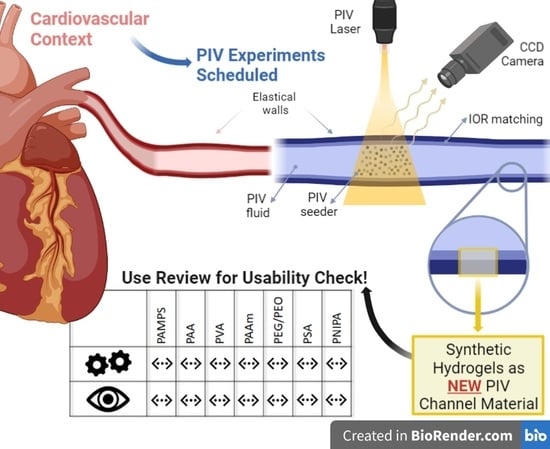
A Review on Novel Channel Materials for Particle Image Velocimetry Measurements—Usability of Hydrogels in Cardiovascular Applications
Abstract
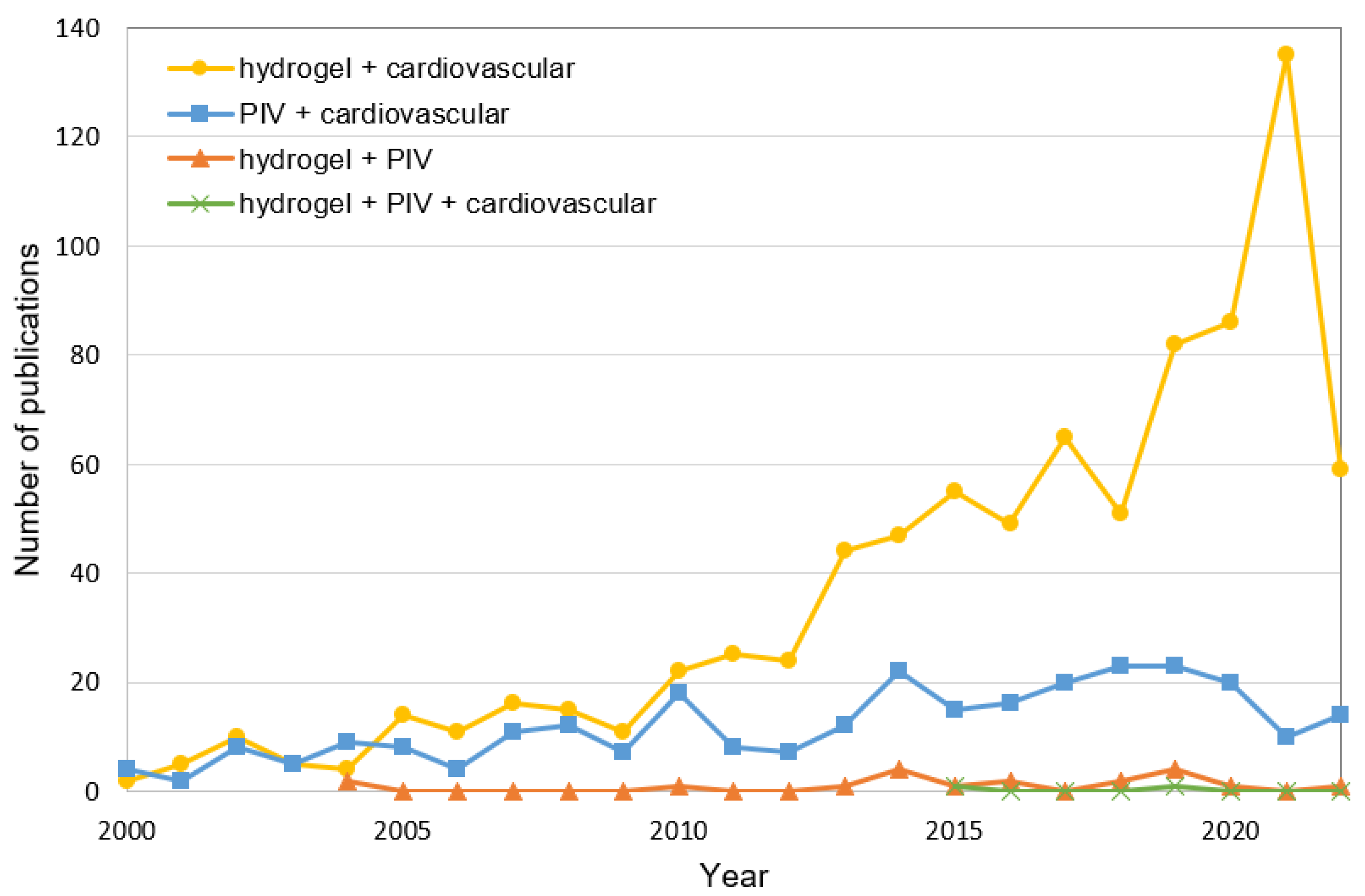
:1. Introduction
2. Particle Image Velocimetry
2.1. Refractive Index Matching
2.2. PIV for Cardiovascular Applications
3. Hydrogels
3.1. Hydrogel Synthesis
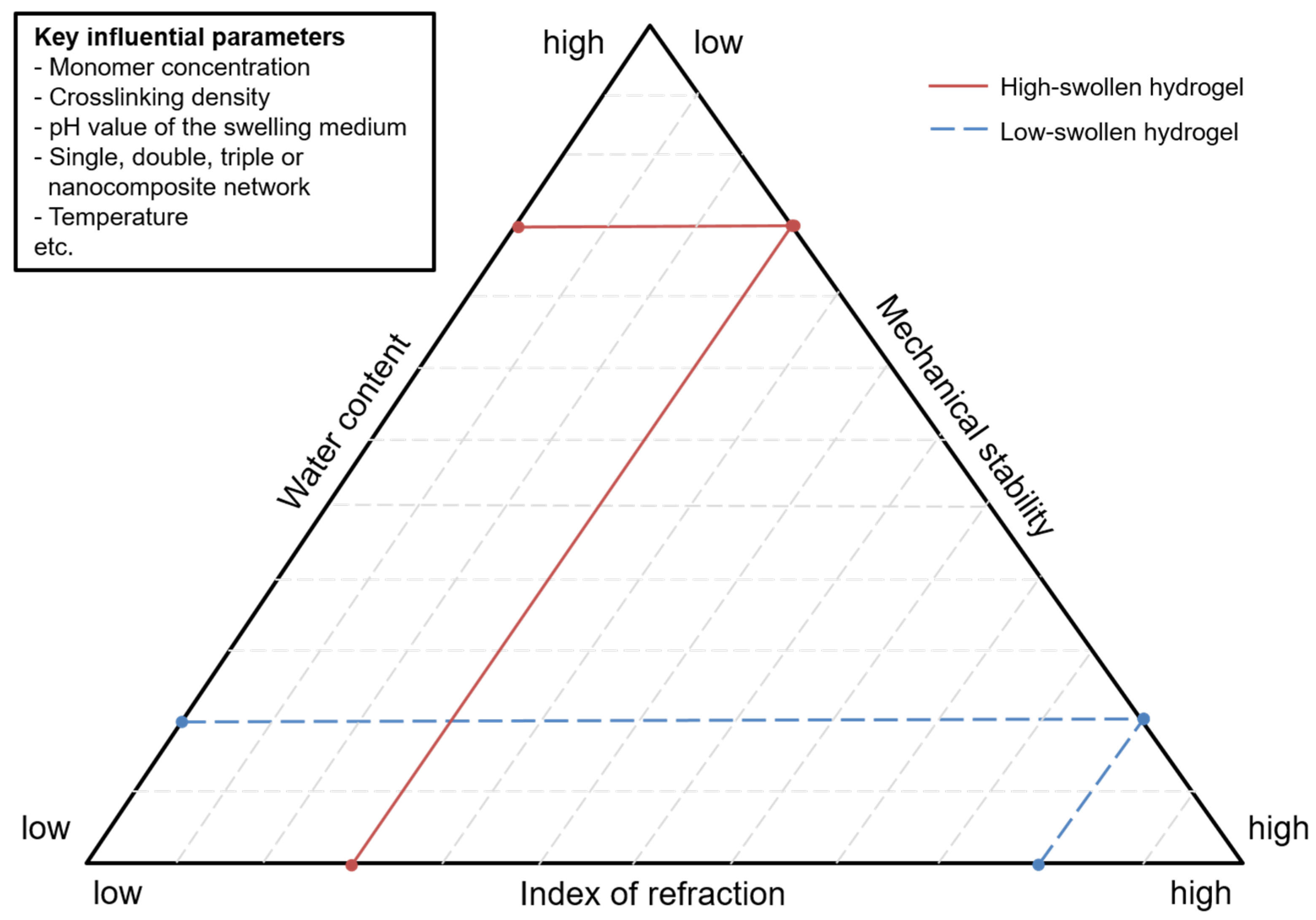
3.2. Hydrogel Swelling
3.3. Measurement Methods for Optical and Mechanical Hydrogel Properties
3.4. Advantages and Disadvantages of Utilizing Hydrogel as a PIV Channel Material
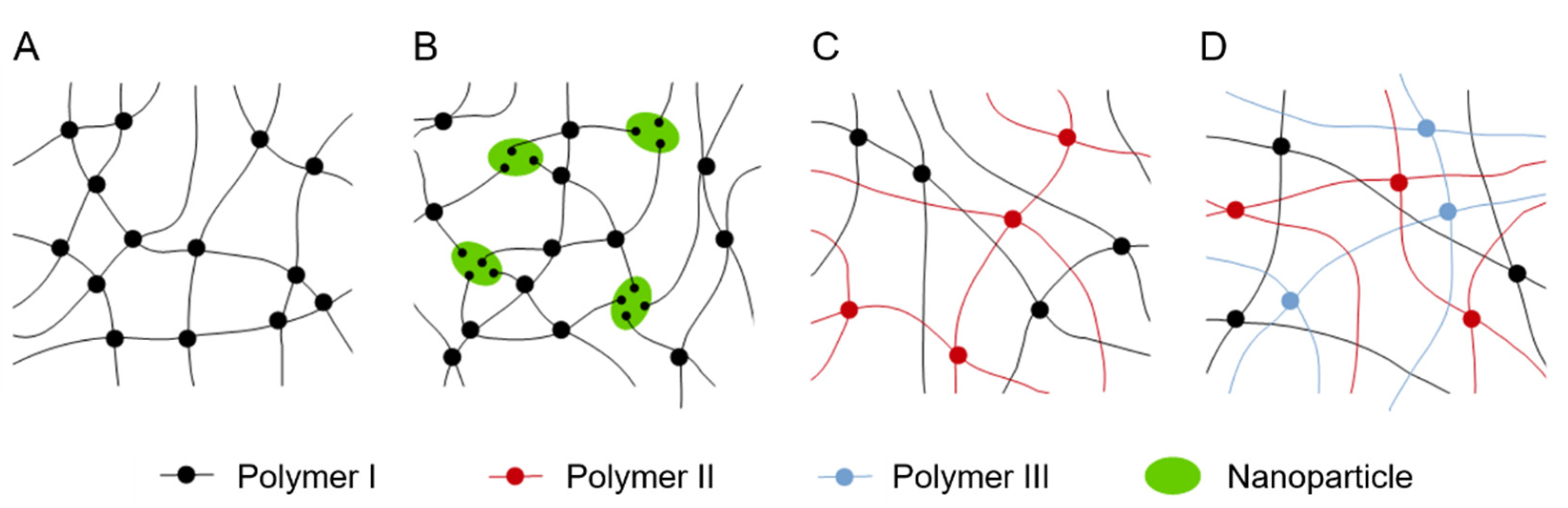
3.5. Double and Triple Networks and Nanocomposite Hydrogels
4. Hydrogels for PIV Channel Materials
4.1. Requirements for Optical Properties
4.2. Requirements for Mechanical Properties
- IOR: <1.55
- Light transmission: >90%
- Elastic modulus: all values
- Tensile/compressive stress at break: all values
- Nominal tensile/compressive strain at break: all values
