Microstructure Analysis and Effects of Single and Mixed Activators on Setting Time and Strength of Coal Gangue-Based Geopolymers
Abstract
:1. Introduction
2. Results and Discussion
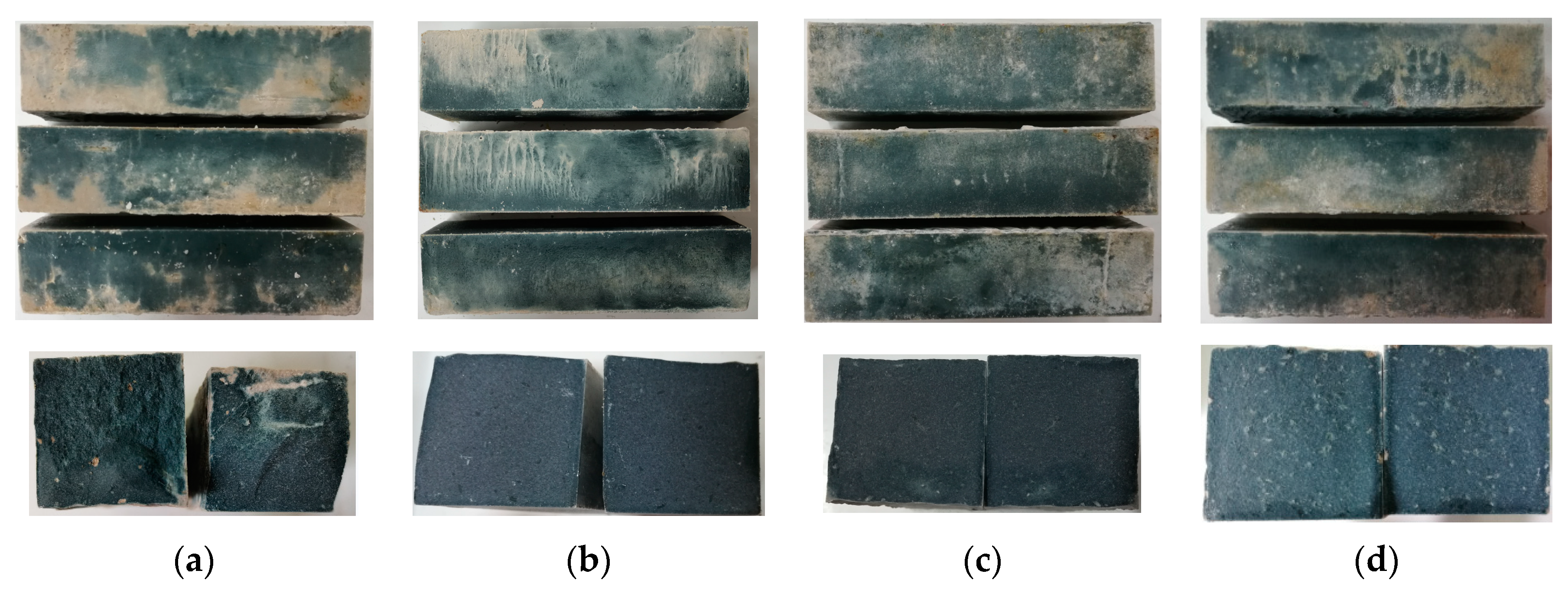
2.1. Appearance Observation
2.2. Effects of Single Activator
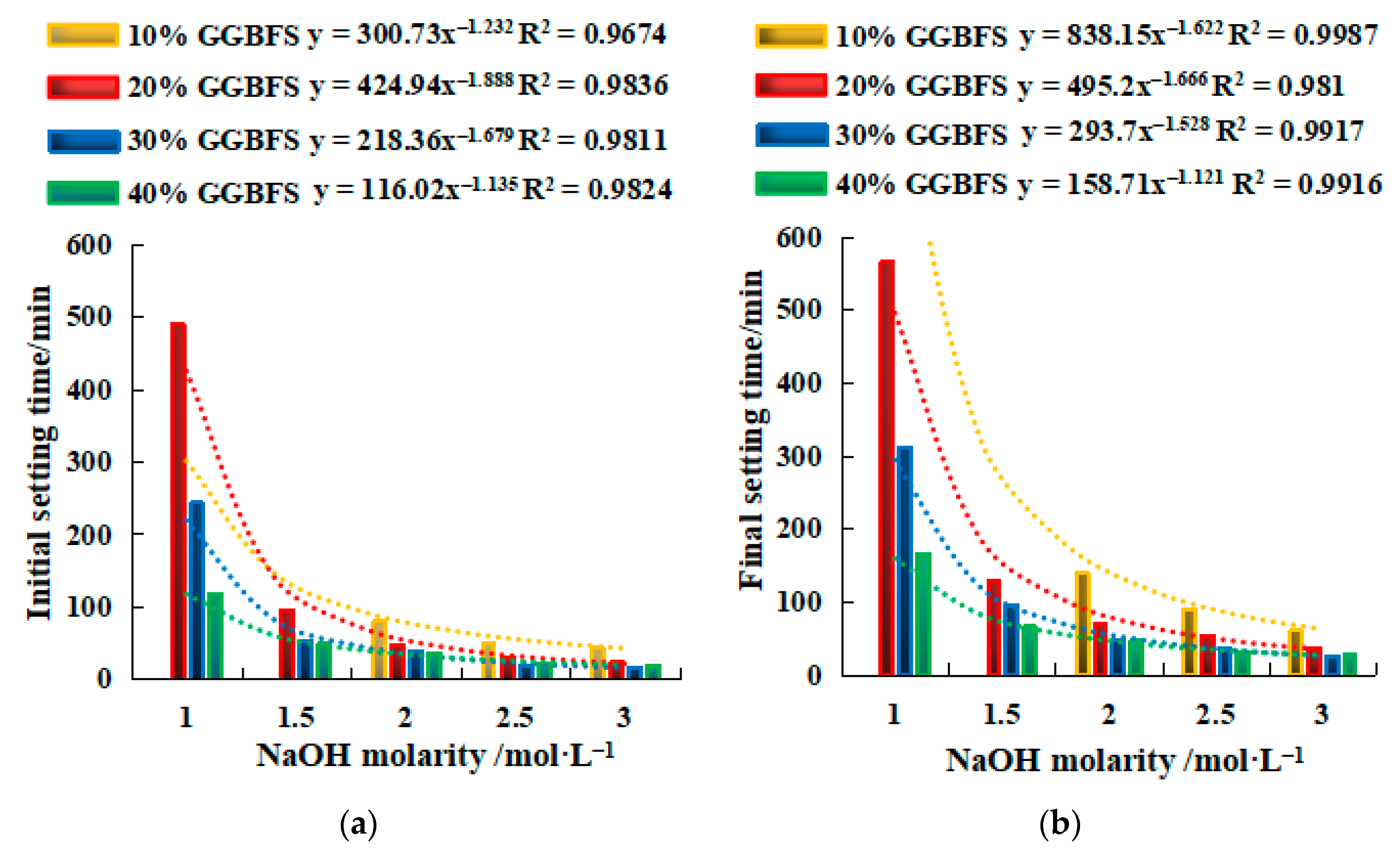
2.2.1. Setting Time of SH Geopolymer
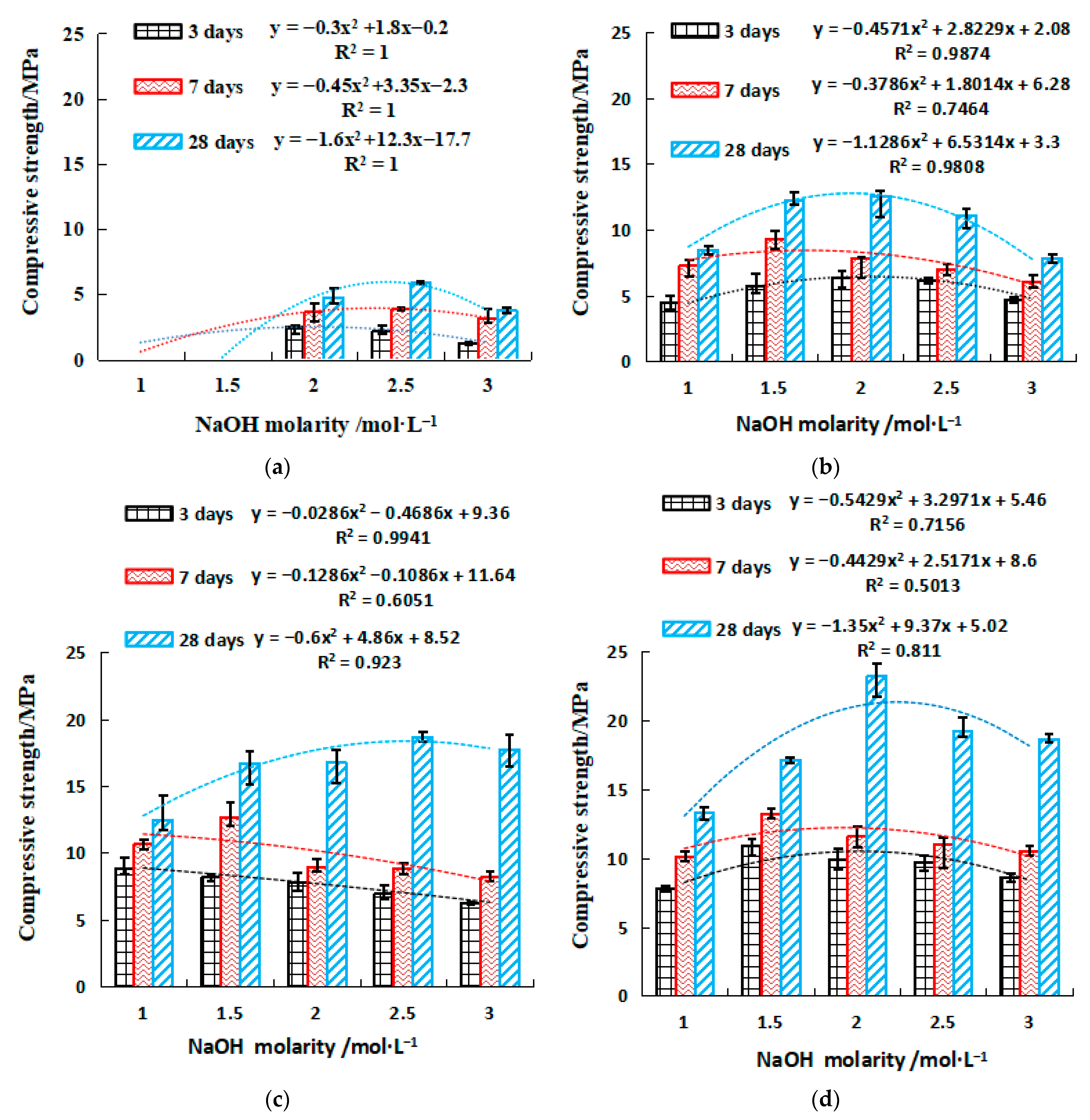
2.2.2. Strength of SH Geopolymer
2.3. Effects of Mixed Activator
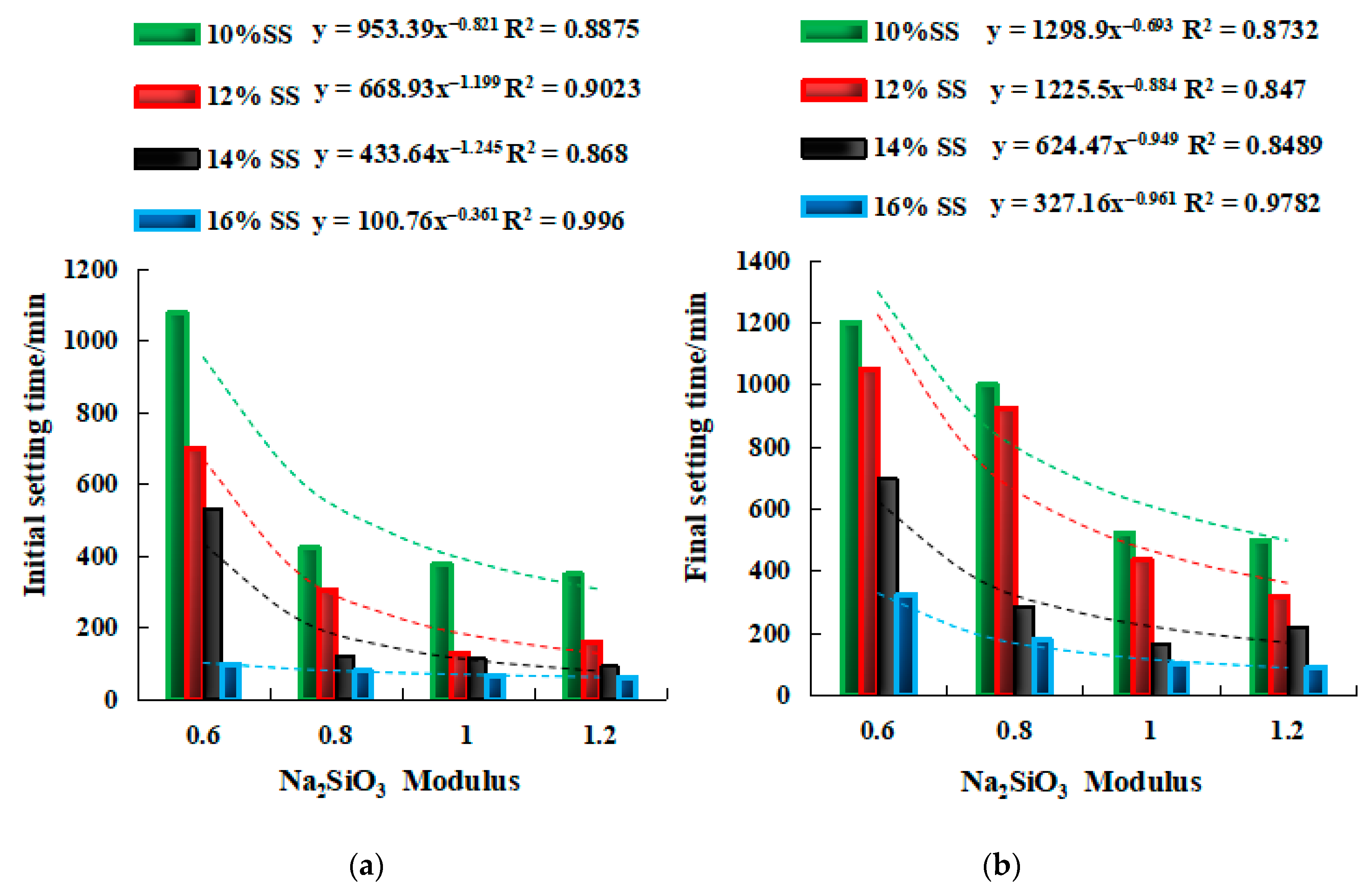
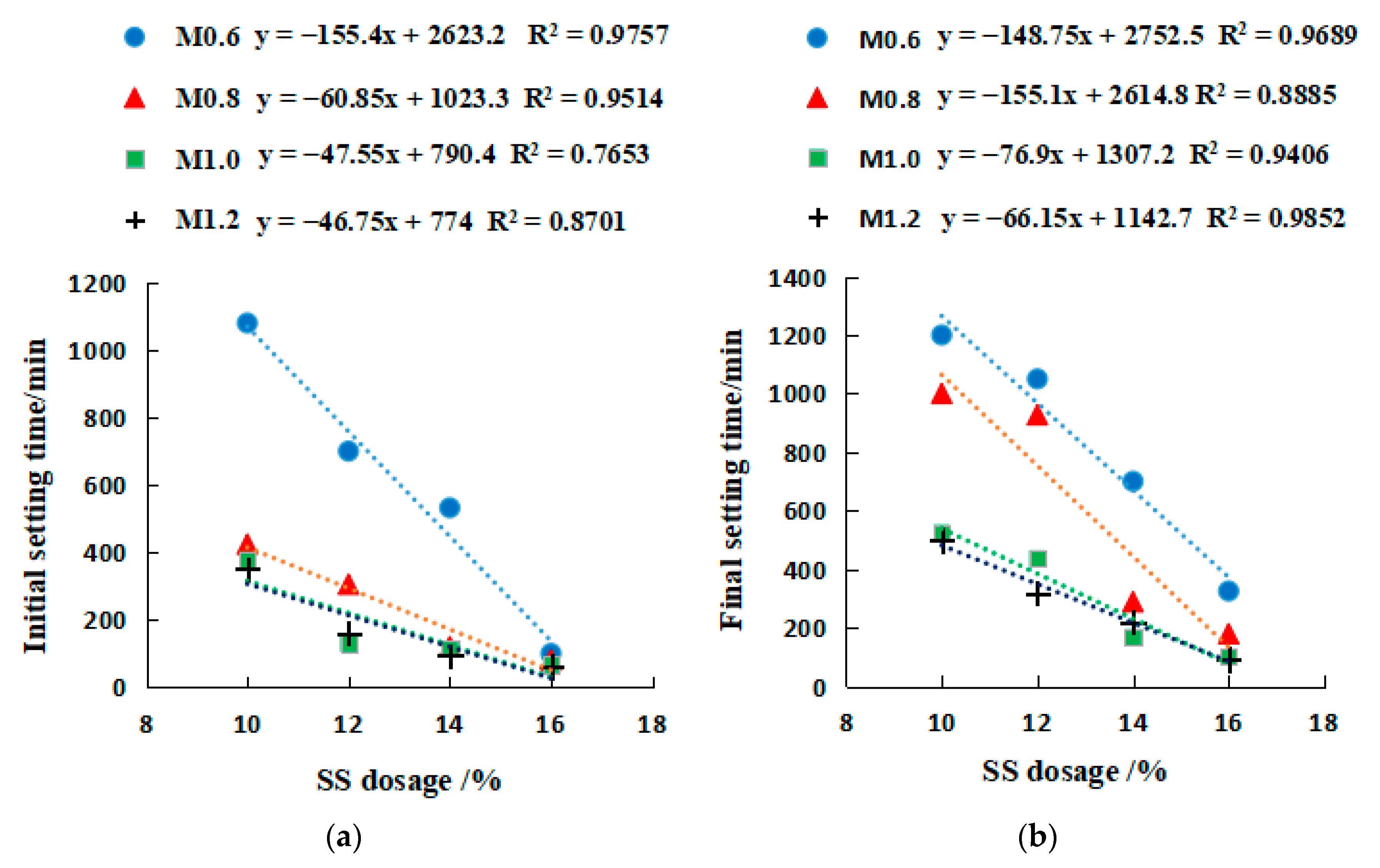
2.3.1. Setting Time of SSG Geopolymer
