An Attempt to Relate Oleogel Properties to Wax Ester Chemical Structures
Abstract
:1. Introduction
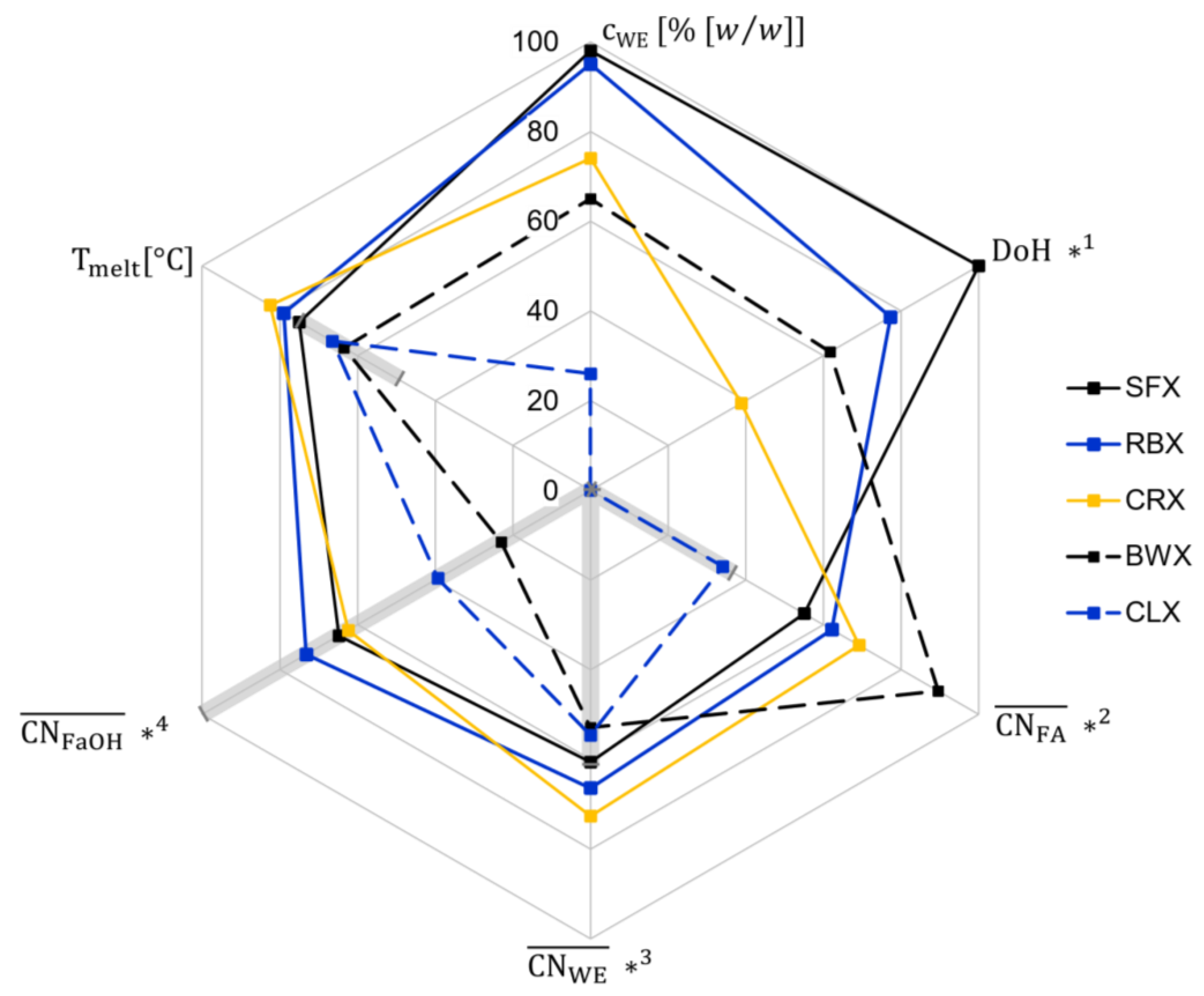
2. Results and Discussion
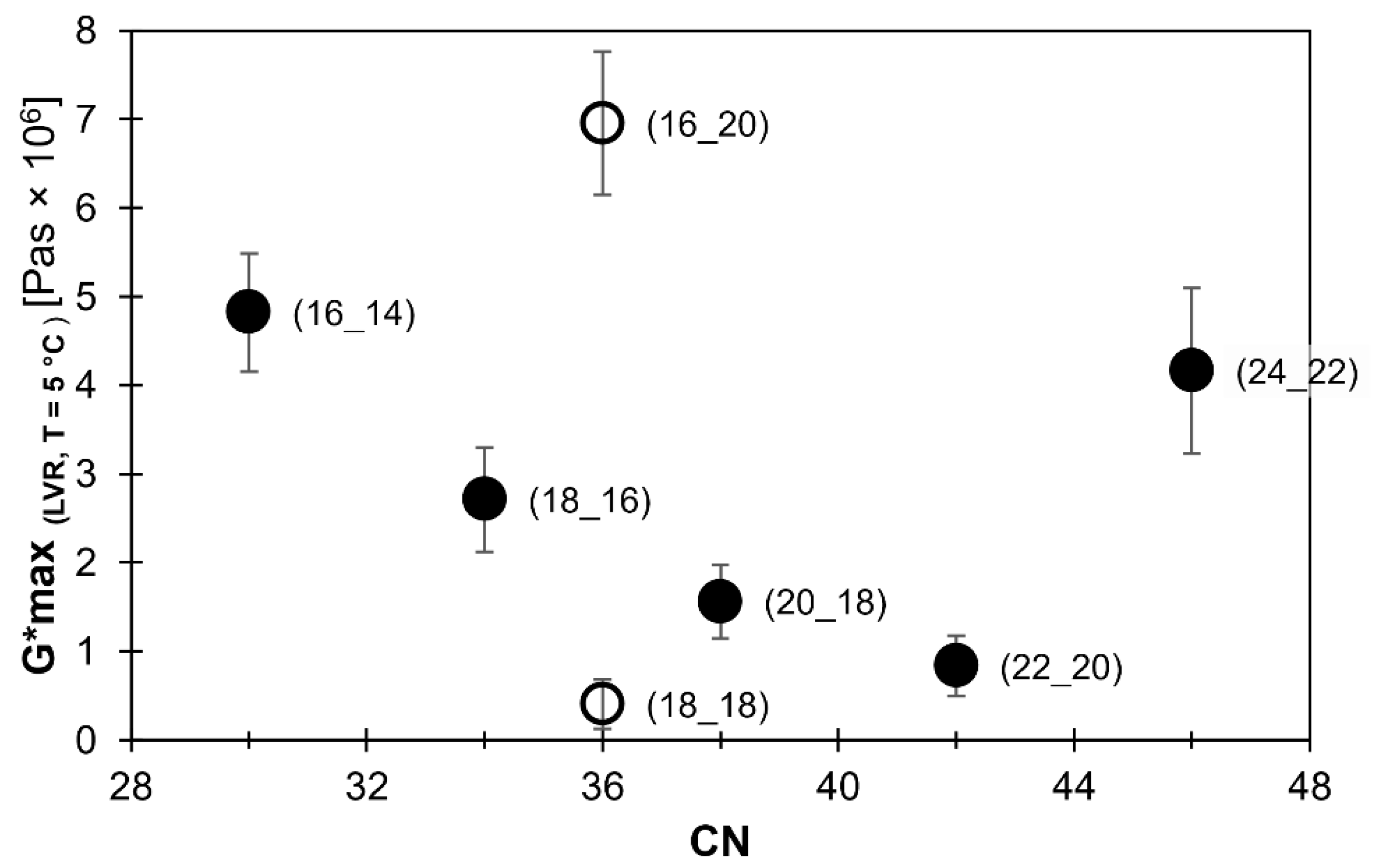
2.1. Viscoelastic Behavior