- Water content: >50 wt%
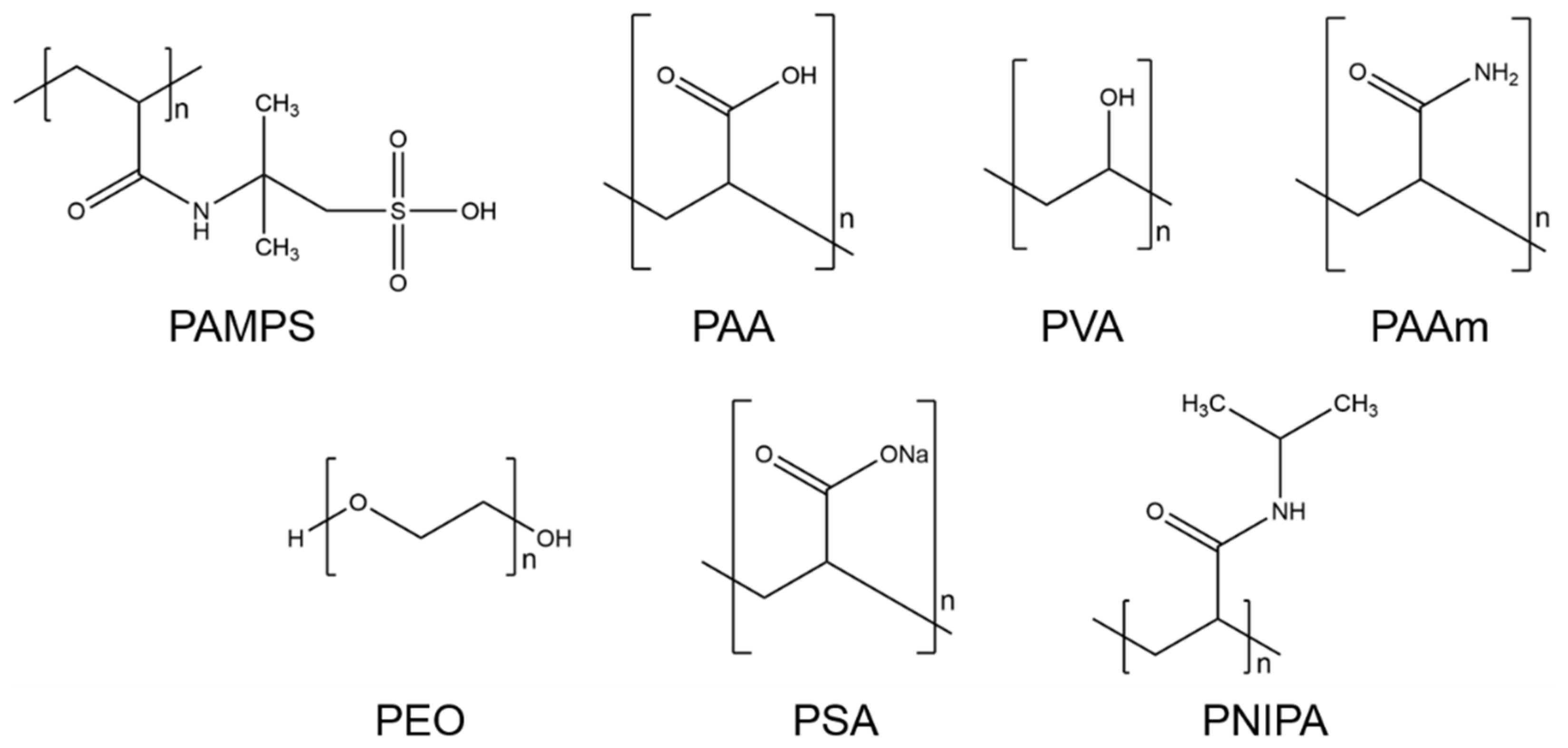
4.3. Selection of Hydrogels
- Poly-2-acrylamido-2-methyl-1-propanesulfonic acid (PAMPS)PAMPS is a synthetic polymer that consists of acrylic 2-Acrylamido-2-methylpropane sulfonic acid (AMPS). The chemical formula of PAMPS is (C7H13NO4S)n. This polymer dissolves well in pure water [71] and is hydrophilic [72]. Furthermore, PAMPS is a thermally stable homopolymer, which induces stability towards thermal degradation [71].
- Polyacrylic acid (PAA)PAA is the polymer of acrylic acid, a compound with the formula (C3H4O2)n. PAA exhibits high water retention, and upon absorbing water, it expands over its original size [73]. This hydrophilic property, as well as its propensity as an emulsifying agent, makes it widely marketable. It is commonly used in commercial products for its thickening and suspension properties, e.g., for disposable diapers, adhesives, paints, pharmaceutical drugs, and beauty products [73,74,75,76].
- Polyvinyl alcohol (PVA)The chemical formula of PVA is (C2H4O)n. This polymer is synthetic and highly water soluble. It is produced by the hydrolysis of polyvinyl acetone [73,76,77]. Furthermore, highly polar and hydrophilic solvents can be used to dissolve PVA [73]. This polymer is typically used for rigid and clear optical films, adhesives, and transdermal drug delivery systems. Because of its excellent physical and chemical properties, such as high biocompatibility, low toxicity, and being chemically inert, PVA is broadly used in industrial applications [73,77].
- Polyacrylamide (PAAm)PAAm can be synthesized from the monomer acrylamide by free-radical polymerization [73,76]. The chemical formula is (C3H5NO)n. This polymer can be used as a superabsorbent material. Lightly cross-linked PAAm can absorb and retain large amounts of water and forms a soft gel when saturated [78]. It has other excellent properties for industrial use. For example, PAAm is chemically inert, has low toxicity, and is stable in a wide pH-value range [73,76].