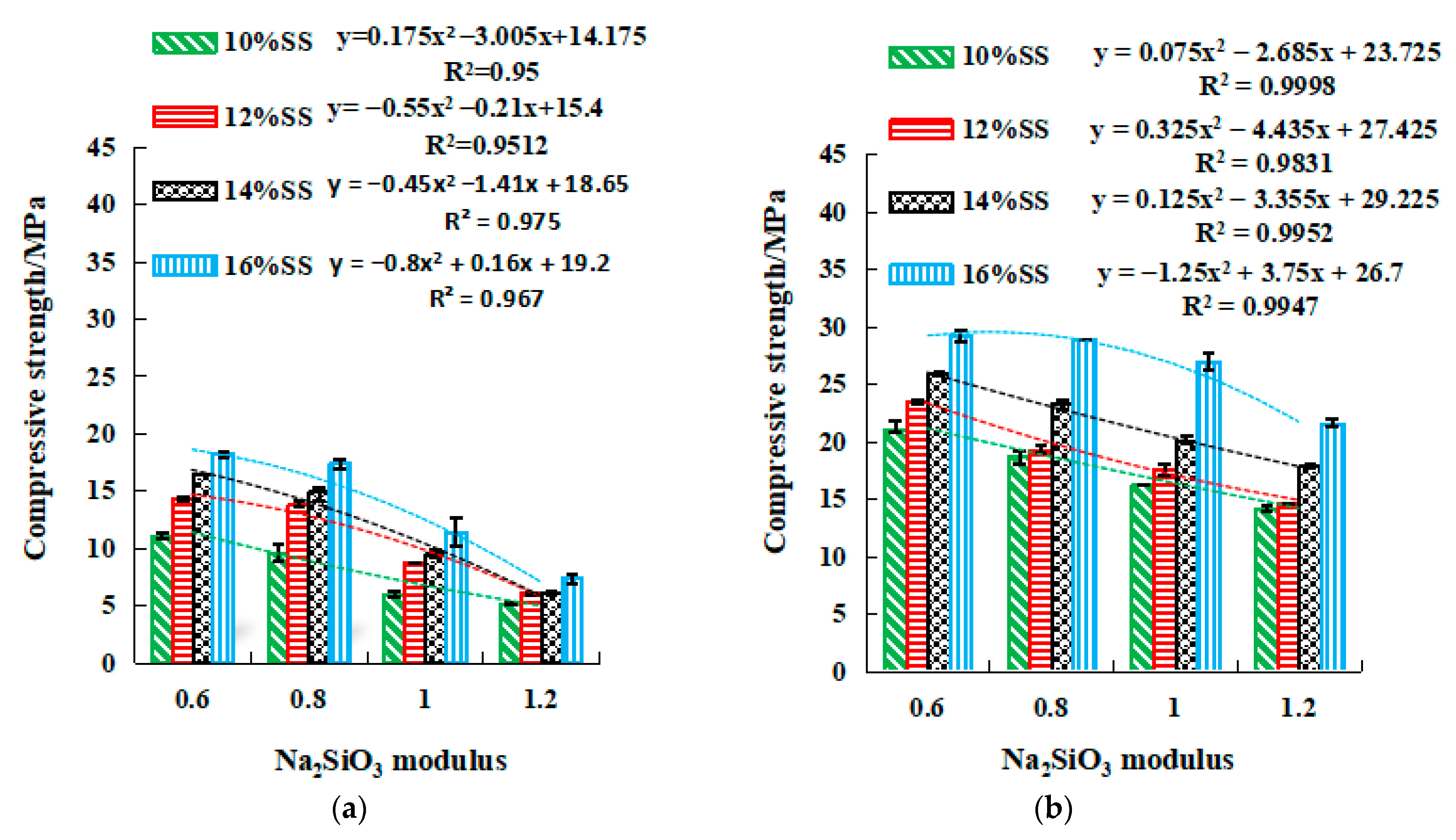
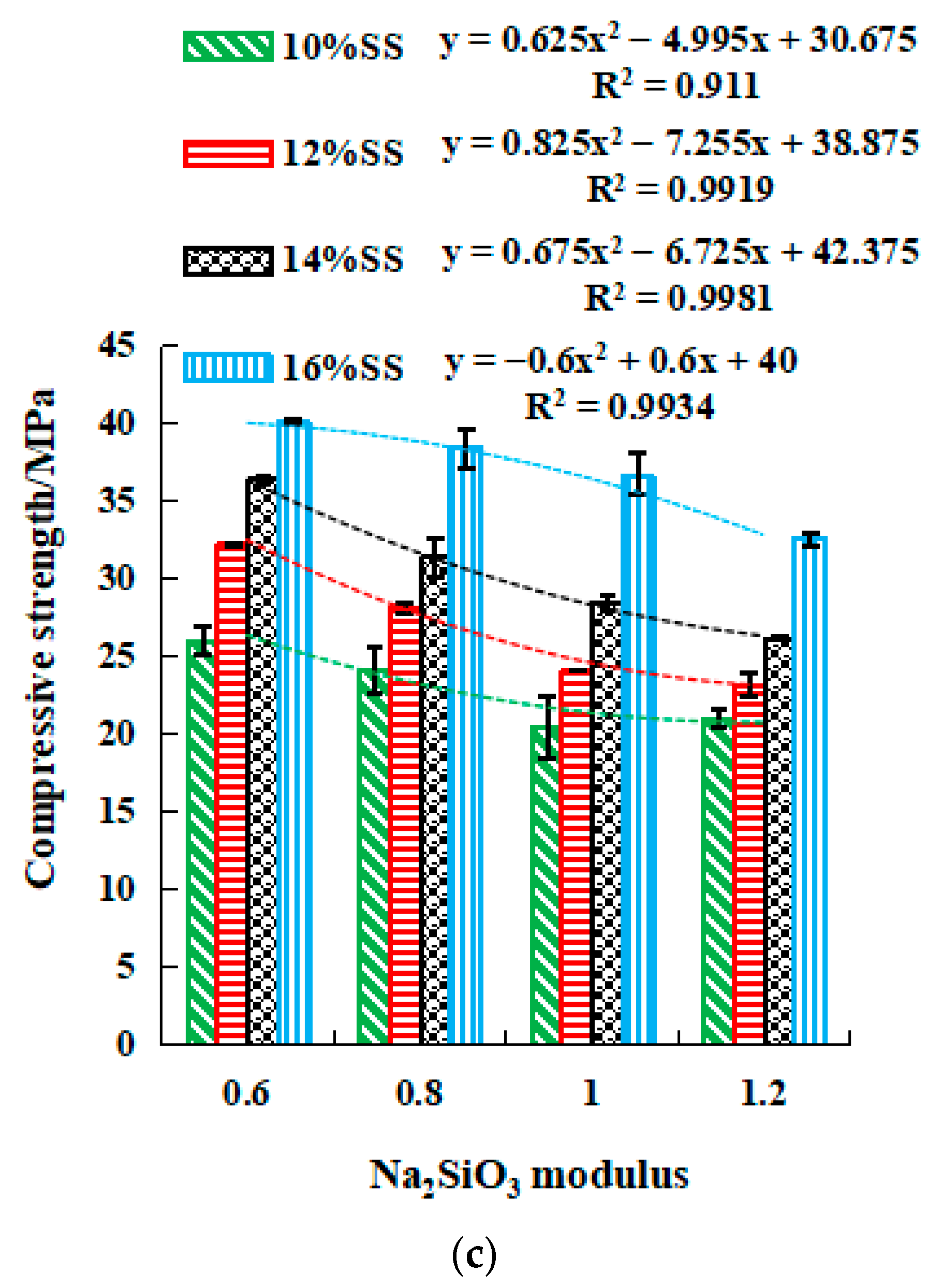
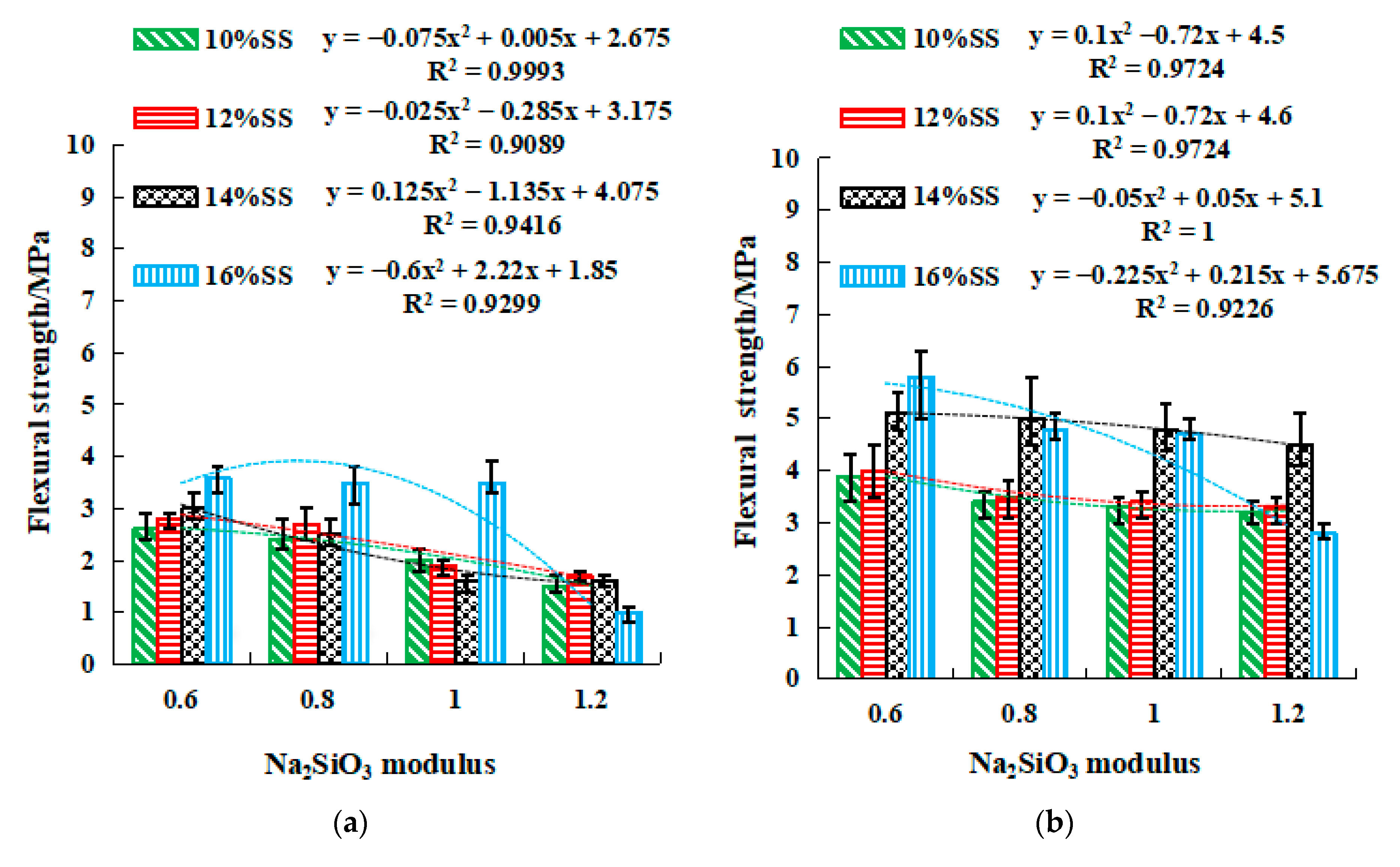
2.3.2. Strength of SSG Geopolymer
3. Characterization
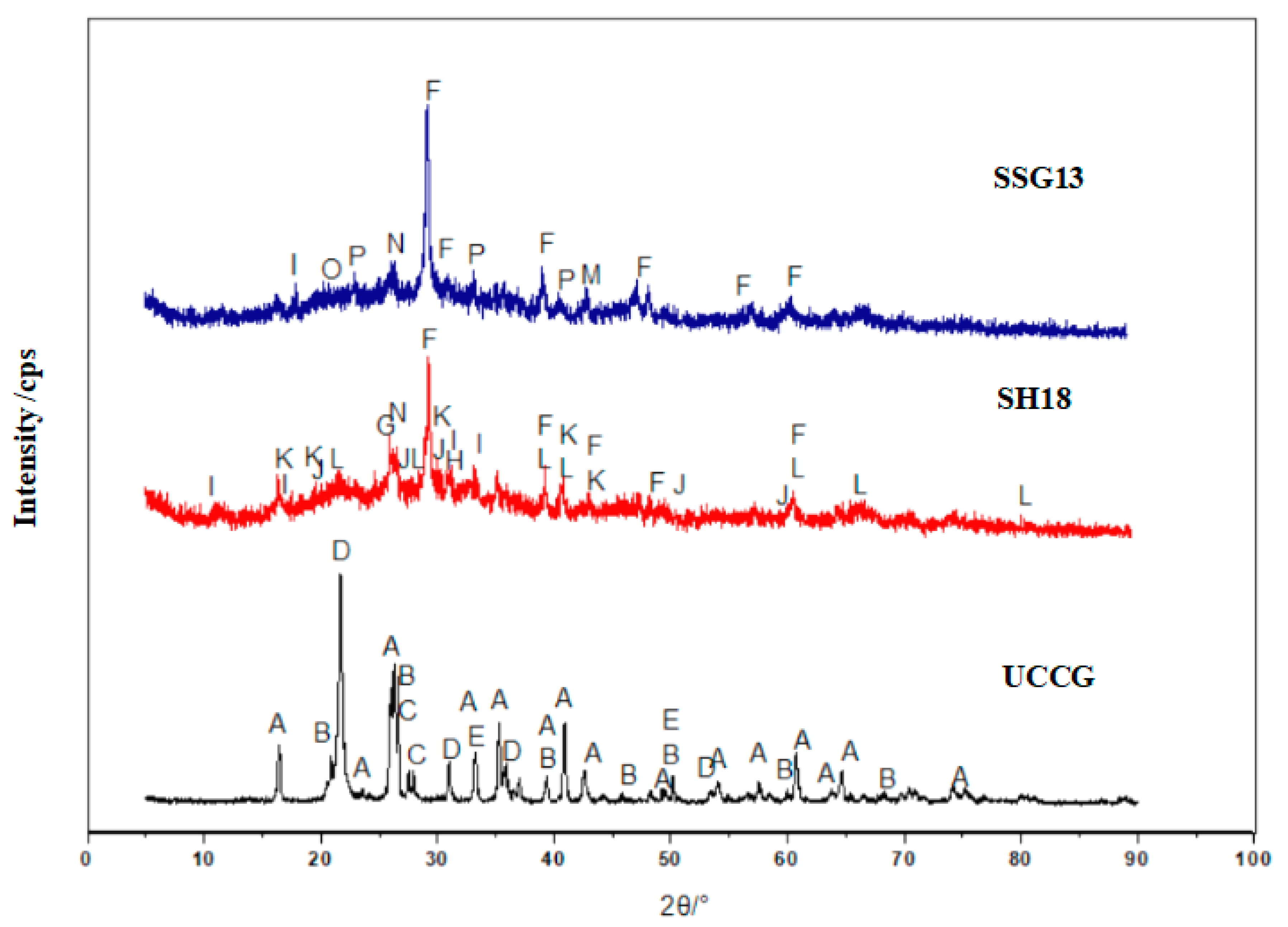
3.1. XRD Tests
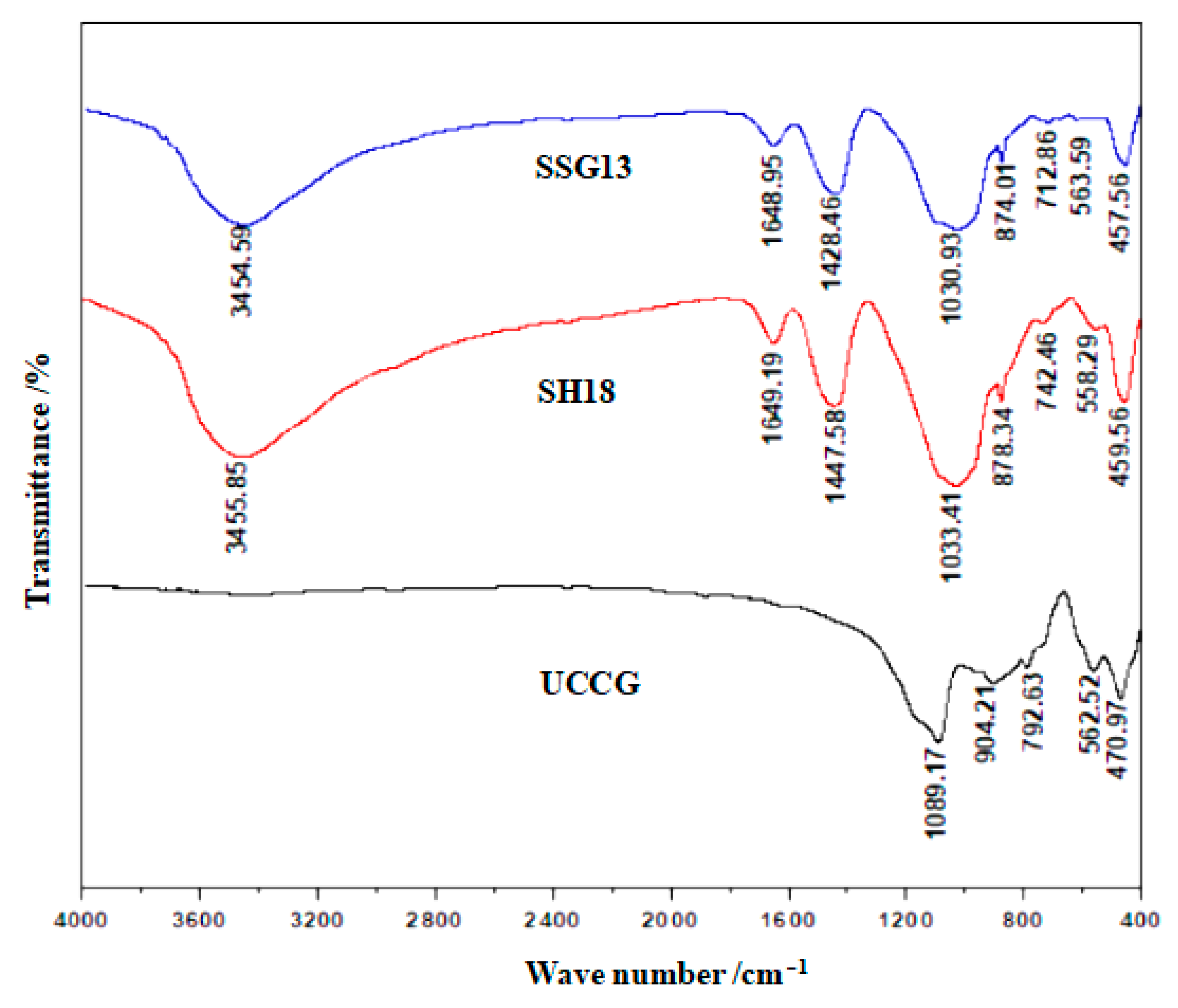
3.2. FTIR Tests
3.3. SEM and EDS Test
4. Conclusions
- (1)
- The setting time of a single activator can be predicted by the molarity of NaOH (y = ax−b), and the setting time of a mixed activator can be predicted by the Na2SiO3 modulus (y = ax−b) and the dosage of SS activator (y = ax + b).
- (2)
