Hvilsom, A.; Contri, R.V.; Mäder, K. Characterization of thermosensitive chitosan-based hydrogels by rheology and electron paramagnetic resonance spectroscopy. Eur. J. Pharm. Biopharm. 2008, 68, 26–33. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Sohni, S.; Khan, S.A.; Akhtar, K.; Khan, S.B.; Asiri, A.M.; Hashim, R.; Omar, A.M. Room temperature preparation of lignocellulosic biomass supported heterostructure (Cu+ Co@ OPF) as highly efficient multifunctional nanocatalyst using wetness co-impregnation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 184–195. [Google Scholar] [CrossRef]
- Khan, S.A.; Bello, B.A.; Khan, J.A.; Anwar, Y.; Mirza, M.B.; Qadri, F.; Farooq, A.; Adam, I.K.; Asiri, A.M.; Khan, S.B. Albizia chevalier based Ag nanoparticles: Anti-proliferation, bactericidal and pollutants degradation performance. J. Photochem. Photobiol. B Biol. 2018, 182, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.H.M.; Khan, S.A. Stabilization of Various Zero-Valent Metal Nanoparticles on a Superabsorbent Polymer for the Removal of Dyes, Nitrophenol, and Pathogenic Bacteria. ACS Omega 2020, 5, 7379–7391. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, T.; Raja, K.; Pangerteni, D.; Patriati, A.; Putra, E. Structural organization of poly (vinyl alcohol) hydrogels obtained by freezing/thawing and γ-irradiation processes: A small-angle neutron scattering (SANS) study. Proc. Chem. 2012, 4, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.; Rajabnezhad, S.; Kohli, K. Hydrogels as potential drug delivery systems. Sci. Res. Essays 2009, 4, 1175–1183. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Bahram, M.; Nurallahzadeh, N.; Mohseni, N. pH-sensitive hydrogel for coacervative cloud point extraction and spectrophotometric determination of Cu (II): Optimization by central composite design. J. Iran. Chem. Soc. 2015, 12, 1781–1787. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Salman, S.; Al-Ghamdi, Y.O. Nanotherapeutic Strategies and New Pharmaceuticals (Part 1); Bentham Sciences Book: Al Sharjah, United Arab Emirates, 2021; pp. 1–228. [Google Scholar]
- Waseeq Ur Rehman, S.A.K.; Ahmad, Z.; Khattak, M.T.N.; Hussain, S.; Al-Ghamdi, Y.O.; Jabli, M.; Anwar, Y. Advances in Nanotherapeutic Agents. In Nanotherapeutic Strategies and New Pharmaceuticals (Part 1); Bentham Sciences: Al Sharjah, United Arab Emirates, 2021; pp. 1–16. [Google Scholar]
- Rabilloud, T.; Chevallet, M.; Luche, S.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteom. 2010, 73, 2064–2077. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Kennedy, J.E.; Higginbotham, C.L. Mechanical properties and thermal behaviour of PEGDMA hydrogels for potential bone regeneration application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1219–1227. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Gillette, B.M.; Jensen, J.A.; Tang, B.; Yang, G.J.; Bazargan-Lari, A.; Zhong, M.; Sia, S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008, 7, 636–640. [Google Scholar] [CrossRef]
- Nomura, H.; Katayama, Y.; Shoichet, M.S.; Tator, C.H. Complete spinal cord transection treatedby implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery 2006, 59, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateescu, A.; Wang, Y.; Dostalek, J.; Jonas, U. Thin hydrogel films for optical biosensor applications. Membranes 2012, 2, 40–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Mao, X.; Chen, G.; Wang, Z.; Zhang, Y.; Zhu, X.; Li, G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem. Sci. 2018, 9, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, V.A.; Yan, J.; Simonian, A.L.; Revzin, A. Micropatterned nanocomposite hydrogels for biosensing applications. Electroanalysis 2011, 23, 1142–1149. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D printing of hydrogels: A review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered hydrogels in cancer therapy and diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gui, J. Natural and artificial polyploids in aquaculture. Aquac. Fish. 2017, 2, 103–111. [Google Scholar] [CrossRef]
- Andreyev, J.; Ross, P.; Donnellan, C.; Lennan, E.; Leonard, P.; Waters, C.; Wedlake, L.; Bridgewater, J.; Glynne-Jones, R.; Allum, W. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014, 15, e447–e460. [Google Scholar] [CrossRef]
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable hydrogels for localized cancer therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Das, S.; Chawan, N.S.; Hazra, A. Nosocomial infections in the intensive care unit: Incidence, risk factors, outcome and associated pathogens in a public tertiary teaching hospital of Eastern India. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2015, 19, 14. [Google Scholar]
- Zumbuehl, A.; Ferreira, L.; Kuhn, D.; Astashkina, A.; Long, L.; Yeo, Y.; Iaconis, T.; Ghannoum, M.; Fink, G.R.; Langer, R. Antifungal hydrogels. Proc. Natl. Acad. Sci. USA 2007, 104, 12994–12998. [Google Scholar] [CrossRef] [Green Version]
- AbouSamra, M.M.; Basha, M.; Awad, G.E.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymeric carrier for the treatment of topical candidiasis. J. Drug Deliv. Sci. Technol. 2019, 49, 365–374. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.-S.; Hu, X.-H.; Chu, L.-Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. https://doi.org/10.3390/gels8030167
Ahmad Z, Salman S, Khan SA, Amin A, Rahman ZU, Al-Ghamdi YO, Akhtar K, Bakhsh EM, Khan SB. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels. 2022; 8(3):167. https://doi.org/10.3390/gels8030167
Chicago/Turabian StyleAhmad, Zubair, Saad Salman, Shahid Ali Khan, Abdul Amin, Zia Ur Rahman, Youssef O. Al-Ghamdi, Kalsoom Akhtar, Esraa M. Bakhsh, and Sher Bahadar Khan. 2022. "Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications" Gels 8, no. 3: 167. https://doi.org/10.3390/gels8030167