- Polyethylene glycol (PEG) and polyethylene oxide (PEO)PEG with low molecular weight (200 to 20,000 g/mol [79]) is an organic epoxide with the formula (C2H4O)n [80]. The polymer is known as PEO for higher molecular weights up to 5 million g/mol [79]. Because of its low toxicity, PEG is one of the most used synthetic hydrogels in biomedical applications [73]. PEG polymers are water soluble and can be coupled with hydrophobic molecules to act as surfactants. These polymers are also soluble in methanol, ethanol, benzene, acetonitrile, and dichloromethane [81].
- Sodium polyacrylate (PSA)This cross-linked PSA, with the chemical formula (C3H3NaO2)n, is a sodium salt of polyacrylic acid produced by free-radical polymerization [82]. This polymer can absorb a large amount of water because it contains ions, such as carboxyl groups and sodium, in the polymer chain [83]. These give PSA hydrophilic properties that allow it to be classified as a superabsorbent polymer. PSA is widely used in commercial applications, such as cosmetic products, and in general, e.g., in diapers as a thickening agent and in coatings [82,83].
- Poly-N-isopropyl acrylamide (PNIPA)PNIPA is one of the most often utilized temperature-sensitive hydrogels and has the formula (C6H11NO)n [84]. PNIPA changes its shape by undergoing a discontinuous phase transition at a critical temperature. When this occurs, the polymer chains change from hydrophobic to hydrophilic behavior and make the hydrogel swell. In addition, PNIPA is a biocompatible polymer. Therefore, its applications are found in the biomedical and optical fields [85].
5. Review of the Optical and Mechanical Properties of the Selected Hydrogels
6. Discussion
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raffel, M.; Willert, C.E.; Wereley, S.T.; Kompenhans, J. Principle of Particle Image velocimetry (PIV). In Particle Image Velocimetry-A Practical Guide, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; pp. 3–8. [Google Scholar]
- Wang, C.; Liu, J.; Li, J.; Guo, Y.; Jiang, N. Turbulence characterization of instantaneous airflow in an aisle of an aircraft cabin mockup. Build Environ. 2017, 116, 207–217. [Google Scholar] [CrossRef]
- Firat, E.; Ozkan, G.M.; Akilli, H. PIV measurements in the near wakes of hollow cylinders with holes. Exp. Fluids 2017, 58, 39. [Google Scholar] [CrossRef]
- Bellani, G.; Variano, E.A. Slip velocity of large neutrally buoyant particles in turbulent flows. New J. Phys. 2012, 14, 125009. [Google Scholar] [CrossRef]
- Owida, A.; Do, H.; Yang, W.; Morsi, Y.S. PIV Measurements and Numerical Validation of End-To-Side Anastomosis. J. Mech. Med. Biol. 2010, 10, 123–138. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 10 July 2022).
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2020: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2021, 69, 294–307. [Google Scholar] [CrossRef]
- Engel, C. Untersuchung der Laufradströmung in einem Radialventilator mittels Particle Image Velocimetry (PIV). Ph.D. Thesis, University Duisburg-Essen, Duisburg, Germany, 12 July 2007. [Google Scholar]
- Raffel, M.; Willert, C.E.; Wereley, S.T.; Kompenhans, J. Examples of Application. In Particle Image Velocimetry-A Practical Guide, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; pp. 259–388. [Google Scholar]
- Bai, K.; Katz, J. On the refractive index of sodium iodide solutions for index matching in PIV. Exp. Fluids 2014, 55, 1704. [Google Scholar] [CrossRef]
- Reis, J.C.R.; Lampreia, I.M.S.; Santos, Â.F.S.; Moita, M.L.C.J.; Douhéret, G. Refractive Index of Liquid Mixtures: Theory and Experiment. Chem. Phys. Chem. 2010, 11, 3722–3733. [Google Scholar] [CrossRef]
- Thormählen, I.; Straub, J.; Grigull, U. Refractive Index of Water and Its Dependence on Wavelength, Temperature, and Density. J. Phys. Chem. Ref. Data 1985, 14, 933–945. [Google Scholar] [CrossRef]
- Hamann, C.; Kurth, S.; Mulleners, K. Towards quantifying effects of refractive index mismatch on PIV results. In Proceedings of the 11th Symposium on Particle Image Velocimetry-PIV15, Santa Barbara, CA, USA, 14–16 September 2015. [Google Scholar]
- Ong, C.W.; Kabinejadian, F.; Xiong, F.; Wong, Y.R.; Toma, M.; Nguyen, Y.N.; Chua, K.J.; Cui, F.S.; Ho, P.; Leo, H. Pulsatile flow investigation in development of thoracic aortic aneurysm: An in-vitro validated fluid structure interaction analysis. J. Appl. Fluid Mech. 2019, 12, 1855–1872. [Google Scholar] [CrossRef]
- Stanley, N.; Ciero, A.; Timms, W.; Hewlin, R. Development of 3-D Printed Optically Clear Rigid Anatomical Vessels for Particle Image Velocimetry Analysis in Cardiovascular Flow. ASME Int. Mech. Eng. Con. Expo. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Franzetti, G.; Bonfanti, M.; Homer-Vanniasinkam, S.; Diaz-Zuccarini, V.; Balabani, S. Experimental evaluation of the patient-specific haemodynamics of an aortic dissection model using particle image velocimetry. J. Biomech. 2022, 134, 110963. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Biadillah, Y.; Mongrain, R.; Brunette, J.A.; Tardif, J.-C.; Bertrand, O.F. A Method for Matching the Refractive Index and Kinematic Viscosity of a Blood Analog for Flow Visualization in Hydraulic Cardiovascular Models. J. Biomech. Eng. 2004, 126, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ruedinger, K.L.; Medero, R.; Roldán-Alzate, A. Fabrication of Low-Cost Patient-Specific Vascular Models for Particle Image Velocimetry. Cardiovasc. Eng. Technol. 2019, 10, 500–507. [Google Scholar] [CrossRef]