- The strength value of NaOH and the molar concentration have a quadratic parabola relationship (y = ax2 + bx + c), and the NaOH molarity of the corresponding activator at a certain intensity can be calculated by substituting the relational equation. Similar trends are observed for Na2SO3 modulus and strength in mix activator. Meanwhile, the dosage of SS (NaOH and Na2SO3) and strength followed a linear relationship (y = ax + b). Therefore, in the mixed activator, the corresponding modulus and the amount of alkali doping at a pre-reaching strength can be calculated through these equations.
- (3)
- To achieve the highest strengths, the optimum mixing ratio in the mixed activator samples was 0.6 modulus, 16% SS, and 6% desulfurization gypsum, the initial and final setting times were 100 min and 325 min, and the 28-day compressive and flexural strengths were 40.1 MPa and 7.8 MPa, respectively. For the single activator samples, the optimum molarity of NaOH was 2 mol/L, the initial and final setting times were 37 min and 47 min, and the 28-day compressive and flexural strength were 23.2 MPa and 7.5 MPa, respectively.
- (4)
- The gangue-based geopolymers are mainly N–A–S–H, C–A–S–H, and C–N–A–S–H gels. The single activator produced more C–A–S–H gels, while the mixed activator generated more N–A–S–H and C–N–A–S–H gels. The mixed activator is recommended in the development of the proposed geopolymers, over that of the single activator, as it yielded higher compressive and flexural strengths. The main reason behind this is that the mixed activator engaged a more intense hydration reaction, therefore producing more cementing gels.
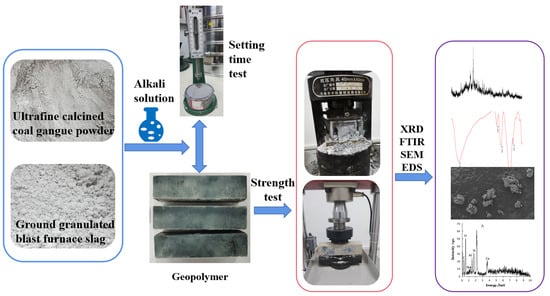
5. Materials and Method
5.1. Materials
5.2. Methods
5.2.1. Mix Design Considering Effects of Activators
Mixing with a Single Activator
Mixing with Mixed Activator
5.2.2. Geopolymer Gels Preparation and Tests Method
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Ghani, N.T.; Elsayed, H.A.; Abdel Moied, S. Geopolymer synthesis by the alkali-activation of blast furnace steel slag and its fire-resistance. HBRC J. 2018, 14, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Glasby, T.; Day, J.; Genrich, R.; Aldred, J. EFC geopolymer concrete aircraft pavements at Brisbane West Wellcamp Airport. Concrete 2015, 2015, 1–9. Available online: https://www.geopolymer.org/wp-content/uploads/GP-AIRPORT.pdf (accessed on 20 January 2022).