2.2. Microstructure
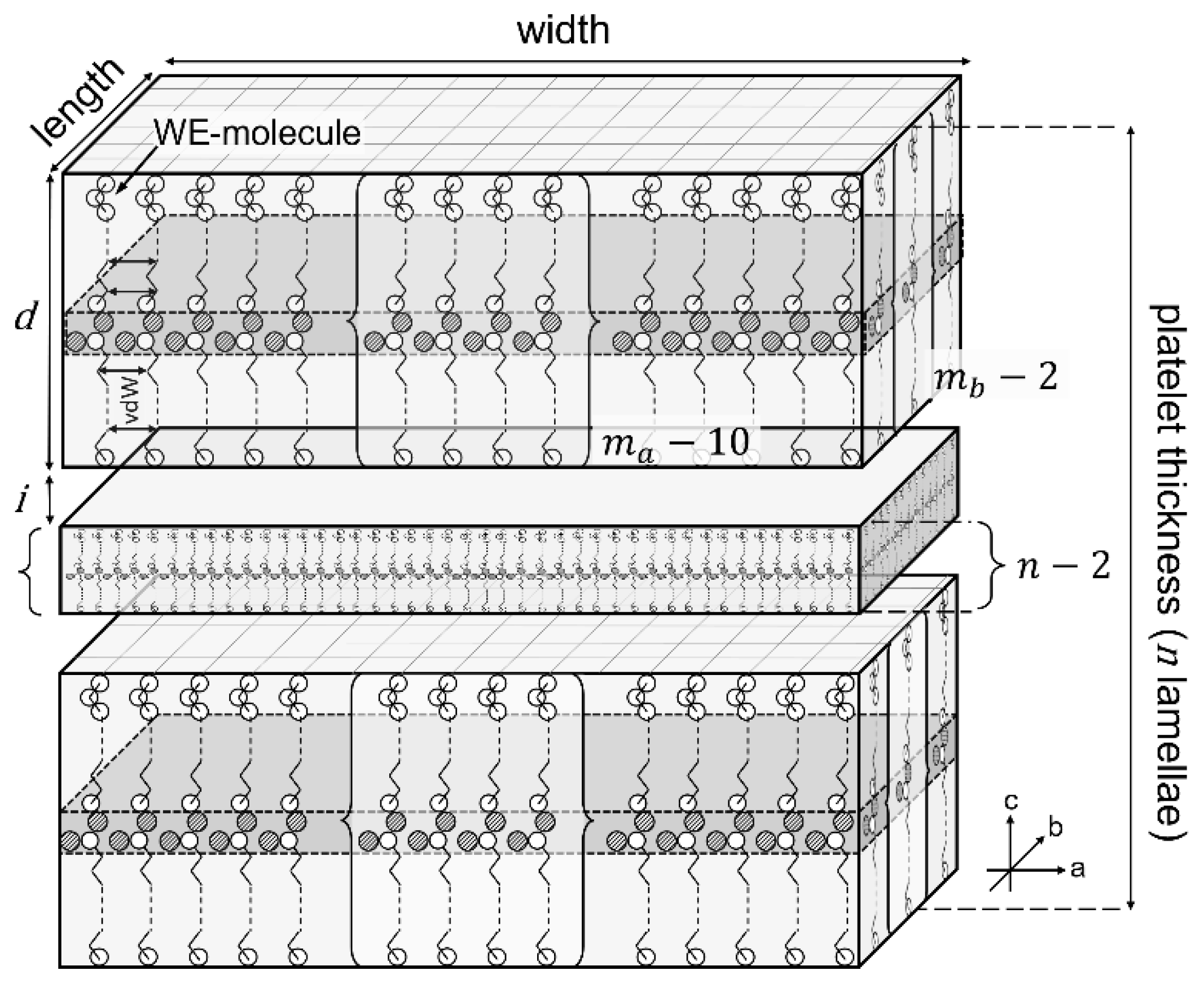
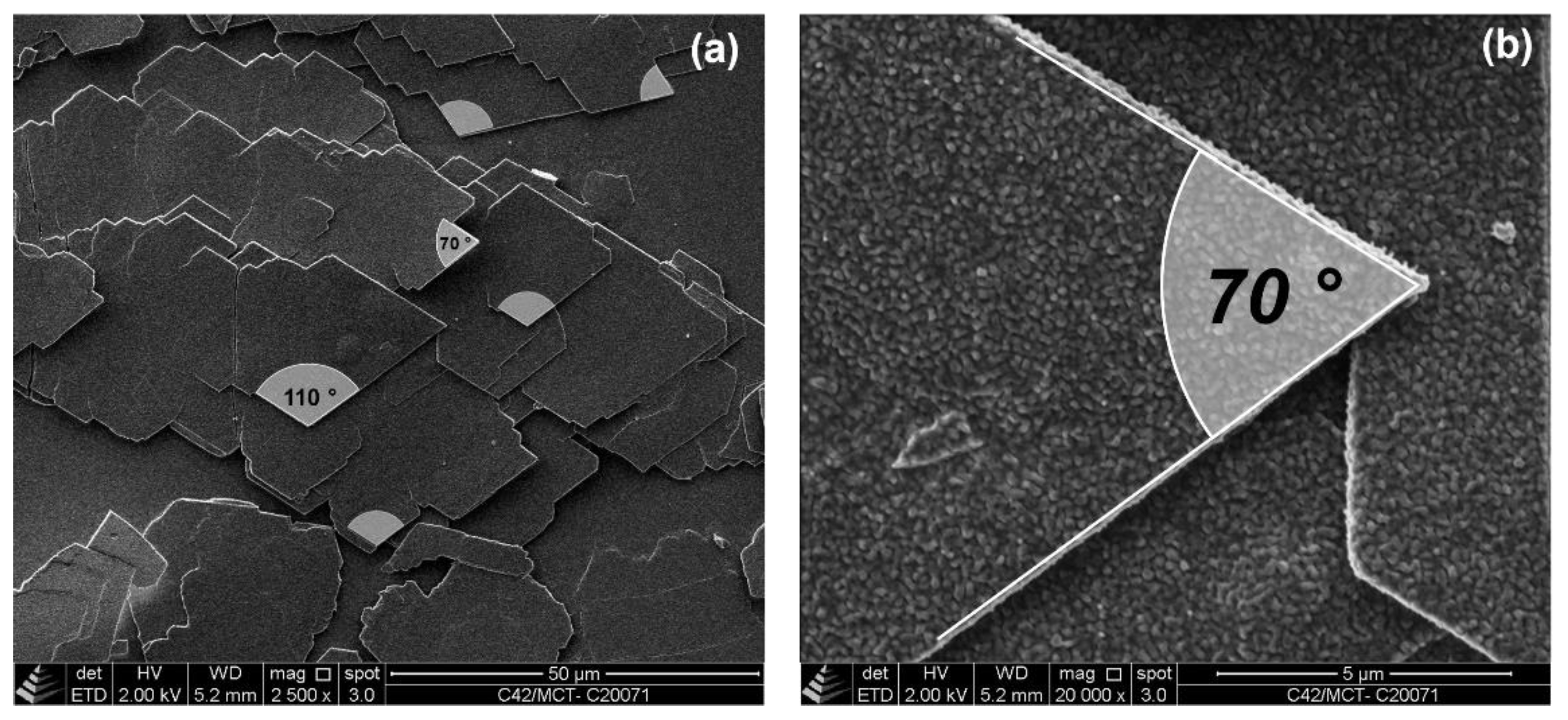
2.2.1. Cryo-Scanning Electron Microscopy (C-SEM)
2.2.2. Bright Field Microscopy (BFM)
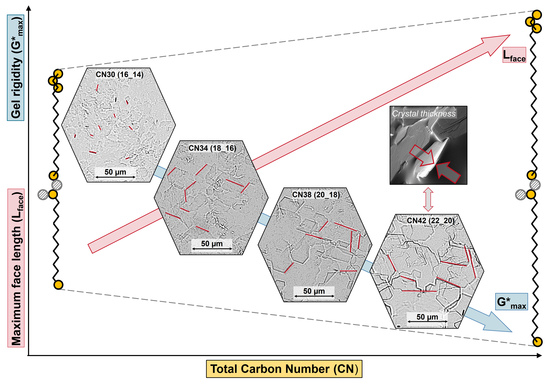