- Yousif, M.Y.; Holdsworth, D.W.; Poepping, T.L. Deriving a Blood-Mimicking Fluid for Particle Image Velocimetry in Sylgard-184 Vascular Models. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 1412–1415. [Google Scholar] [CrossRef]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M.; Edwards, A.; Carson, R.J. The mechanical behavior of vascular grafts: A review. J. Biomater. Appl. 2001, 15, 241–278. [Google Scholar] [CrossRef]
- Najjari, M.R.; Hinke, J.A.; Bulusu, K.V.; Plesniak, M.W. On the rheology of refractive-index-matched, non-Newtonian blood-analog fluids for PIV experiments. Exp. Fluids 2016, 57, 96. [Google Scholar] [CrossRef]
- Goldsmith, H.L.; Turitto, V.T. Rheological Aspects of Thrombosis and Haemostasis: Basic Principles and Applications. Thromb. Haemost. 1986, 55, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Abd Alla, S.G.; Sen, M.; El-Naggar, A.W.M. Swelling and mechanical properties of superabsorbent hydrogels based on Tara gum/acrylic acid synthesized by gamma radiation. Carbohydr. Polym. 2012, 89, 478–485. [Google Scholar] [CrossRef]
- Chang, C.J.; Swift, G. Poly(Aspartic Acid) Hydrogel. J. Macromol. Sci. Part A 1999, 36, 963–970. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook, 1st ed.; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer Science+Business Media: New York, NY, USA; Dordrecht, The Netherlands; Heidelberg, Germany; London, UK, 2010; pp. 1–16. [Google Scholar]
- Molchanov, V.S.; Shibaev, A.V.; Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Philippova, O.E.; Khokhlov, A.R. Antiseptic Polymer–Surfactant Complexes with Long-Lasting Activity against SARS-CoV-2. Polymers 2022, 14, 2444. [Google Scholar] [CrossRef]
- Ratner, B.; Hoffman, A. Synthetic hydrogels for biomedical applications. In Hydrogels for Medical and Related Applications, 1st ed.; Andrade, J.D., Ed.; American Chemical Society: Washington, DC, USA, 1976; Volume 31, pp. 1–36. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug. Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Peppas, N.A. Hydrogels. In Biomaterials Science-An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: London, UK; San Diego, CA, USA, 2004; pp. 100–107. [Google Scholar]
- Feil, H.; Bae, H.; Feijen, J. Mutual Influence of pH and Temperature on the Swelling of Ionizable and Thermosensitive Hydrogels. Macromolecules 1992, 25, 5528–5530. [Google Scholar] [CrossRef]
- Esch, C.M.; Galperin, A.; Krolitzki, B.; Glasmacher, B.; Shen, A.; Ratner, B.D. Proof of Concept of a New Glucose Sensing Technology: Color-Changing Hydrogels Including Au Nanoparticles. Biomed. Tech. 2013, 58, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Liao, L.; Li, X.; Cui, Y. Study on the Swelling, Shrinking and Bending Behavior of Electric Sensitive Poly(2-acrylamido-2-methylpropane sulfonic acid) Hydrogel. Mod. Appl. Sci. 2009, 3, 3091–3093. [Google Scholar] [CrossRef]
- Hussain, S. Textbook of Dental Materials, 1st ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2008. [Google Scholar]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Maciel Filho, R. pHEMA hydrogels: Synthesis, kinetics and in vitro tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Weitzman, J.S.; Samuel, L.C.; Craig, A.E.; Zeller, R.B.; Monismith, S.G.; Koseff, J.R. On the use of refractive-index-matched hydrogel for fluid velocity measurement within and around geometrically complex solid obstructions. Exp. Fluids 2014, 55, 1862. [Google Scholar] [CrossRef]
- Franklin, J.; Wang, Z.Y. Refractive index matching: A general method for enhancing the optical clarity of a hydrogel matrix. Chem. Mater. 2002, 14, 4487–4489. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter. 2012, 8, 6048–6056. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018, 28, 1802576. [Google Scholar] [CrossRef]
- Pu, X.; Liu, M.; Chen, X.; Sun, J.; Du, C.; Zhang, Y.; Zhai, J.; Hu, W.; Wang, Z.L. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Shams Es-haghi, S.; Offenbach, I.; Debnath, D.; Weiss, R.A.; Cakmak, M. Mechano-optical behavior of loosely crosslinked double-network hydrogels: Modeling and real-time birefringence measurement during uniaxial extension. Polymer 2017, 115, 239–245. [Google Scholar] [CrossRef]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators-Springer Series on Chemical Sensors and Biosensors, 1st ed.; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–14. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Jorsch, C. Implantierbare Sensoren auf Hydrogelbasis. Ph.D. Thesis, Technischen Universität Dresden, Dresden, Germany, 12 May 2017. [Google Scholar]
- Tummala, G.K.; Rojas, R.; Mihranyan, A. Poly(vinyl alcohol) Hydrogels Reinforced with Nanocellulose for Ophthalmic Applications: General Characteristics and Optical Properties. J. Phys. Chem. B 2016, 120, 13094–13101. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.; Noolandl, J.; Ta, C.; Frank, C.W. Interpenetrating Polymer Network Hydrogel Contact Lenses. U.S. Patent 7857447 B2, 28 December 2010. [Google Scholar]
- Myung, D.; Koh, W.; Bakri, A.; Zhang, F.; Marshall, A.; Ko, J.; Noolandi, J.; Carrasco, M.; Cochran, J.R.; Frank, C.W.; et al. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices 2007, 9, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Horie, K. Large Photoinduced Refractive Index Changes of Transparent Polymer Films Containing Photoeliminable Diazo and Azido Groups. Macromolecules 1999, 32, 1103–1110. [Google Scholar] [CrossRef]