- Bligh, R.; Glasby, T. Development of geopolymer precast floor panels for the Global Change Institute at University of Queensland. In Proceedings of the Concrete Institute of Australia Biennial Conference, Concrete, 2013; Available online: https://www.wagner.com.au/media/1515/gci-paper_concrete-2013-conference_australia.pdf (accessed on 20 January 2022).
- Kalombe, R.M.; Ojumu, V.T.; Eze, C.P.; Nyale, S.M.; Kevern, J.; Petrik, L.F. Fly Ash-Based Geopolymer Building Materials for Green and Sustainable Development. Materials 2020, 13, 5699. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Sanjayan, J. Method of formulating geopolymer for 3D printing for construction applications. Mater. Des. 2016, 110, 382–390. [Google Scholar] [CrossRef]
- Gupta, S.; Kashani, A.; Mahmood, A.H.; Han, T. Carbon sequestration in cementitious composites using biochar and flyash–Effect on mechanical and durability properties. Constr. Build. Mater. 2021, 291, 123363. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H.; Bi, M.S.; Gao, W.; Shu, C.M. Experimental study of thermophysical properties of coal gangue at initial stage of spontaneous combustion. J. Hazard. Mater. 2020, 400, 123251. [Google Scholar] [CrossRef]
- Lü, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Ma, Z.; Sun, X. Eco-friendly treatment of coal gangue for its utilization as supplementary cementitious materials. J. Clean. Prod. 2021, 285, 124834. [Google Scholar] [CrossRef]
- Liu, C.; Shui, Z.; Gao, X.; Ma, S. Performance evaluation of alkali-activated coal gangue-blast furnace slag composite. Bull. Chin. Ceram. Soc. 2020, 39, 2877–2884. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, C.; Huang, P.; Sun, Q.; Li, M.; Chai, J. Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer. Energies 2020, 13, 2504. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Liu, C.; Deng, X.; Liu, J.; Hui, D. Mechanical properties and microstructures of hypergolic and calcined coal gangue based geopolymer recycled concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Runtti, H.; Luukkonen, T.; Niskanen, M.; Tuomikoski, S.; Kangas, T.; Tynjälä, P.; Tolonen, E.T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; et al. Sulphate removal over barium-modified blast-furnace-slag geopolymer. J. Hazard. Mater. 2016, 317, 373–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 2028109. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wan, J.; Sun, H.; Li, L. Investigation on the activation of coal gangue by a new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, D.; Chen, J.; Yang, N. Effect of heart activated and mechanical force activated method on coal gangue cementing performance. J. Build. Mater. 2011, 14, 371–375. [Google Scholar] [CrossRef]
- Hui-Teng, N.; Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Ern Hun, K.; Razi, H.M.; Yong-Sing, N. Formulation, mechanical properties and phase analysis of fly ash geopolymer with ladle furnace slag replacement. J. Mater. Res. Technol. 2021, 12, 1212–1226. [Google Scholar] [CrossRef]
- Yeoh, M.L.Y.; Ukritnukun, S.; Rawal, A.; Davies, J.; Kang, B.J.; Burrough, K.; Aly, Z.; Dayal, P.; Vance, E.R.; Gregg, D.J.; et al. Mechanistic impacts of long-term gamma irradiation on physicochemical, structural, and mechanical stabilities of radiation-responsive geopolymer pastes. J. Hazard. Mater. 2021, 407, 124805. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Wan, S.; Hu, B.; Tong, J.; Sun, H.; Chen, Y.; Zhang, J.; Hou, H. Immobilizatiaon of heavy metals in municipal solid waste incineration fly ash with red mud-coal gangue. J. Mater. Cycles Waste Manag. 2020, 22, 1953–1964. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, A. Review on property of geopolymer binder and its engineering application. J. Archit. Civ. Eng. 2020, 37, 13–38. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, X.; Zhang, D.; Wang, M. Geopolymer activator and its excitation principle. Inorg. Chem. Ind. 2020, 54, 16–20. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.; Chen, H.; Wang, J.; Shi, J.; Li, Z.; Yu, M. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Wang, Q.; Ni, W.; Li, K.; Fu, P.; Hu, W.; Li, Z. Feasibility of using fly ash–slag-based binder for mine backfilling and its associated leaching risks. J. Hazard. Mater. 2020, 400, 123191. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Hosseini, S.; Brake, N.A.; Nikookar, M.; Günaydın-Şen, Ö.; Snyder, H.A. Mechanochemically activated bottom ash-fly ash geopolymer. Cem. Concr. Compos. 2021, 118, 103976. [Google Scholar] [CrossRef]
- Heikal, M.; Nassar, M.Y.; El-Sayed, G.; Ibrahim, S.M. Physico-chemical, mechanical, microstructure and durability characteristics of alkali activated Egyptian slag. Constr. Build. Mater. 2014, 69, 60–72. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Qin, L.; Qu, B.; Shi, C.; Zhang, Z. Effect of Ca/Si Ratio on the formation and characteristics of synthetic aluminosilicate hydrate gels. Mater. Rep. 2020, 34, 12058–12064. [Google Scholar] [CrossRef]
- ISO 679: 2009; International Organization for Standardization. Cement-Test Methods-Determination of Strength. International Standards Organization: Geneva, Switzerland, 2009.