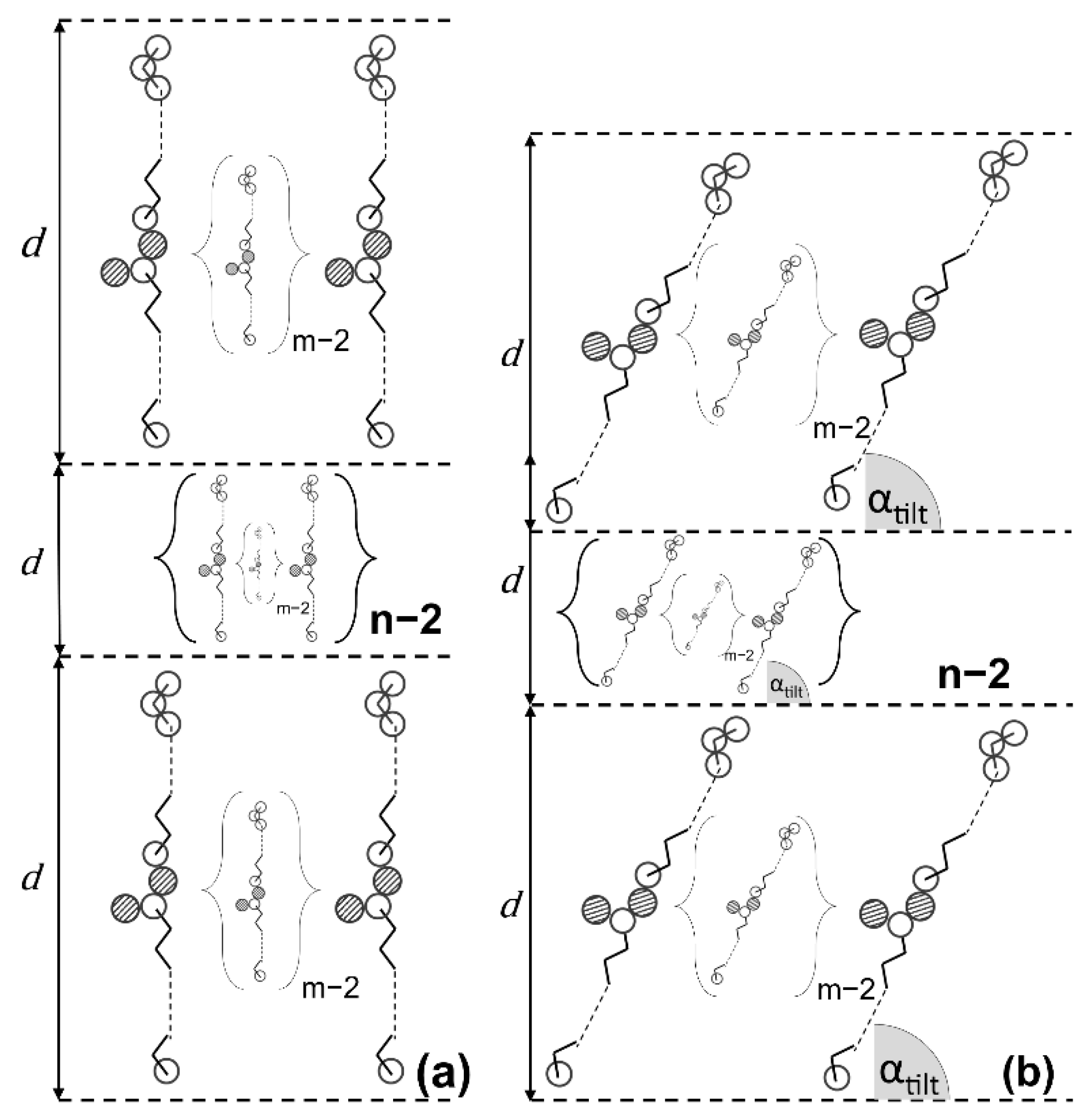
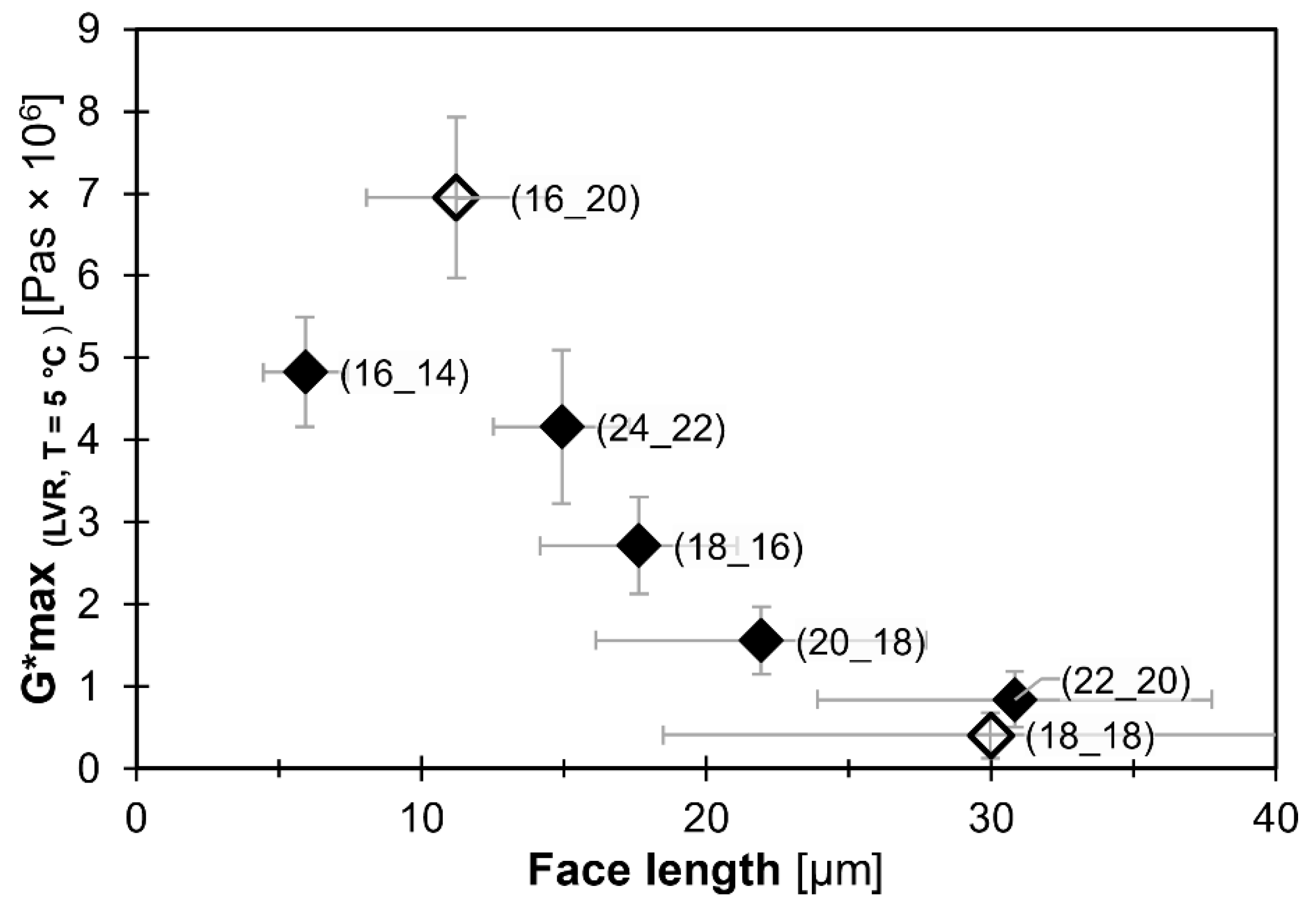
2.3. Relationship between Microstructure and Viscoelastic Behavior
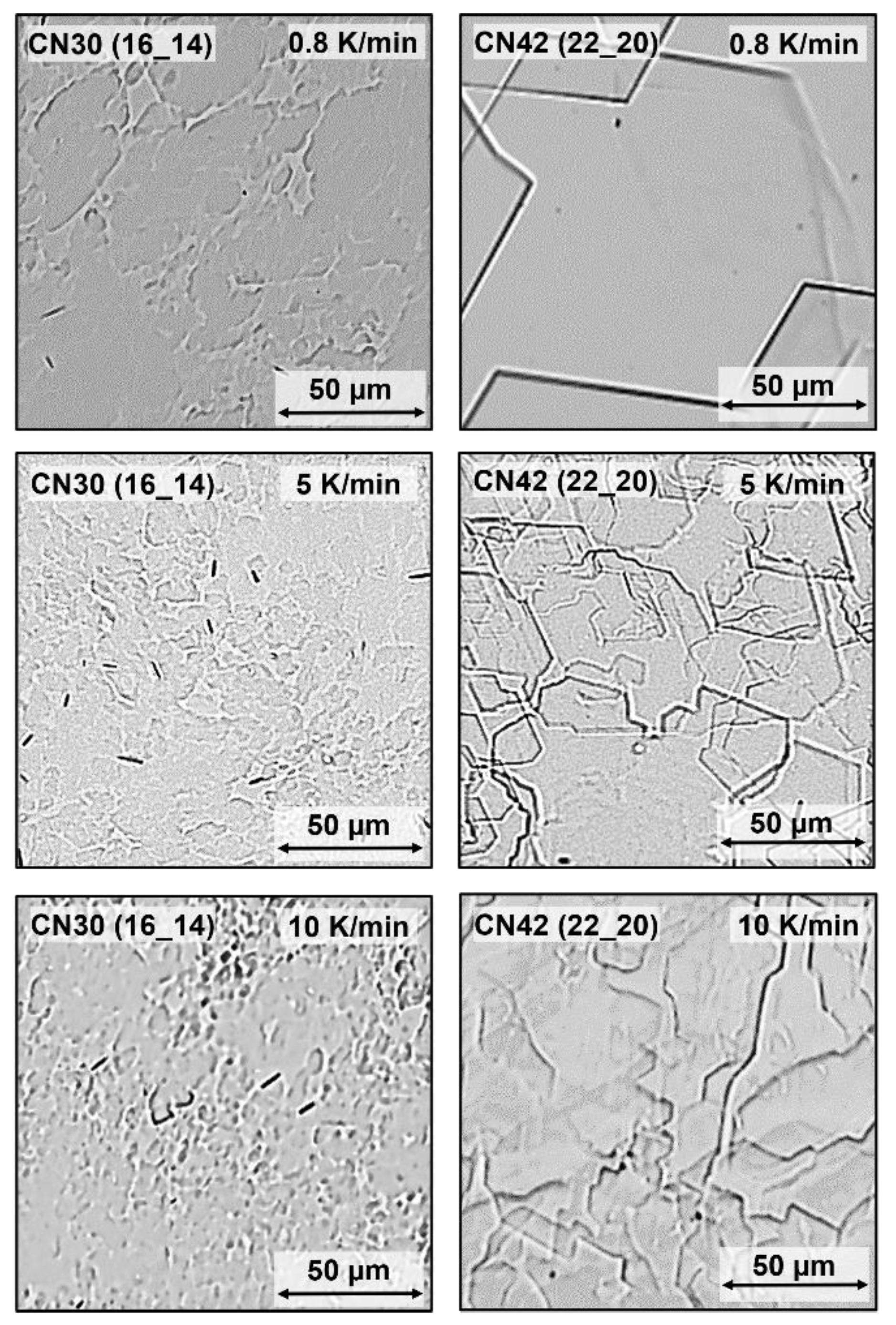
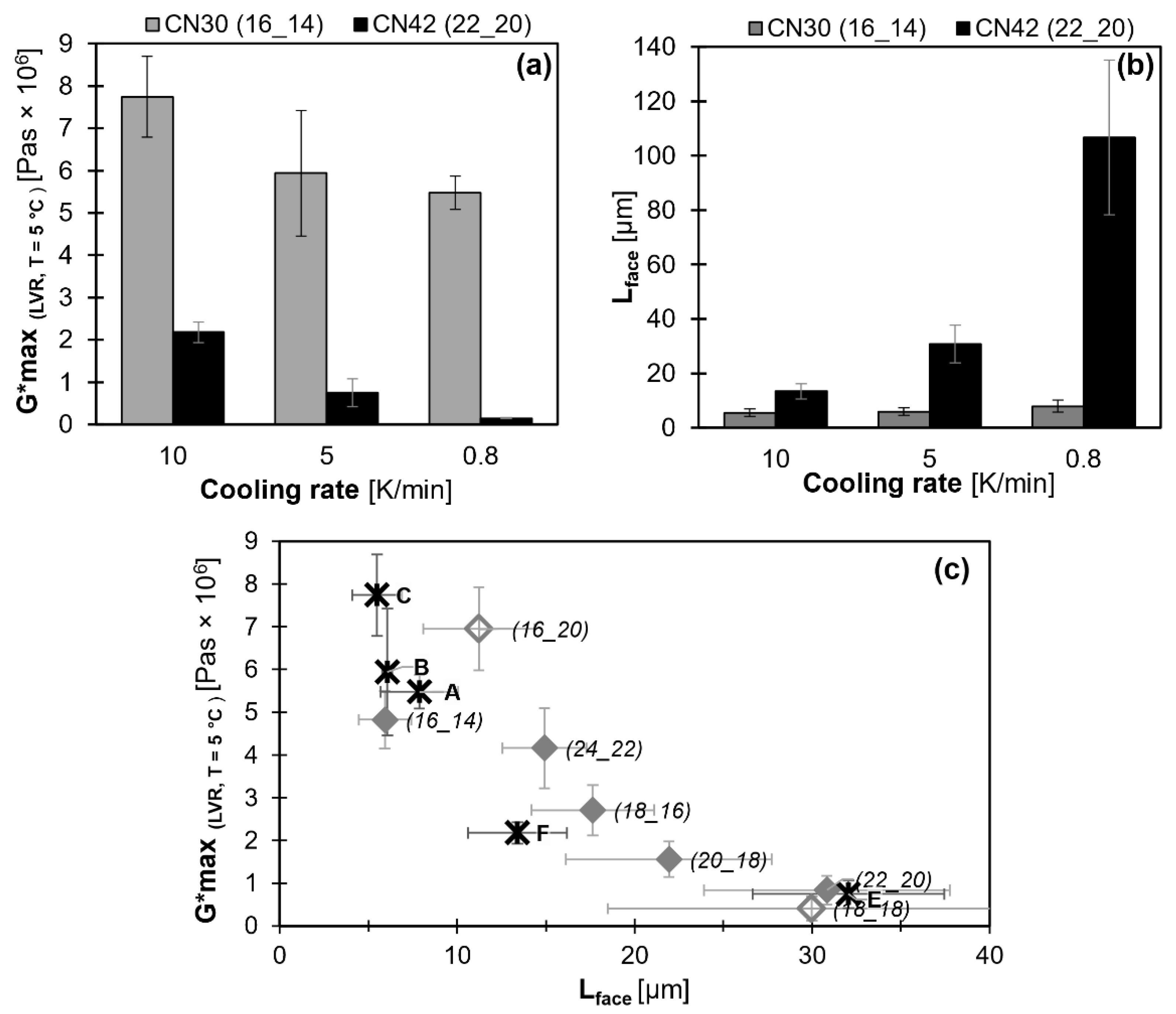
2.4. Effect of Cooling Rate
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Methods
4.2.1. Oleogel Preparation
4.2.2. Viscoelastic Behavior (Rheology)
4.2.3. Microstructure
Scanning Electron Microscopy (SEM)
Bright Field Microscopy (BFM)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flöter, E.; Wettlaufer, T.; Conty, V.; Scharfe, M. Oleogels-Their Applicability and Methods of Characterization. Molecules 2021, 26, 1673. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.I.; Co, E.D. Marangoni AG Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil. Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- No 231/2012, Commission Regulation (EU). Publications Office of the European Union: Brussels, Belgium, 2012.