- Dai, T.; Qing, X.; Lu, Y.; Xia, Y. Conducting hydrogels with enhanced mechanical strength. Polymer 2009, 50, 5236–5241. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Su, H.W.; Chen, W.C. Photosensitive high-refractive-index poly(acrylic acid)-graft-poly(ethylene glycol methacrylate) Nanocrystalline Titania hybrid films. Macromol. Chem. Phys. 2008, 209, 1778–1786. [Google Scholar] [CrossRef]
- Bose, R.K.; Lau, K.K.S. Mechanical properties of ultrahigh molecular weight PHEMA hydrogels synthesized using initiated chemical vapor deposition. Biomacromolecules 2010, 11, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Li, H.J. Control of the coil-to-globule transition and ultrahigh mechanical properties of PNIPA in nanocomposite hydrogels. Angew. Chem.-Int. Ed. 2005, 44, 6500–6504. [Google Scholar] [CrossRef]
- Zhao, Y.; Ju, X.J.; Zhang, L.P.; Wang, W.; Faraj, Y.; Zou, L.B.; Xie, R.; Liu, Z.; Chu, L.Y. Transparent thermo-responsive poly(N-isopropylacrylamide)-l-poly(ethylene glycol)acrylamide conetwork hydrogels with rapid deswelling response. New J. Chem. 2019, 43, 9507–9515. [Google Scholar] [CrossRef]
- Su, E.; Okay, O. Hybrid cross-linked poly(2-acrylamido-2-methyl-1-propanesulfonic acid) hydrogels with tunable viscoelastic, mechanical and self-healing properties. React. Funct. Polym. 2018, 123, 70–79. [Google Scholar] [CrossRef]
- Can, V.; Abdurrahmanoglu, S.; Okay, O. Unusual swelling behavior of polymer-clay nanocomposite hydrogels. Polymer 2007, 48, 5016–5023. [Google Scholar] [CrossRef]
- Kizilay, M.Y.; Okay, O. Effect of initial monomer concentration on spatial inhomogeneity in poly(acrylamide) gels. Macromolecules 2003, 36, 6856–6862. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pereira, B.P.; Yusof, N.; Selvaratnam, L.; Yu, Z.; Abbas, A.A.; Kamarul, T. Unconfined compression properties of a porous poly(vinyl alcohol)-chitosan-based hydrogel after hydration. Acta Biomater. 2009, 5, 1919–1925. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K.; Kandalam, U.; Rocca, J.G. Swelling and Mechanical Properties of Modified HEMA-based Superporous Hydrogels. J. Bioact. Compat. Polym. 2010, 25, 483–497. [Google Scholar] [CrossRef]
- Okay, O.; Sariisik, S.B. Swelling behavior of poly (acrylamide-co-sodium acrylate) hydrogels in aqueous salt solutions: Theory versus experiments. Eur. Polym. J. 2000, 36, 393–399. [Google Scholar] [CrossRef]
- Tanaka, Y.; Gong, J.P.; Osada, Y. Novel hydrogels with excellent mechanical performance. Prog. Polym. Sci. 2005, 30, 1–9. [Google Scholar] [CrossRef]
- Baker, J.P.; Hong, L.H.; Blanch, H.W.; Prausnitz, J.M. Effect of Initial Total Monomer Concentration on the Swelling Behavior of Cationic Acrylamide-Based Hydrogels. Macromolecules 1994, 27, 1446–1454. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Oktamuliani, S.; Kanno, N.; Maeda, M.; Hasegawa, K.; Saijo, Y. Validation of Echodynamography in Comparison with Particle-image Velocimetry. Ultrason Imaging 2019, 41, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ohta, M. Influence of plaque stiffness on deformation and blood flow patterns in models of stenosis. Biorheology 2015, 52, 171–182. [Google Scholar] [CrossRef]
- Yousif, M.Y.; Holdsworth, D.W.; Poepping, T.L. A blood-mimicking fluid for particle image velocimetry with silicone vascular models. Exp. Fluids 2011, 50, 769–774. [Google Scholar] [CrossRef]
- Barker, H.P. Optical Coating Having Low Refractive Index. Patent Application No. EP 1336123 A2, 20 August 2003. [Google Scholar]
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2013, 9, 861–888. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–17.
- Aggour, Y.A. Thermal stability of poly (2-acrylamido-2-methylpropanesulphonic acid) and polymer complexes of 2-acrylamido-2-methylpropanesulphonic acid with some transition metal salts. Polym. Degrad. Stab. 1994, 44, 97–99. [Google Scholar] [CrossRef]
- Atta, A.M. Swelling behaviors of polyelectrolyte hydrogels containing sulfonate groups. Polym. Adv. Technol. 2002, 13, 567–576. [Google Scholar] [CrossRef]
- Erothu, H.; Kumar, A.C. Hydrophilic Polymers. In Biomedical Applications of Polymeric Materials and Composites, 1st ed.; Francis, R., Kumar, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 163–185. [Google Scholar] [CrossRef]
- Saeed, A.M. Temperature effect on swelling properties of commercial polyacrylic acid hydrogel beads. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1614–1627. [Google Scholar]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook, 1st ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar] [CrossRef]
- Jaffe, H.L.; Rosenblum, F.M. Poly(Vinyl Alcohol) for Adhesives. In Handbook of Adhesives, 3rd ed.; Skeist, I., Ed.; Chapman & Hall: New York, NY, USA, 1990; pp. 401–407. [Google Scholar]
- Chanda, M.; Roy, S.K. Industrial Polymers. In Industrial Polymers, Specialty Polymers and Their Applications, 1st ed.; CRC Press Taylor & Francis Group: New York, NY, USA, 2008; pp. 1–161. [Google Scholar]
- Webb, S.W.; Stanley, D.A.; Scheiner, B.J. An Infrared Examination of Ion-Exchanged Montmorillonite Treated with Polyethylene Oxide-Report of Investigation 9036; U.S. Department of the Interior, Bureau of Mines: Pittsburgh, PA, USA, 1986; pp. 1–16.