| Point | Ca | O | C | Na | Al | Si | Ca/Si | Al/Si | Na/Si |
|---|---|---|---|---|---|---|---|---|---|
| A | 9.78 | 47.14 | 30.05 | 0.93 | 4.86 | 7.24 | 1.35 | 0.67 | 0.13 |
| B | 13.79 | 44.94 | 28.32 | 3.15 | 3.51 | 6.29 | 2.19 | 0.56 | 0.50 |
| C | 10.77 | 48.52 | 23.65 | 3.24 | 5.38 | 8.44 | 1.28 | 0.64 | 0.38 |
| D | 14.61 | 43.67 | 14.61 | 1.97 | 4.82 | 7.79 | 1.88 | 0.62 | 0.25 |
| E | 5.45 | 28.13 | 55.11 | 2.54 | 1.86 | 6.57 | 0.83 | 0.28 | 0.39 |
| F | 8.10 | 36.55 | 41.11 | 3.15 | 2.62 | 8.45 | 0.96 | 0.31 | 0.37 |
| G | 3.50 | 24.38 | 64.66 | 1.96 | 2.00 | 3.50 | 1.0 | 0.57 | 0.56 |
| H | 7.00 | 29.13 | 49.31 | 1.17 | 3.58 | 9.81 | 0.71 | 0.36 | 0.12 |
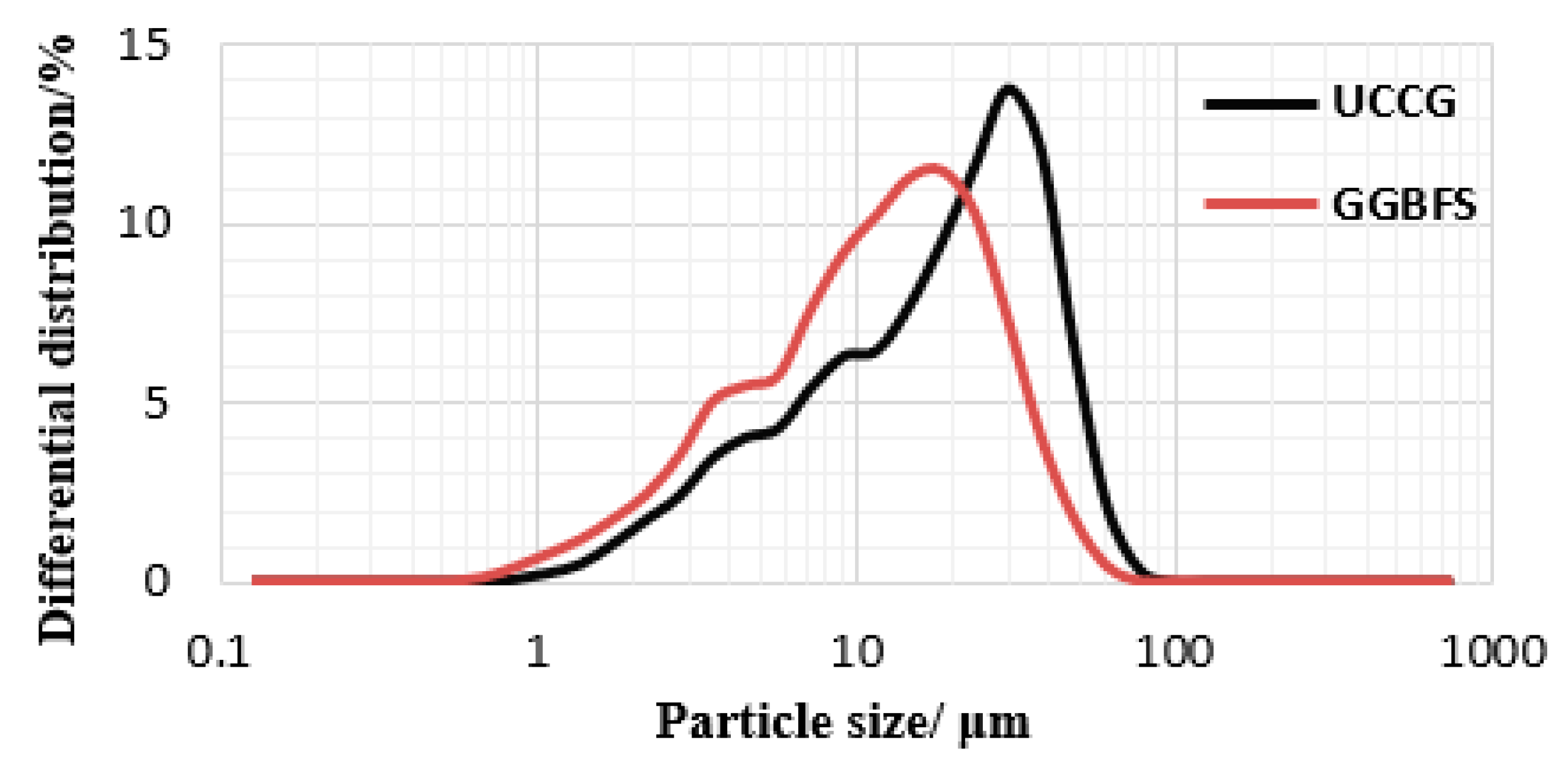
| Materials | D10 | D50 | D90 | D97 | D100 | D(3,2) | D(4,3) | Span |
|---|---|---|---|---|---|---|---|---|
| UCCG | 3.647 | 17.157 | 37.319 | 47.252 | 76.767 | 8.838 | 19.154 | 1.963 |
| GGBFS | 2.755 | 10.687 | 26.483 | 36.201 | 60.654 | 6.167 | 12.945 | 2.220 |
| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | K2O | Na2O | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| UCCG | 54.2 | 41.6 | 0.98 | 0.55 | 0.10 | 0.02 | 0.96 | 0.91 | 0.46 | 0.22 |
| GGBFS | 34.9 | 16.7 | 1.05 | 33.5 | 6.0 | 1.7 | - | - | - | 6.15 |
| Sample No. | SH1 | SH2 | SH3 | SH4 | SH5 | SH6 | SH7 | SH8 | SH9 | SH10 |
| GGBFS/% | 10 | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 20 | 20 |
| CNaOH/mol·L−1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Sample No. | SH11 | SH12 | SH13 | SH14 | SH15 | SH16 | SH17 | SH18 | SH19 | SH20 |
| GGBFS/% | 30 | 30 | 30 | 30 | 30 | 40 | 40 | 40 | 40 | 40 |
| CNaOH/mol·L−1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Sample No. | SSG1 | SSG2 | SSG3 | SSG4 | SSG5 | SSG6 | SSG7 | SSG8 |
| SS alkali/% | 10 | 10 | 10 | 10 | 12 | 12 | 12 | 12 |
| Na2SiO3 modulus | 0.6 | 0.8 | 1.0 | 1.2 | 0.6 | 0.8 | 1.0 | 1.2 |
| Sample No. | SSG9 | SSG10 | SSG11 | SSG12 | SSG13 | SSG14 | SSG15 | SSG16 |
| SS alkali/% | 14 | 14 | 14 | 14 | 16 | 16 | 16 | 16 |
| Na2SiO3 modulus | 0.6 | 0.8 | 1.0 | 1.2 | 0.6 | 0.8 | 1.0 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, Y.; Lin, C. Microstructure Analysis and Effects of Single and Mixed Activators on Setting Time and Strength of Coal Gangue-Based Geopolymers. Gels 2022, 8, 195. https://doi.org/10.3390/gels8030195
Yang X, Zhang Y, Lin C. Microstructure Analysis and Effects of Single and Mixed Activators on Setting Time and Strength of Coal Gangue-Based Geopolymers. Gels. 2022; 8(3):195. https://doi.org/10.3390/gels8030195
Chicago/Turabian StyleYang, Xiaoyun, Yan Zhang, and Cheng Lin. 2022. "Microstructure Analysis and Effects of Single and Mixed Activators on Setting Time and Strength of Coal Gangue-Based Geopolymers" Gels 8, no. 3: 195. https://doi.org/10.3390/gels8030195