- Title 21: Foods and Drugs CFR: § 172.890. In Direct Food Substances Affirmed as Generally Recognized as Safe; 2012; Volume 01.04.2021. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol3/pdf/CFR-2021-title21-vol3.pdf (accessed on 17 August 2022).
- Doan, C.D. Structure-function of wax-based oleogels prepared in rice bran oil. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Holloway, P.J. The composition of beeswax alkyl esters. J. Am. Oil. Chem. Soc. 1969, 46, 189–190. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Wettlaufer, T.; Brykczynski, H.; Flöter, E. Wax-Based Oleogels—Properties in Medium Chain Triglycerides and Canola Oil. Eur. J. Lipid. Sci. Technol. 2021, 124, 2100114. [Google Scholar] [CrossRef]
- Brykczynski, H.; Wettlaufer, T.; Flöter, E. Revisiting pure component wax esters as basis of wax-based oleogels. J. Am. Oil. Chem. Soc. 2022. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. Edible Oleogels: Structure and Health Implications; AOCS Press: Urbana, IL, USA, 2011. [Google Scholar]
- Blake, A.I.; Toro-Vazquez, J.F.; Hwang, H.-S. Wax Oleogels; Academic Press: London, UK; AOCS Press: London, UK, 2018; pp. 133–171. [Google Scholar]
- Patel, A.R. Natural Waxes as Oil Structurants. In Alternative Routes to Oil Structuring; Patel, A.R., Ed.; Springer International Publishing: Cham, Germany, 2015; pp. 15–27. [Google Scholar]
- Ensikat, H.J.; Boese, M.; Mader, W.; Barthlott, W.; Koch, K. Crystallinity of plant epicuticular waxes: Electron and X-ray diffraction studies. Chem. Phys. Lipids 2006, 144, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K. Lipids: Molecular organization, physical functions and technical applications. In Oily Press lipid library; Oily Press: Dundee, UK, 1994; Volume 5. [Google Scholar]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Crystallization kinetics of organogels prepared by rice bran wax and vegetable oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Wesdorp, L.H. (Eds.) Structure and Properties of Fat Crystal Networks, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks. Food Biophys. 2015, 10, 456–465. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. The Effect of Shear on the Microstructure and Oil Binding Capacity of Wax Crystal Networks. Food Biophys. 2015, 10, 403–415. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Hetzer, B.; Flöter, E. Characterization of Oleogels Based on Waxes and Their Hydrolyzates. Eur. J. Lipid. Sci. Technol. 2021, 123, 2000345. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yılmaz, E. Preparation and Characterization of Virgin Olive Oil-Beeswax Oleogel Emulsion Products. J. Am. Oil. Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Rocha JC, B.; Lopes, J.D.; Mascarenhas MC, N.; Arellano, D.B.; Guerreiro LM, R.; da Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.; Evans, K.O.; Koga, C.; Lee, Y. Morphology and networks of sunflower wax crystals in soybean oil organogel. Food Struct. 2015, 5, 10–20. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Flöter, E. Effect of Cooling Rate on the Oleogel Properties of Wax–Wax-Hydrolyzate Mixtures. Food Biophys. 2022. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Blake, A.I. Marangoni AG Plant wax crystals display platelet-like morphology. Food Struct. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Avendaño-Vásquez, G.; De la Peña-Gil, A.; Charó-Alvarado, M.E.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Self-Assembly of Symmetrical and Asymmetrical Alkyl Esters in the Neat State and in Oleogels. Front. Sustain. Food Syst. 2020, 4, 132. [Google Scholar] [CrossRef]
- Iyengar, B.T.; Schlenk, H. Melting points of synthetic wax esters. Lipids 1969, 4, 28–30. [Google Scholar] [CrossRef]