- Flory, J. Molecular Size Distribution in Ethylene Oxide Polymers. J. Am. Chem. Soc. 1940, 62, 1561–1565. [Google Scholar] [CrossRef]
- Winger, M.; de Vries, A.H.; van Gunsteren, W.F. Force-field dependence of the conformational properties of α,ω-dimethoxypolyethylene glycol. Mol. Phys. 2009, 107, 1313–1321. [Google Scholar] [CrossRef]
- Intratec. Starch-Graft Sodium Polyacrylate Production-Report Sodium Polyacrylate E11A-Basic Cost Analysis; Intratec Solutions LLC: San Antonio, TX, USA, 2019; pp. 1–36. [Google Scholar]
- Gooch, J.W. Particulate Applied Barrier Dressing. In Biocompatible Polymeric Materials and Tourniquets for Wounds, 1st ed.; Springer Science+Business Media: New York, NY, USA, 2010; pp. 32–47. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase Transition of Aqueous Solutions of Poly(N-isopropylacrylamide) and Poly(N-isoprop ylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Garner, B.W.; Cai, T.; Ghosh, S.; Hu, Z.; Neogi, A. Refractive index change due to volume-phase transition in polyacrylamide gel nanospheres for optoelectronics and bio-photonics. Appl. Phys. Express 2009, 2, 057001. [Google Scholar] [CrossRef]
- Shi, X.; Xu, S.; Lin, J.; Feng, S.; Wang, J. Synthesis of SiO2-polyacrylic acid hybrid hydrogel with high mechanical properties and salt tolerance using sodium silicate precursor through sol-gel process. Mater. Lett. 2009, 63, 527–529. [Google Scholar] [CrossRef]
- Harrass, K.; Krüger, R.; Möller, M.; Albrecht, K.; Groll, J. Mechanically strong hydrogels with reversible behaviour under cyclic compression with MPa loading. Soft Matter 2013, 9, 2869–2877. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Zhou, L.; Chen, S. Mechanical properties, water-swelling behavior, and morphology of water-swellable rubber prepared using crosslinked sodium polyacrylate. J. Appl. Polym. Sci. 2006, 102, 1489–1496. [Google Scholar] [CrossRef]
- Lin, H.-R.; Ling, M.-H.; Lin, Y.-J. High strength and low friction of a PAA-alginate-silica hydrogel as potential material for artificial soft tissues. J. Biomater. Sci. Polym. Ed. 2009, 20, 637–652. [Google Scholar] [CrossRef]
- Kaneko, D.; Tada, T.; Kurokawa, T.; Gong, J.P.; Osada, Y. Mechanically strong hydrogels with ultra-low frictional coefficients. Adv. Mater. 2005, 17, 535–538. [Google Scholar] [CrossRef]
- Kajiya, T.; Daerr, A.; Narita, T.; Royon, L.; Lequeux, F.; Limat, L. Dynamics of the contact line in wetting and diffusing processes of water droplets on hydrogel (PAMPS–PAAM) substrates. Soft Matter 2011, 7, 11425–11432. [Google Scholar] [CrossRef]
- Refractive Index of Polymers by Index. Available online: http://scientificpolymer.com/technical-library/refractive-index-of-polymers-by-index/ (accessed on 8 July 2022).
- Hyon, S.H.; Cha, W.I.; Ikada, Y. Preparation of transparent poly(vinyl alcohol) hydrogel. Polym. Bull. 1989, 22, 119–122. [Google Scholar] [CrossRef]
- Gu, Z.Q.; Xiao, J.M.; Zhang, X.H. The development of artificial articular cartilage-PVA-hydrogel. Biomed. Mater. Eng. 1998, 8, 75–81. [Google Scholar] [PubMed]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Ferruzzi, G.G.; Pan, N.; Casey, W.H. Mechanical Properties of Gellan and Polyacrylamide Gels With Implications for Soil Stabilization. Soil Sci. 2011, 165, 778–792. [Google Scholar] [CrossRef]
- Chen, P.; Wu, R.; Wang, J.; Liu, Y.; Ding, C.; Xu, S. One-pot preparation of ultrastrong double network hydrogels. J. Polym. Res. 2012, 19, 9825. [Google Scholar] [CrossRef]
- Sultana, S.; Sumon, K.; Noor, H.; Ajmotgir, W.; Sarker, K.; Hasan, R. Swelling and Physico-Mechanical Properties of Synthesized Sodium Polyacrylate Hydrogels. Int. J. Adv. Res. 2017, 5, 84–92. [Google Scholar] [CrossRef]
- Fei, R.; George, J.T.; Park, J.; Means, A.K.; Grunlan, M.A. Ultra-strong thermoresponsive double network hydrogels. Soft Matter 2013, 9, 2912. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Hashemi, S.A.; Zohuriaan-Mehr, M.J. Synthesis of fast-swelling superabsorbent hydrogels: Effect of crosslinker type and concentration on porosity and absorption rate. Eur. Polym. J. 2003, 39, 1341–1348. [Google Scholar] [CrossRef]



| Property | Measurement Method | Description | Relevance to PIV Channel Material |
|---|---|---|---|
| Optical | |||
| Index of refraction (IOR) | Refractometry [35,40,44,45,46,47] | Determination of the angle of refraction by the change in light direction in different materials | IOR matching between flow channel material and fluid |
| Infrared absorption | Fourier transform infrared spectroscopy (FTIR) [37,48,49,50,51,52] | Measuring the infrared absorption and emission spectra | Chemical hydrogel composition and structure |