- Aleby, S.; Fischmeister, I.; Iyengar, B.T.R. The infrared spectra and polymorphism of long chain esters: IV. Some esters from tetradecanol, hexadecanol, octadecanol, eicosanol, docosanol and dodecanoic, tetradecanoic, hexadecanoic, octadecanoic and eicosanoic acid. Lipids 1971, 6, 421–425. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Callau, M.; Sow-Kébé, K.; Nicolas-Morgantini, L.; Fameau, A.L. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J. Colloid. Interface Sci. 2020, 560, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, F.G.; Bot, A.; Flöter, E. Structuring of edible oils by long-chain FA, fatty alcohols, and their mixtures. J. Am. Oil. Chem. Soc. 2004, 81, 1–6. [Google Scholar] [CrossRef]
- Choi, K.O.; Hwang, H.S.; Jeong, S.; Kim, S.; Lee, S. The thermal, rheological, and structural characterization of grapeseed oil oleogels structured with binary blends of oleogelator. J. Food Sci. 2020, 85, 3432–3441. [Google Scholar] [CrossRef]
- Ahmadi, L.; Wright, A.J.; Marangoni, A.G. Structural and Mechanical Behavior of Tristearin/Triolein-rich Mixtures and the Modification Achieved by Interesterification. Food Biophys. 2009, 4, 64–76. [Google Scholar] [CrossRef]
- Acevedo, N.C. Characterization of the Nanostructure of Triacylglycerol Crystal Networks. In Structure-Function Analysis of Edible Fats; Marangoni, A.G., Ed.; AOCS Press: Champaign, IL, USA, 2018; pp. 1–19. [Google Scholar]
- Nederveen, C. Dynamic mechanical behavior of suspensions of fat particles in oil. J. Colloid Sci. 1963, 18, 276–291. [Google Scholar] [CrossRef]
- Aydın, A.A. High-chain fatty acid esters of 1-octadecanol as novel organic phase change materials and mathematical correlations for estimating the thermal properties of higher fatty acid esters’ homologous series. Sol. Energy Mater. Sol. Cells 2013, 113, 44–51. [Google Scholar] [CrossRef]
- Aydın, A.A. High-chain fatty acid esters of 1-hexadecanol for low temperature thermal energy storage with phase change materials. Sol. Energy Mater. Sol. Cells 2012, 96, 93–100. [Google Scholar] [CrossRef]
- Aydın, A.A.; Okutan, H. High-chain fatty acid esters of myristyl alcohol with even carbon number: Novel organic phase change materials for thermal energy storage—1. Sol. Energy Mater. Sol. Cells 2011, 95, 2752–2762. [Google Scholar] [CrossRef]
- Floeter, E.; Haeupler, M.; Sato, K. Molecular Interactions and Mixing Phase Behavior of Lipid Crystals. In Crystallization of Lipids: Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals, 1st ed.; Sato, K., Ed.; Wiley: Hoboken, NJ, USA, 2018; Volume 149, pp. 61–104. [Google Scholar]
- Kohlhaas, R. Röntgenographische Untersuchung von definierten Einkristallen des Palmitinsäure-Cetylesters. Z. Für Krist. Cryst. Mater. 1938, 98, 418–438. [Google Scholar] [CrossRef]
- Kreger, D.R.; Schamhart, C. On the long crystal-spacings in wax esters and their value in micro-analysis of plant cuticle waxes. Biochim. Et Biophys. Acta 1956, 19, 22–44. [Google Scholar] [CrossRef]
- Sato, K. (Ed.) Crystallization of Lipids: Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals, 1st ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yao, Y.; Zhou, H.; Liu, W.; Li, C.; Wang, S. The Effect of Cooling Rate on the Microstructure and Macroscopic Properties of Rice Bran Wax Oleogels. J. Oleo Sci. 2021, 70, 135–143. [Google Scholar] [CrossRef] [PubMed]



| Total Carbon Number (CN) | % [m/m] | % [w/w] |
|---|---|---|
| 30 | 10 | 8.93 |
| 34 | 10 | 9.93 |
| 36 | 10 | 10.42 |
| 38 | 10 | 10.91 |
| 42 | 10 | 11.86 |
| 46 | 10 | 12.80 |
| WE (FaOH_FA) | Lface [µm] | Standard Deviation |
|---|---|---|
| CN 30 (16_14) | 5.9 | ±1.5 |
| CN 34 (18_16) | 17.6 | ±3.5 |
| CN 36 (18_18) | 30.0 | ±11.5 |
| CN 36 (16_20) | 11.2 | ±3.1 |
| CN 38 (18_20) | 21.9 | ±5.8 |
| CN 42 (22_20) | 30.8 | ±6.9 |
| CN 46 (24_22) | 14.9 | ±2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brykczynski, H.; Hetzer, B.; Flöter, E. An Attempt to Relate Oleogel Properties to Wax Ester Chemical Structures. Gels 2022, 8, 579. https://doi.org/10.3390/gels8090579
Brykczynski H, Hetzer B, Flöter E. An Attempt to Relate Oleogel Properties to Wax Ester Chemical Structures. Gels. 2022; 8(9):579. https://doi.org/10.3390/gels8090579
Chicago/Turabian StyleBrykczynski, Henriette, Birgit Hetzer, and Eckhard Flöter. 2022. "An Attempt to Relate Oleogel Properties to Wax Ester Chemical Structures" Gels 8, no. 9: 579. https://doi.org/10.3390/gels8090579