| Raman scattering | Raman spectroscopy [48] | Measuring the inelastic scattering of monochromatic light on molecules or solids | Chemical hydrogel composition and structure |
| Light absorption | Ultraviolet and visible spectroscopy (UV/VIS) [38,39,53] | Light absorption in the visible and ultraviolet radiation range caused by electron transitions between different states in the molecule | Chemical hydrogel composition and structure; transparency of hydrogel |
| Mechanical | |||
| Tensile/compressive stress | Universal testing machine (UTM) [37,48,49,52,53,54,55,56,57,58,59] | Determining the behavior of material samples under axial, tensile, or compression load | Mechanical durability and stiffness depending on hydration |
| Water vapor uptake and submission | Dynamic vapor sorption (DVS) [49,51,55,56,59,60] | Measuring material absorbability by varying the surrounding water vapor concentration | Hydrogel swelling and shrinking |
| Elastic Modulus in MPa | Tensile Stress in MPa | Tensile Strain in % | |
|---|---|---|---|
| Physiological | 0.85–1.75 | 0.51–3.08 | 28–91 |
| Pathological | 3.13–4.27 | 1.11–3.59 | 27–60 |
| Elastic Modulus in MPa | Tensile (*)/Compressive Stress at Break in MPa | Nominal Tensile (*)/Compressive Strain at Break in % | Water Content in wt.% | Index of Refraction | Light Transmission in % | Ref. | |
|---|---|---|---|---|---|---|---|
| Poly-2-acrylamido-2-methyl-1-propanesulfonic acid (PAMPS) | |||||||
| PAMPS/PAAm PAMPS/PAAm PAMPS/PAAm + silica nano- particle PAMPS/PAAm/PAMPS (cross-linked) PAMPS/PAAm/PAMPS (non-cross-linked) PAMPS/PAMPS PAMPS/PAA PAMPS/PTFEA PAMPS/PTFEA/PAAm PAMPS/MBAm + laponite PAMPS/PAAm | 0.84 - 0.06–0.33 2 2.1 - - - - 0.69 - | 4.6 17.2 18.6–73.5 4.8 9.2 3 2.3 1.6 * 21 27 - | 65 92 94–97 57 70 80 75 4.9 * 97 - - | 84.8 90 - 82.5 84.8 93 92 52 93 - - | - - - - - - - - - - 1.346–1.350 | - - - - - - - - - - - - | [90] [49] [37] [90] [90] [49] [49] [49] [49] [54] [91] |
| Polyacrylic acid (PAA) | |||||||
| PAA/PAAm PAA/alginate PAA/alginate + silica nano- particles PAA + sodium silicate | - - - 0.0128–0.0456 | 2.1 1.32 7.72–9.73 - | 95 82.81 47.63–75.33 - | 89 98.5 98.1–98.2 99.1–99.8 | - - - - | - - - - | [49] [89] [89] [86] |
| PAA PAA/PEGMA + nanotitania hybrid film | - - | - - | - - | - - | 1.527 1.501–1.528 | - - | [92] [50] |
| Polyvinyl alcohol (PVA) | |||||||
| PVA | - | 2.45 * | 650 * | 85 | - | - | [93] |
| PVA | 0.38–2.28 * and 8.99–14.84 | 2.23–4.47 * | 207.8–317.4 * | 78.4–86.5 | - | - | [94] |
| PVA + saline | 0.7–18.4 | 1.4–2.1 | 45–62 | 75–80 | - | - | [95] |
| PVA + nanocellulose | - | - | - | 90.7–94.2 | 1.3330–1.3359 | - | [44] |
| Polyacrylamide (PAAm) | |||||||
| PAAm PAAm/PAAm PAAm/sPEOPO PAAm/PVA PAAm PAAm + sucrose PAAm PAAm/PAAm | 0.63 * - 11.6–59.1 0.062–0.087 - - - - | 1.1 * 5.4 2.0–5.6 - - - - - | 81 * 92 88.6–93.2 469–500 * - - - - | - 92 92.3–95.2 - 89.8 - 75–95 92.23 | - - - - - 1.385–1.420 1.338–1.380 1.343 | - - - 92 98.2–98.9 - - - | [96] [49] [87] [38] [39] [36] [68] [40] |
| Polyethylene glycol (PEG) and oxide (PEO) | |||||||
| PEG/PAA PEG/PAA PEG/PAA PEG-DA/PAA PEG-DA/MPEG PEO PEO/PEG | 0.5–1.5 * - - - - - - | 2–13 * 2.5–10.9 1.1 * 8 - - - | - 93.8–97.2 - 90 - - - | 83–99 90 85 - 50–95 80–95 - | 1.35 - 1.35 - 1.3388–1.4136 1.339–1.356 1.4539/1.459 | 90 - 96 - 97.6–100 - - | [45] [97] [46] [63] [68] [68] [92] |
| Sodium polyacrylate (PSA) | |||||||
| PSA PSA/PAA/PBA PSA/PAAm | - - - | 0.2–2.2 * 1.1–7.7 * - | 5–115 * 1170–1730 * - | - - 80.79–99.02 | - - 1.3327 | - - - | [98] [88] [35] |
| Poly-N-isopropyl acrylamide (PNIPA) | |||||||
| PNIPA + inorganic clay P(NIPA-co-AMPS)/PNIPA PNIPAm/PEGAAm PNIPA | 0.4 * 0.085–0.311 4.10 - | 1 * 2.532–17.50 0.175 * - | 1000 * 71–95 56 * - | 80–90 - 80 - | - - - 1.32–1.39 | - - 90 - | [52] [99] [85] [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, C.M.; Kuhn, A.I.; Hentschel, G.; Glasmacher, B. A Review on Novel Channel Materials for Particle Image Velocimetry Measurements—Usability of Hydrogels in Cardiovascular Applications. Gels 2022, 8, 502. https://doi.org/10.3390/gels8080502
Winkler CM, Kuhn AI, Hentschel G, Glasmacher B. A Review on Novel Channel Materials for Particle Image Velocimetry Measurements—Usability of Hydrogels in Cardiovascular Applications. Gels. 2022; 8(8):502. https://doi.org/10.3390/gels8080502
Chicago/Turabian StyleWinkler, Christina Maria, Antonia Isabel Kuhn, Gesine Hentschel, and Birgit Glasmacher. 2022. "A Review on Novel Channel Materials for Particle Image Velocimetry Measurements—Usability of Hydrogels in Cardiovascular Applications" Gels 8, no. 8: 502. https://doi.org/10.3390/gels8080502